Abstract
Purpose: Hyperthermia induces tumour cell apoptosis through the mitochondrial apoptotic pathway; however, the signal transduction mechanism underlying this process still needs to be fully elucidated. Phospholipid scramblase 3 (PLS3), a target of protein kinase C-δ (PKC-δ), resides in mitochondria and plays pivotal roles in regulating apoptotic response. Activated PLS3 facilitates cardiolipin (CL) translocation from the mitochondrial inner membrane to the outer leaflet of the mitochondrial outer membrane and triggers apoptosis.
Materials and methods: The tongue squamous cell carcinoma Tca8113 cells were transfected or co-transfected using Lipofectamine 2000 with plasmids pCMV-6×His-PLS3, pCMV-6×His-PLS3 (T21A), pHA-PKC-δ, pHA-PKC-δ-KD (K376R), pHA-Hsp27, and empty control plasmid pcDNA3.1. The transfected cells were heated in water bath at 43°C for 20 min, 40 min and 60 min. Assessments of apoptosis and redistribution of mitochondrial cardiolipin were performed by flow cytometry. PLS3, PKC-δ, Hsp27, phosphorylation of PLS3 and PLS3/PKC-δ interaction were detected by western blotting.
Results: In our study the results show that elevated levels of the wild-type PLS3, but not the PLS3 (T21A) mutant, is able to increase hyperthermia-induced CL translocation and apoptosis. Wild-type PKC-δ facilitates PLS3 phosphorylation, PKC-δ/PLS3 interaction, and CL translocation, which consequently promote apoptosis. In contrast, heat shock protein 27 (Hsp27) blocks PKC-δ-induced PLS3 phosphorylation, suppresses PKC-δ/PLS3 interaction and CL translocation, and inhibits apoptosis.
Conclusions: Our findings suggest that phosphorylation of PLS3 by PKC-δ is involved in the hyperthermia-induced apoptotic signal transduction pathway in Tca8113 cells, and that Hsp27 blocks this pathway to suppress hyperthermia-induced apoptosis.
Introduction
Cancer is treated with a combination of therapeutic modalities, i.e. surgery, radiotherapy, chemotherapy, immunotherapy, and hyperthermia. Hyperthermia is an important component of combined tumour therapy and is a very effective and promising treatment Citation[1], Citation[2]. External hyperthermic stimuli activate the mitochondrial apoptotic pathway, and induce tumour cell apoptosis Citation[3–5]; however, the related apoptotic signal transduction pathways and mechanisms of regulation have not yet been fully elucidated.
Cardiolipin (CL) is an anionic phospholipid that is present in the outer surface of the mitochondrial inner membrane (IMM) where it is also synthesised. Due to the location of CL synthase, 90% of CL resides mainly within the inner mitochondrial membrane Citation[6]. Within the mitochondria cytochrome c is bound to IMM by its association with CL which plays an important role in the retention of IMM-bound cytochrome c within the intermembrane space. CL is involved in the apoptotic signal transduction mediated by cytochrome c release from mitochondria Citation[7], Citation[8]. The oxidation or degradation of CL and the translocation of CL from the mitochondrial inner membrane to the outer leaflet of the mitochondrial outer membrane collectively trigger a decrease in the amount of CL within the mitochondrial inner membrane, promote cytochrome c release from the mitochondria, and induce cellular apoptosis Citation[6], Citation[7], Citation[9]. Changes in CL have been demonstrated in the early stages of apoptosis, and these changes are considered to be an indicator of cellular apoptosis Citation[8], Citation[10].
Phospholipid translocation plays a pivotal role in multiple aspects of apoptosis Citation[11], Citation[12]. CL, a unique mitochondrial lipid, must be translocated from the inner membrane (IM) to the outer leaflet of the outer membrane (OM) in order to induce apoptosis Citation[9]. Phospholipid scramblase (PLS) is a family of enzymes responsible for bidirectional movement of phospholipids between two compartments Citation[13]. Four members of the PLS family have been identified: PLS1 is present in the plasma membrane, PLS2 is reported to be in the nucleus, PLS3 is in mitochondria, and PLS4 has not been characterised. PLS3 is involved in the synthesis of CL Citation[14]. PLS3 is a critical regulator of mitochondrial structure, respiratory function, and distribution of CL, and is responsible for translocating CL from the IMM to the OM of mitochondria Citation[15]. PLS3 is essential for apoptotic signal transduction. Over-expression of wild-type PLS3 promotes UV-induced apoptosis, and results in CL translocation and increased CL distribution on the outer leaflet of the outer mitochondrial membrane Citation[15]. Over-expression of wild-type PLS3 not only promotes tumour necrosis factor-α (TNF-α)-induced apoptosis but also CL translocation Citation[16] In addition, PLS3 phosphorylation facilitates CL translocation and cytochrome c release, and subsequently increases the activities of caspase-3 and caspase-9, and enhances TRAIL-induced apoptosis Citation[17]. These findings suggest that apoptotic stimulation can induce cell apoptosis through the PLS3 → CL signal transduction pathway.
Previous studies have shown that protein kinase C-δ (PKC-δ), a member of the PKC family of serine-threonine kinases phosphorylates PLS3 Citation[9], Citation[17], Citation[18]. PKC is a family of mammalian cytoplasmic lipid-dependent serine/threonine protein kinases containing at least 10 members. Differences in PKC isozyme protein structure and substrate preferences have allowed the family to be divided into three subfamilies: the conventional group (α, βI, βII and γ), the novel group (δ, ε, η and θ), and the atypical group (ζ and ι /λ) Citation[19]. PKC regulates a wide variety of cellular functions, including cell proliferation, differentiation, and apoptosis Citation[19], Citation[20]. PKC-δ, one member of this novel subfamily, is present in the cytoplasm, and plays a crucial role in apoptotic regulation Citation[21]. PKC-δ can be activated by a variety of stimuli. Activated PKC-δ translocates to various organelles, including the nucleus, mitochondria, the plasma membrane, Golgi complex, and endoplasmic reticulum. PKC-δ induces various downstream events in each organelle that eventually lead to apoptosis Citation[21]. Its function depends on the specific cell type in which it is expressed, the stimulus present, and the cellular compartment where it is translocated Citation[21], Citation[22]. PLS3 is a physiologic target of PKC-δ when PKC-δ translocates to mitochondria Citation[18]. PLS3 is the direct mitochondrial effector of PKC-δ and an important downstream factor for apoptotic pathways Citation[20]. The activation and translocation of PKC-δ into mitochondria are crucial for apoptosis Citation[21]. Activated PKC-δ interacts with PLS3, and activates PLS3 via phosphorylation, causing the translocation of CL from IMM to the outer leaflet of the outer membrane and inducing apoptosis Citation[9],Citation[15–19], Citation[22], Citation[23]. PKC-δ activates PLS3 through phosphorylation of Thr21. After Thr21 is mutated to alanine, the PLS3 mutant, PLS3 (T21A), cannot be activated by PKC-δ. It has been shown that the T21A mutation of PLS3 eliminates PKC-δ-facilitated PLS3 phosphorylation, abrogates PKC-δ/PLS3 interaction, blocks the PKC-δ/PLS3-induced apoptotic pathway, and inhibits apoptosis Citation[23], Citation[24].
Heat shock proteins are highly homologous molecular chaperone proteins that are pivotal for cell survival. Development of cellular heat tolerance is related to production of heat shock proteins Citation[25]. Suppression of heat shock protein expression may be a strategy for cancer treatment Citation[26], Citation[27]. HSPs include multiple families, among which low molecular weight HSPs, such as Hsp27 (27 kD), are closely associated with tumour progression. Hsp27 decreases the mitochondrial release of cytochrome c, blocks the activation of the caspase apoptotic cascade Citation[28], and plays an important role in the development of resistance to heat, radio, and chemo therapies []. Inhibition or down-regulation of Hsp27 enhances thermo- and radio-sensitivity of tumour cells []. Poor prognosis has often been observed among cancer patients with over-expression of Hsp27. It has been reported that Hsp27 is a direct or indirect substrate of PKC-δ Citation[36], Citation[37]. Activation of Hsp27 via phosphorylation can diminish or block PKC-δ-induced apoptotic signal transduction Citation[38].
In this study we set up a hyperthermia-induced apoptosis model using the Tca8113 cell line derived from human tongue squamous cell carcinoma. We investigated the interaction between PKC-δ, PLS3, and Hsp27 and the related molecular mechanism during hyperthermia-induced Tca8113 apoptosis, with a focus on CL translocation in mitochondria, the apoptosis regulatory centre. Our results show that hyperthermia induces PLS3 phosphorylation by PKC-δ and translocation of CL, leading to apoptosis. Furthermore, Hsp27 inhibits phosphorylation of PLS3, which blocks CL translocation and reduces apoptosis.
Materials and methods
Materials
The human tongue squamous cell carcinoma cell line Tca8113 was purchased from Shanghai Cell Bank. Eukaryotic expression plasmid pCMV-6×His-PLS3, pCMV-6×His-PLS3 (T21A), pHA-PKC-δ, pHA-PKC-δ-KD (K376R), pHA-Hsp27, and empty control plasmid pcDNA3.1 were gifts from Ray Lee. Anti-phosphothreonine (PT) monoclonal antibody was purchased from Sigma-Aldrich (St Louis, MO). Anti-Hsp27 polyclonal antibody and anti-β-actin polyclonal antibody were from Cell Signaling Technology (Beverly, MA). Anti-PLS3 polyclonal antibody was obtained from Proteintech Group (Chicago, IL). Anti-PKC-δ monoclonal antibody was from BD Biosciences (Palo Alto, CA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA). The study protocol was approved by the Affiliated Stomatological Hospital of Kunming Medical College.
Cell culture, transfection, and heat shock
Tca8113 cells were cultured in RPMI 1640 medium containing 10% bovine foetal serum (1 × 105 U/L penicillin and streptomycin 1 × 102 mg/L) at 37°C and 5% CO2. Tca8113 Cells at 90% confluence were transfected or co-transfected with different plasmids expression vectors using Lipofectamine 2000 according to the manufacturer's protocol. Twenty-four hours after transfection, the transfected cells and parent cells were heat shocked in a temperature-controlled water bath at 43°C. Unless otherwise specified, the duration of heat treatment was 40 min and the cells were returned to normal culture conditions afterwards for a 4-h or 24-h incubation before being harvested for further analysis.
Cell lysate preparation
Tca8113 cells were harvested and washed with cold PBS, and then resuspended in lysis buffer (50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, and 1 mM phenylmethylsulphonyl fluoride). After incubation on ice for 5 min, cells were centrifuged at 10,000 × g at 4°C for 10 min, and the supernatant was collected as cell lysate and the protein concentration was measured with the Bio-Rad protein assay (Bio- Rad Laboratories, Hercules, CA) and adjusted to same concentrations for each sample.
Western blotting analysis
Equal amounts of protein were separated by 10% SDS-PAGE and electroblotted onto Immobilon-P membrane (Millipore, Bedford, MA). The membrane was incubated with the primary antibody at 4°C overnight, and then incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. After washing with TBS/T (1 × TBS and 0.1% Tween-20), the membrane was visualised by chemiluminescence (Pierce, Rockford, IL).
His pull-down
This method was used as described previously Citation[23]. The cells were washed with cold PBS and resuspended in lysis buffer (Pierce His Protein Interaction Pull-Down Kit, Hermo Fisher Scientific, CA) (50 mM NaH2PO4, pH 8.0, containing 500 mM NaCl, 20 mM imidazole, 1% Triton X-100, and 20 mM 2-mercaptoethanol (pH 8.0)) for 5 min and sonicated briefly on ice. Cell lysates were centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was collected and incubated with Ni-NTA magnetic agarose beads (QIAGEN, Hilden, Germany) for 2 h. After incubation, the Ni-beads were collected by centrifugation at 800 × g, at 4°C for 2 min, thoroughly washed with cold wash buffer (50 mM NaH2PO4, 500 mM NaCl, 20 mM imidazole, 1% Triton X-100 (pH 8.0)) five times, and resuspended in SDS for western blotting analysis.
Immuno-coprecipitation
Whole cell lysates were incubated with 1.25 µg/mL anti-PKC-δ antibody at 4°C for 2 h, and then PKC-δ was precipitated by a 2 h incubation after addition of protein A/G. The immunoprecipitate was washed with RIPA buffer (20 mM Tris-HCl, pH 8.0, containing 1% NP-40, 0.2% deoxycholate, and 120 mM NaCl), and resuspended in SDS for western blotting analysis.
Analysis of apoptosis
The cells were harvested, fixed with 70% cold ethanol at −20°C overnight. Cells were then washed with PBS and stained with 0.025 mg/mL propidium iodide (PI), 0.05% Triton X-100, 0.1 mg/mL RNase A for 30 min at room temperature under dark. The DNA content was then determined using flow cytometry. The hypodiploid peak in the early G0/G1 phase was considered the apoptosis peak and used for the calculation of the percentage of apoptotic cells.
Analysis of CL distribution on the outer leaflet of the mitochondrial outer membrane
10-N-nonyl acridine orange (NAO) (Molecular Probes, Eugene, OR), binds to CL at the saturation level with a stoichiometric ratio of 2 : 1 and emits red fluorescence at 630 nm. When cells are incubated with increasing concentrations of NAO, CL on the outer leaflet of the outer mitochondrial membrane is saturated first, followed by CL residing on the inner leaflet of the outer membrane and on the inner membrane. Increasing NAO concentration over the saturation level reduced fluorescence intensity Citation[39]. The cells were harvested and fixed with 1% paraformaldehyde at room temperature for 10 min. After PBS wash, cells were incubated with various concentrations of NAO for 15 min, the low concentrations of NAO was used to saturate CL on the outer leaflet of the outer mitochondrial membrane. The fluorescence intensity peak was determined using flow cytometry, and the median value at the first saturation point represents the CL content on the outer membrane. NAO at 35 µM was used to saturate all mitochondrial CL. The median fluorescence intensity value of the peak reflects the total mitochondrial CL content in the target cells. The percentage of CL present on the outer leaflet of the outer mitochondrial membrane was determined. This method was used by Garcia-Fernandez et al. to quantify the percentages of CL in the different mitochondrial membrane compartments Citation[40].
Statistics
Calculations were performed using SPSS 11.0 software. The differences between two groups were analysed using the least significant difference (LSD) pairwise multiple comparison test.
Results
Analysis of hyperthermia-induced mitochondrial CL translocation and apoptosis in Tca8113 cells
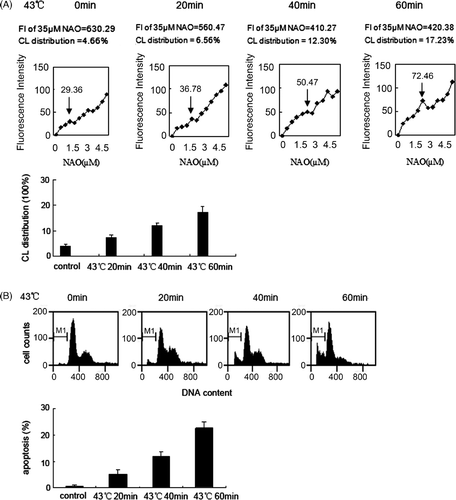
Tca8113 cells were incubated at 43°C for 0, 20, 40, and 60 min, and then cultured under normal conditions for 4 h before harvesting. The cells were stained with NAO, and the percentage of outer leaflet CL to the total amount of CL was determined by flow cytometry. After heat treatment, the cells were cultured under normal conditions for 24 h before harvesting. The cells were stained with propidium iodide (PI) and the percentage of apoptotic cells was determined by flow cytometry. The percentages of mitochondrial outer leaflet CL after incubations of 0, 20, 40, and 60 min were 4.66%, 6.56%, 12.3%, and 17.23%, respectively, and the corresponding apoptotic indices were 0.78%, 5.21%, 11.89%, and 22.7%. These results indicated that both the percentage of mitochondrial outer leaflet CL and the apoptotic index increase with an increase in the length of heat treatment ().
Figure 1. Analysis of apoptosis and mitochondrial CL distribution in parents Tca8113 cells. (A) The parents cells were heated at 43°C for 20 min, 40 min, 60 min, and then incubated at 37°C for 4 h. Cells were stained with different concentrations of NAO, and the median fluorescence intensity at 640 nm was determined by flow cytometry. The median fluorescence intensity value at the first site of plateaus represented saturation of the amount of CL in the outer leaflet of mitochondrial outer membrane (↓ point in the figure).The median fluorescence intensity value of 35 µM NAO was considered to be 100% and used to calculate the percentage in first saturated site NAO concentration. (B) The parents Tca8113 cells were heated at 43°C for 20 min, 40 min, and 60 min, and then cultured at 37°C for 24 h. The apoptotic cells were determined by flow cytometry. Columns, mean from three independent experiments. Bars, ±SD.
Wild-type PLS3 facilitates hyperthermia-induced mitochondrial CL translocation in Tca8113 cells
Tca8113 cells were transfected with the control, wild-type PLS3, and mutant PLS3 (T21A) plasmids for 24 h, respectively. Western blotting of the whole cell lysates revealed that the levels of PLS3 and PLS3 (T21A) were equally expressed (). Cells were then heat shocked for 40 min, and the CL distribution on the outer leaflet of the outer mitochondrial membrane was analysed with NAO staining by flow cytometry. The amount of CL distributed on the outer leaflet of the outer membrane in all three transfected cell lines were increased after heat shock (). Compared to the pre-heat treatment distribution, the percentage of outer leaflet CL increased to greater than two-fold in the Tca8113-control cells (5.9% to 14.22%), about five-fold in the Tca8113-PLS3 cells (5.05% to 24.61%), and greater than three-fold in Tca8113-PLS3 (T21A) cells (4.99% to 16.27%). Among the three transfected cell lines, Tca8113-PLS3 cells showed the largest statistically significant increase of CL on the outer leaflet after heat shock (P < 0.01), whereas Tca8113-Control and Tca8113-PLS3 (T21A) cells displayed similar amounts of CL (P > 0.05) (). These observations indicate that CL translocation in the Tca8113 cells is associated with PLS3 phosphorylation.
Figure 2. Tca8113 cells were transfected with the control, wild-type PLS3, and mutant PLS3 (T21A) plasmids for 24 h, respectively. Western blotting showed comparable expression of PLS3 in Tca8113- PLS3 cells and Tca8113-PLS3 (T21A) cells.
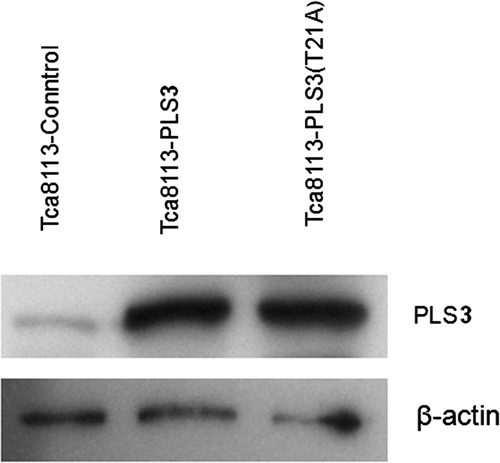
Figure 3. Wild-type PLS3 facilitates heat-induced mitochondrial CL translocation. Tca8113 cells were transfected with the control, wild-type PLS3, and mutant PLS3 (T21A) plasmids for 24 h, respectively. The transfected cells were heated at 43°C for 40 min and then incubated at 37°C for 4 h. Cells were harvested and stained with different concentrations of NAO, and the median fluorescence intensity at 640 nm was determined by flow cytometry. ↓ represent the same means as in . The median fluorescence intensity value of 35 µM NAO was considered to be 100% and used to calculate the percentage in first saturated site NAO concentration. Columns, mean from three independent experiments. Bars, ±SD. *p < 0.01.
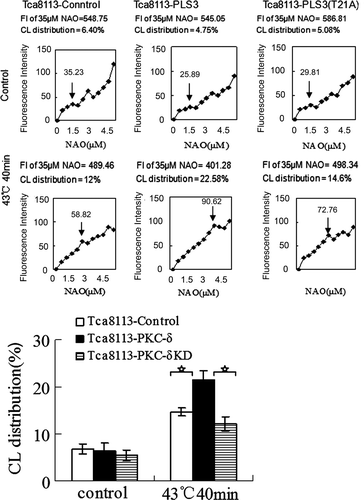
Table I. Redistribution of CL in PLS3- transfected cell groups (%).
Wild-type PLS3 enhances hyperthermia-induced apoptosis in Tca8113 cells
The effect of PLS3 on hyperthermia-induced apoptosis was evaluated by varying the duration of heat shock. The transfected Tca8113 cell lines were incubated at 43°C for 20 min, 40 min, and 60 min, and then cultured under normal conditions for 24 h before harvesting. The cells were stained with PI, and the apoptotic levels were determined by flow cytometry. After heat treatment, the percentage of apoptotic cells increased from 6.25% to 13.56% (20 min), 21.67% (40 min), and 31.94% (60 min) for the Tca8113-control cells. For the Tca8113-PLS3 cells, the percentage of apoptotic cells increased from 6.45% to 19.64% (20 min), 31.11% (40 min), and 39.31% (60 min). Lastly, the percentage of apoptotic cells increased from 7.82% to 12.74% (20 min), 21.93% (40 min), and 30.79% (60 min) in the Tca8113-PLS3 (T21A) cells (). For the same heat treatment duration, the percentage of apoptotic cells in the Tca8113-PLS3 cell line was significantly different (P < 0.01) from those of the other two transfected cell lines, which showed a similar percentage of apoptotic cells (P > 0.05) (). These results indicate that PLS3 facilitates hyperthermia-induced apoptosis, and that the PLS3 (T21A) mutant does not have this effect.
Figure 4. Wild-type PLS3 promotes heat-induced apoptosis. The control, wild-type PLS3, and mutant PLS3 (T21A) plasmids transfected Tca8113 cells were heated at 43°C for 20 min, 40 min, and 60 min, and harvested for apoptosis assays by flow cytometry after cells being cultured at 37°C for 24 h. The apoptotic population was determined by propidium iodide staining. The sub-G1 population was marked by M1 gate and used to represent the apoptotic population. Columns, mean from three independent experiments. Bars, ±SD. *p < 0.01.
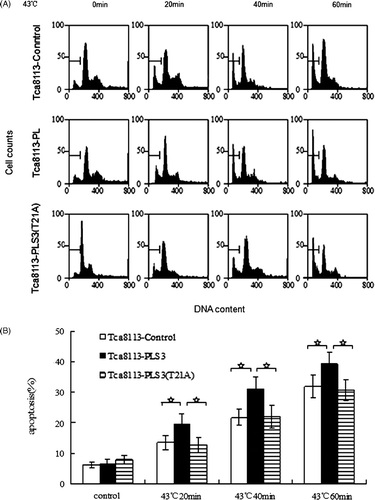
Table II. Apoptosis in of PLS3- transfected cell groups (%).
PLS3 phosphorylation was induced by heat
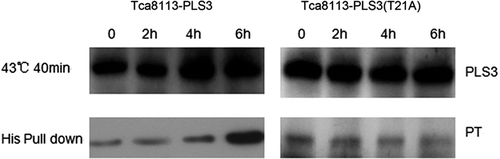
To investigate the correlation between PLS3 phosphorylation and hyperthermia-induced apoptosis, we analysed levels of PLS3 phosphorylation in the wild-type PLS3 and mutant PLS3 (T21A) plasmids transfected cells after heat shock. PLS3 was extracted from the lysate of transfected Tca8113 cells harvested 2 h, 4 h and 6 h after heat treatment according to the His pull-down procedure, and phosphothreonine content was analysed by western blotting using anti-phosphothreonine monoclonal antibody. In the cells transfected with wild-type PLS3, phosphorylation of PLS3 was increased 4 h after heat treatment, with a further significant increase at 6 h. In contrast, no appreciable change of the PLS3 phosphorylation level was observed in cells transfected with the PLS3 (T21A) mutant (). This finding further confirms that PLS3 phosphorylation is closely associated with hyperthermia-induced apoptosis of the tongue squamous carcinoma cells.
Figure 5. Heat-induced phosphorylation of PLS3. The Tca8113-PLS3 and Tca8113-PLS3 (T21A) transfected cells were heat shocked for 40 min and returned to 37°C. PLS3 was extracted from the cell lysate of transfected cells after 2 h, 4 h, and 6 h at 37°C for western blotting assays. Immunoblot analysis shows an increase in phosphorylation level of PLS3 with increasing time at 37°C following heat shock of Tca8113-PLS3 cells, but no change was observed following heat shock of Tca8113-PLS3 (T21A) cells.
Wild-type PKC-δ facilitates hyperthermia-induced mitochondrial CL translocation in Tca8113 cells
Tca8113 cells were transfected with control, wild-type PKC-δ, and PKC-δ-KD (K376R) mutant plasmids. Western blotting of the whole cell lysates revealed that the levels of PKC-δ were comparable in cells transfected with PKC-δ and PKC-δ-KD(K367R) (). Cells were heated at 43°C for 40 min and NAO stained for CL distribution analysis. The amount of CL on the outer leaflet of the outer mitochondrial membrane was elevated in all three transfected cell lines (), with an increase to greater than two-fold in the Tca8113-Control cells (6.8% to 14.7%), greater than three-fold in the Tca8113-PKC-δ cells (6.3% to 21.44%), and greater than two-fold in Tca8113-PKC-δ (K376R) cells (from 5.49% to 12.06%) relative to the amount before heat treatment. The Tca8113-PKC-δ cells showed the largest increase of CL on the outer leaflet after heat shock, which was significantly different from the other two transfected cell lines (P < 0.01). No appreciable difference in the amount of CL on the outer leaflet of the mitochondrial outer membrane after heat shock was observed between the Tca8113-control cells and Tca8113-PKC-δ (K376R) cells (P > 0.05) (). The inactivated PKC-δ (K376R) slightly repressed CL translocation. The above results show that hyperthermia-induced mitochondrial CL translocation is PKC-δ dependent.
Figure 6. Tca8113 cells were transfected with the control, wild-type PKC-δ, and PKC-δ-KD plasmids for 24 h, respectively. Western blotting showed comparable expression of PLS3 in Tca8113- PLS3 cells and Tca8113-PLS3 (T21A) cells.
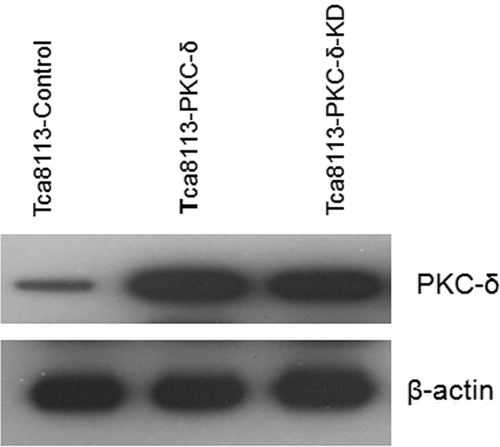
Figure 7. Wild-type PKC-δ facilitates hyperthermia-induced mitochondrial CL translocation. The control, PKC-δ, and PKC-δ-KD plasmids transfected cells were heated at 43°C for 40 min, and then harvested and stained with different concentrations of NAO after cells being incubated at 37°C for 4 h. The median fluorescence intensity at 640 nm was determined by flow cytometry. ↓ represent the same means as in . The median fluorescence intensity value of 35 µM NAO was considered to be 100% and used to calculate the percentage in first saturated site NAO concentration. Columns, mean from three independent experiments. Bars, ±SD. *p < 0.01.
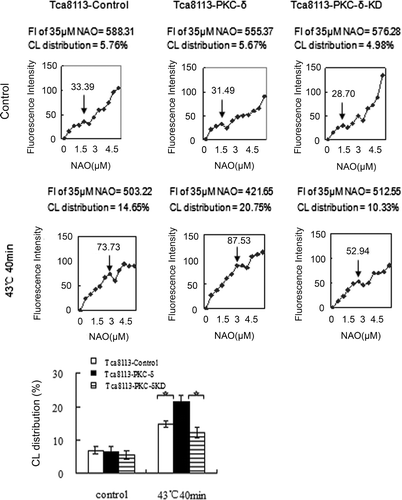
Table III. Redistribution of CL in PKC-δ- transfected cell groups (%).
Wild-type PKC-δ enhances hyperthermia-induced apoptosis in Tca8113 cells
Apoptosis analysis with PI staining was performed on heat-shocked cells cultured under normal conditions for 24 h. Elevated apoptotic levels were observed in all three transfected cell lines (). Compared to the pre-treatment amount, the percentage of apoptotic cells increased from 8.48% to 21.04% for the Tca8113-control cells, from 7.81% to 32.45% for the Tca8113-PKC-δ cells, and from 9.9% to 18.91% for the Tca8113-PKC-δ (K376R) cells. The heat-shocked Tca8113-PKC-δ cells exhibited an apoptotic level that was significantly different from the other two cell lines (P < 0.01), whereas the Tca8113-PKC-δ (K376R) cells and Tca8113-control cells displayed similar apoptotic levels (P > 0.05) (). These results suggest that wild-type PKC-δ facilitates hyperthermia-induced apoptosis, whereas inactivated PKC-δ does not enhance apoptosis.
Figure 8. Wild-type PKC-δ facilitates hyperthermia-induced apoptosis. The three transfected Tca8113 cells were heated at 43°C for 40 min, and harvested for apoptosis assays by flow cytometry after cells being cultured at 37°C for 24 h. The apoptotic population was determined by propidium iodide staining. The sub-G1 population was marked by M1 gate and used to represent the apoptotic population. Columns, mean from three independent experiments. Bars, ±SD. *p < 0.01.
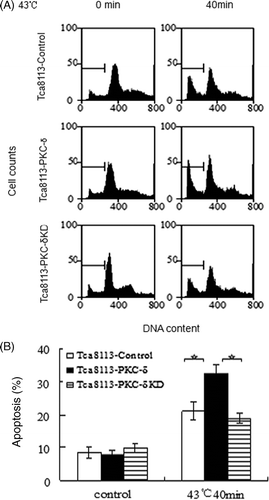
Table IV. Apoptosis in PKC-δ- transfected cell groups (%).
Phosphorylation of PLS3 by wild-type PKC-δ is enhanced by heat
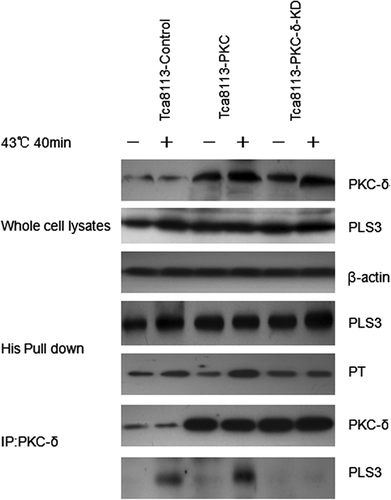
As described above, hyperthermia-induced mitochondrial CL translocation and apoptosis in the Tca8113 cells are associated with PLS3 phosphorylation and are PKC-δ dependent. We speculated that PKC-δ regulates hyperthermia-induced apoptosis through PLS3 phosphorylation. To test this hypothesis we co-transfected the control, wild-type PKC-δ, and PKC-δ-KD (K376R) plasmids with the PLS3 plasmid into the Tca8113 cells, and subjected the cells to heat shock. After heat treatment, equal amounts of PLS3 protein was extracted from the transfected cells following the His pull-down procedure and analysed for phosphorylation level by western blotting using anti-phosphothreonine monoclonal antibody. Compared to the control group, the PLS3 phosphorylation level was increased in the presence of co-transfected PKC-δ, whereas no obvious change was observed with inactivated PKC-δ. Furthermore, immuno-coprecipitation analysis detected an interaction between PLS3 and PKC-δ, which was enhanced by elevated PKC-δ expression. No PLS3/PKC-δ interaction was observed with the inactivated PKC-δ mutant ().
Figure 9. Heat enhances phosphorylation of PLS3 by PKC-δ. The Tca8113 cells were co-transfected with wild-type PLS3 plasmid and the control, wild-type PKC-δ or PKC-δ-KD plasmid for 24 h, respectively. The co-transfected cells were heated at 43°C for 40 min and then cultured at 37°C for 4 h, whole cell lysates were incubated with Ni beads to pull down His-tagged PLS3, and analysed with the antibodies against phosphothreonine and PLS3. PKC-δ was immunoprecipitated from whole cell lysates and analysed with antibodies against PKC-δ and PLS3. The interaction of PLS3/PKC-δ was enhanced by elevated PKC-δ expression, but no PLS3/PKC-δ interaction was observed with the PKC-δ-KD.
Hsp27 decreases outer mitochondrial CL distribution, and suppresses apoptosis
Tca8113 cells were transfected with control and pHA-Hsp27 plasmids for 24 h. Western blotting of the whole cell lysates revealed that the level of Hsp27 was elevated in the pHA-Hsp27 transfected cells (). The Hsp27-transfected Tca8113 cells were analysed for CL distribution and apoptosis following heat shock. Both CL distribution on the outer leaflet of the outer mitochondrial membrane and the apoptotic level were enhanced by heat treatment. The amount of CL on the outer leaflet of the mitochondrial outer membrane increased from 5.47% to 15.22% in the Tca8113-control cells and from 4.85% to 10.24% in the Tca8113-Hsp27 cells (, ). The percentage of apoptotic cells increased from 10.37% to 22.49% in the Tca8113-control cells and from 9.91% to 15.88% in the Tca8113-Hsp27 cells (, ). Both the amount of CL on the outer leaflet of the mitochondrial outer membrane and the apoptotic level of the Tca8113-Hsp27 cells were significantly different from the levels in the Tca8113-control cells (P < 0.05). These results show that Hsp27 suppresses CL translocation and apoptosis
Figure 10. Tca8113 cells were transfected with the control, Hsp27 plasmids for 24 h, respectively. Western blotting showed elevated and stable expression of Hsp27 in Tca8113-Hsp27 cells.
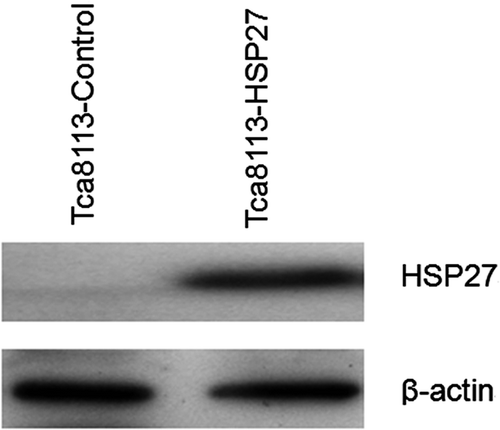
Figure 11. Hsp27 decreases outer mitochondrial CL distribution. Tca8113 cells were transfected with the control and Hsp27 plasmids for 24 h, and harvested and stained with different concentrations of NAO after cells being incubated at 37°C for 4 h. The median fluorescence intensity at 640 nm was determined by flow cytometry. ↓ represent the same means as in . The median fluorescence intensity value of 35 μM NAO was considered to be 100% and used to calculate the percentage in first saturated site NAO concentration. Columns, mean from three independent experiments. Bars, ±SD. **p < 0.05.
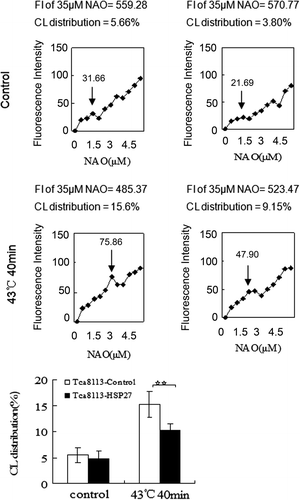
Figure 12. Hsp27 suppresses heat-induced apoptosis. The control and Hsp27 plasmids transfected Tca8113 cells were heated at 43°C for 40 min, and harvested for apoptosis assays by flow cytometry after cells being cultured at 37°C for 24 h. The apoptotic population was determined by propidium iodide staining. The sub-G1 population was marked by M1 gate and used to represent the apoptotic population. Columns, mean from three independent experiments. Bars, SD. **p < 0.05.
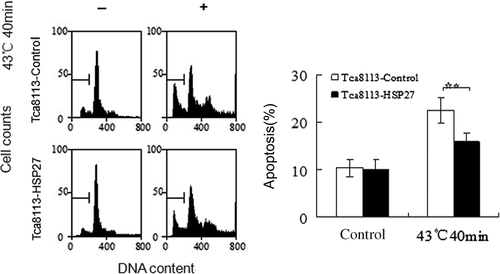
Table V. Redistribution of CL in Hsp27- transfected cell groups (%).
Table VI. Apoptosis in Hsp27- transfected cells groups (%).
Hsp27 suppresses the interaction between PKC-δ and PLS3
This study is designed to investigate the effect of Hsp27 on the PKC-δ/PLS3 interaction and on PLS3 phosphorylation in the hyperthermia-treated Tca8113 cells. The control and Hsp27 plasmids were co-transfected into the Tca8113 cells with the PLS3 plasmid. PLS3 was extracted from heat-shocked cells and its phosphorylation level was analysed. The result showed no appreciable increase in PLS3 phosphorylation in the presence of co-transfected Hsp27. The immuno-coprecipitation analysis using anti-PKC-δ antibody indicated that Hsp27 blocked the PLS3/PKC-δ interaction ().
Figure 13. Hsp27 suppresses the interaction between PKC-δ and PLS3. The Tca8113 cells were co-transfected with the control or Hsp27 plasmids and the PLS3 plasmid for 24 h respectively. The co-transfected cells were heated at 43°C for 40 min and then cultured at 37°C for 4 h, whole cell lysates were incubated with Ni beads to pull down His-tagged PLS3, and analysed with the antibodies against phosphothreonine and PLS3 by western blotting. PKC-δ was immunoprecipitated from whole cell lysates and analysed with antibodies against PKC-δ and PLS3 by western blotting. Western blotting assay showed Hsp27 blocked the PLS3/PKC-δ interaction and decreased the phosphorylation level of PLS3.
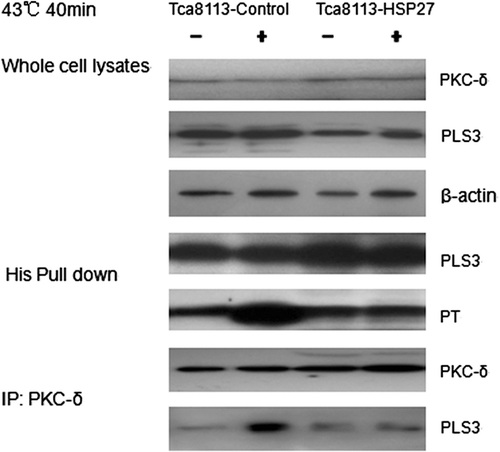
The above results show that Hsp27 inhibits PKC-δ-induced PLS3 phosphorylation, and consequently suppresses hyperthermia-induced mitochondrial CL translocation and Tca8113 cell apoptosis.
Discussion
Hyperthermia treatment of cells in culture enhances pro-apoptotic gene expression, represses anti-apoptotic gene expression, activates apoptosis-related factors, and induces apoptosis Citation[41–43]. This study confirms the thermal induction of the mitochondrial PKC-δ → PLS3 → CL apoptotic signal transduction pathway in Tca8113 cells. This pathway was confirmed using wild-type, mutant and control PLS3 and PKC-δ constructs. Hyperthermia induces translocation of CL and apoptosis in wild-type Tca8113 cells (). Over-expression of the wild-type PLS3 but not over-expression of the non-phosphorylatable PLS3 (T21A) mutant results in increased levels of phosphorylated PLS3, increased CL translocation, and increased apoptosis (). PKC-δ is shown to be the kinase, which is responsible for heat-induced phosphorylation of PLS3 using transfected cells with elevated levels of wild-type PLS3 and elevated levels of wild-type PKC-δ or mutant PKC-δ KD (K376R). Cells with elevated expression of wild-type PKC-δ had increased levels of heat-induced phosphorylated PLS3, PKC-δ/PLS3 interaction, CL translocation and apoptosis compared to controls (). Finally, a series of experiments are performed demonstrating that thermally induced and activated Hsp27 reduces apoptosis by blocking the heat-induced PKC-δ/PLS3 interaction and phosphorylation of PLS3 ().
In our study we transfected Tca8113 cells with wild-type PLS3 and the PLS3 (T21A) mutant plasmids, and evaluated the PLS3 phosphorylation level, apoptotic level, and the CL distribution after heat shock. The results show that the expression level of PLS3 in wild-type PLS3 cells is consistent with that in PLS3 (T21A) mutant cells; however, there was significant difference in the apoptotic index and the amount of CL distributed within the mitochondrial outer membrane between these two transfected cell types. These findings suggested that wild-type PLS3, but not the PLS3 (T21A) mutant, enhances hyperthermia-induced mitochondrial CL translocation as well as apoptosis, Similarly, an increase in the amount of PLS3 can enhance hyperthermia-induced mitochondrial CL translocation and apoptosis ( and ). Meanwhile, obvious phosphorylation of PLS3 was observed in the wild-type PLS3 transfected cells after heat treatment, but not in the PLS3 mutant (T21A) transfected cells (). These findings suggested that hyperthermia-induced mitochondrial CL translocation and apoptosis mediated by PLS3 depends on PLS3 activation by phosphorylation.
The role of PKC-δ in thermal-induced apoptosis was investigated using cells over-expressing wild-type PKC-δ and inactivated PKC-δ (K376R). Cells containing wild-type PKC-δ, but not the inactivated PKC-δ (K376R), are able to facilitate mitochondrial CL translocation and apoptosis after heat treatment, and an increased level of PKC-δ can enhance mitochondrial CL translocation and apoptosis ( and ). The results confirm that PKC-δ is necessary for hyperthermia-induced translocation of CL and apoptosis of Tca8113 cells.
To investigate the mitochondrial CL translocation, cellular apoptosis, PLS3 phosphorylation and PKC-δ/ PLS3 interaction, we heat shocked cells co-transfected with PKC-δ/PLS3 at 43°C. The results showed that wild-type PKC-δ promotes mitochondrial CL translocation and facilitates apoptosis at the same time, enhances the PKC-δ/PLS3 interaction and PLS3 phosphorylation. In contrast, the PKC-δ-KD (K376R) mutant cannot facilitate hyperthermia-induced PLS3 phosphorylation and suppressed CL translocation and apoptosis (). These findings prove the existence of a signal transduction pathway mediated by PKC-δ-regulated PLS3 phosphorylation during hyperthermia-induced apoptosis of Tca8113 cells.
Hyperthermia induces apoptosis; however, thermoresistance is prevalent among cancer hyperthermia, and is a key factor that influences curative effects. Although the molecular mechanism of thermoresistance is still unclear, it is thought to be associated with protein and RNA synthesis, especially of HSPs. Elevation of HSPs in tumour cells reduces thermosensitivity, whereas inhibition or down-regulation of HSPs enhances thermosensitivity and hyperthermia-induced apoptosis Citation[44]. The interaction between Hsp27 and PKC-δ was reported to be correlated with chemo- and radio- resistance. Hsp27 siRNA treatment eliminates Hsp27/PKC-δ interaction and reactivates PKC-δ Citation[34]. In this study we heat shocked control plasmid, Hsp27 plasmid transfected cells and PLS3-Hsp27 co-transfected cells, respectively. We observed that Hsp27 abrogates hyperthermia-induced PKC-δ/PLS3 interaction in Tca8113 cells, which in turn suppresses PLS3 phosphorylation by PKC-δ, mitochondrial CL translocation, and apoptosis (, , ). These results suggest that Hsp27 decreases tumour cell thermosensitivity through inhibiting phosphorylation of PLS3 by PKC-δ.
It has been reported that PKC-δ was activated by reactive oxygen species (ROS) i.e. superoxide anion radicals, hydrogen peroxide and nitric oxide, then translocated to mitochondria and induced apoptosis through ROS-PKC-δ pathway Citation[4], Citation[45], Citation[46]. Heat stress enhances ROS in various cell lines. PKC-δ, which was activated by ROS producing during heat shock, may be one mechanism for targeting heat-activated PKC-δ. PLS3, Hsp27 are direct or indirect substrates of PKC-δ Citation[20], Citation[36], Citation[37]. Hsp27 can bind with PKC-δ Citation[47] and increased binding activity between Hsp27 and PKC-δ causes reduction of PKC-δ kinase activity Citation[38]. This may abrogate PKC-δ/PLS3 interaction and inhibit apoptosis. The mechanism of heat treatment activating PKC-δ and the signal transduction pathways is still unclear, and need to be further elucidated.
In Tca8113 cells, our study proves that during hyperthermia, wild-type PLS3, but not the PLS3 (T21A) mutant, promotes mitochondrial CL translocation and apoptosis. PKC-δ interacts with PLS3 during hyperthermia-induced apoptosis and facilitates PLS3 phosphorylation, CL translocation, and apoptosis. Hsp27 blocks the interaction between PLS3 and PKC-δ, and consequently suppresses PKC-δ-induced PLS3 phosphorylation, down-regulates PLS3 activity, and inhibits CL translocation and apoptosis. These effects may comprise the molecular mechanism of tumour cell thermo-resistance. These results provide a new theoretical basis for hyperthermia of cancer therapy, and presents novel targets for improving its effectiveness.
Declaration of interest
This research was supported in part by the National Natural Science Foundation of China (30760272, 30960422, to YWH), the Ministry of Education Program for New Century Excellent Talents in University (NCET-07-0388, to YWH) and the Project of the Department of Science and Technology of Yunnan Province (2006C0038Q, 2009CC021, to YWH). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- van der Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13: 1173–1184
- Vertree RA, Leeth A, Girouard M, Roach JD, Zwischenberger JB. Whole-body hyperthermia: A review of theory, design and application. Perfusion 2002; 17: 279–290
- Klostergaard J, Leroux ME, Auzenne E, Khodadadian M, Spohn W, Wu JY, Donato NJ. Hyperthermia engages the intrinsic apoptotic pathway by enhancing upstream caspase activation to overcome apoptotic resistance in MCF-7 breast adenocarcinoma cells. J Cell Biochem 2006; 98: 356–369
- Wada S, Cui ZG, Kondo T, Zhao QL, Ogawa R, Shoji M, Arai T, Makino K, Furuta I. A hydrogen peroxide-generating agent, 6-formylpterin, enhances heat-induced apoptosis. Int J Hyperthermia 2005; 21: 231–246
- Yu DY, Zhao QL, Wei ZL, Shehata M, Kondo T. Enhancement of hyperthermia-induced apoptosis by sanazole in human lymphoma U937 cells. Int J Hyperthermia 2009; 25: 364–373
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res 2000; 39: 257–288
- Iverson SL, Orrenius S. The cardiolipin-cytochrome C interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 2004; 423: 37–46
- Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis 2007; 12: 877–885
- Liu J, Durrant D, Yang HS, He Y, Whitby FG, Myszka DG, Lee RM. The interaction between tBid and cardiolipin or monolysocardiolipin. Biochem Biophys Res Commun 2005; 330: 865–870
- Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: Evidence of a role for lipid peroxidation and autophagy. Neuroscience 2002; 115: 587–602
- Degli Esposti M. Mitochondria in apoptosis: Past, present and future. Biochem Soc Trans 2004; 32: 493–495
- Yu A, McMaster CR, Byers DM, Ridgway ND, Cook HW. Stimulation of phosphatidylserine biosynthesis and facilitation of UV-induced apoptosis in Chinese hamster ovary cells overexpressing phospholipid scramblase 1. J Biol Chem 2003; 278: 9706–9714
- Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta 1999; 1439: 317–330
- Van Q, Liu J, Lu B, Feingold KR, Shi Y, Lee RM, Hatch GM. Phospholipid scramblase-3 regulates cardiolipin de novo biosynthesis and its resynthesis in growing HeLa cells. Biochem J 2007; 401: 103–109
- Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, Lee RM. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res 2003; 1: 892–902
- Liu J, Epand RF, Durrant D, Grossman D, Chi NW, Epand RM, Lee RM. Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-alpha-induced apoptosis. Biochemistry 2008; 47: 4518–4529
- Ndebele K, Gona P, Jin TG, Benhaga N, Chalah A, Degli-Esposti M, Khosravi-Far R. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced mitochondrial pathway to apoptosis and caspase activation is potentiated by phospholipid scramblase-3. Apoptosis 2008; 13: 845–856
- Liu J, Chen J, Dai Q, Lee RM. Phospholipid scramblase 3 is the mitochondrial target of protein kinase C delta-induced apoptosis. Cancer Res 2003; 63: 1153–1156
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci 2009; 14: 2386–2399
- Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 2008; 88: 1341–1378
- Reyland ME. Protein kinase Cdelta and apoptosis. Biochem Soc Trans 2007; 35: 1001–1004
- Perletti G, Terrian DM. Distinctive cellular roles for novel protein kinase C isoenzymes. Curr Pharm Des 2006; 12: 3117–3133
- He Y, Liu J, Durrant D, Yang HS, Sweatman T, Lothstein L, Lee RM. N-benzyladriamycin-14-valerate (AD198) induces apoptosis through protein kinase C-delta-induced phosphorylation of phospholipid scramblase 3. Cancer Res 2005; 65: 10016–10023
- He Y, Liu J, Grossman D, Durrant D, Sweatman T, Lothstein L, Epand RF, Epand RM, Lee RM. Phosphorylation of mitochondrial phospholipid scramblase 3 by protein kinase C-delta induces its activation and facilitates mitochondrial targeting of tBid. J Cell Biochem 2007; 101: 1210–1221
- Calderwood SK, Ciocca DR. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int J Hyperthermia 2008; 24: 31–39
- Coss RA. Inhibiting induction of heat shock proteins as a strategy to enhance cancer therapy. Int J Hyperthermia 2005; 21: 695–701
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006; 5: 2592–2601
- Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome C-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome C. Gene Expr 2001; 9: 195–201
- Choi DH, Ha JS, Lee WH, Song JK, Kim GY, Park JH, Cha HJ, Lee BJ, Park JW. Heat shock protein 27 is associated with irinotecan resistance in human colorectal cancer cells. FEBS Lett 2007; 581: 1649–1656
- Lee JH, Sun D, Cho KJ, Kim MS, Hong MH, Kim IK, Lee JS. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J Cancer Res Clin Oncol 2007; 133: 37–46
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 2008; 70: 543–553
- Tsuruta M, Nishibori H, Hasegawa H, Ishii Y, Endo T, Kubota T, Kitajima M, Kitagawa Y. Heat shock protein 27, a novel regulator of 5-fluorouracil resistance in colon cancer. Oncol Rep 2008; 20: 1165–1172
- Teimourian S, Jalal R, Sohrabpour M, Goliaei B. Down-regulation of Hsp27 radiosensitizes human prostate cancer cells. Int J Urol 2006; 13: 1221–1225
- Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther 2007; 6: 299–308
- Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R, Gleave M, Chapet O, Rodriguez-Lafrasse C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther 2009; 17: 1387–1394
- Siwko S, Mochly-Rosen D. Use of a novel method to find substrates of protein kinase C delta identifies M2 pyruvate kinase. Int J Biochem Cell Biol 2007; 39: 978–987
- Takai S, Matsushima-Nishiwaki R, Tokuda H, Yasuda E, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O. Protein kinase C delta regulates the phosphorylation of heat shock protein 27 in human hepatocellular carcinoma. Life Sci 2007; 81: 585–591
- Kim EH, Lee HJ, Lee DH, Bae S, Soh JW, Jeoung D, Kim J, Cho CK, Lee YJ, Lee YS. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res 2007; 67: 6333–6341
- Petit JM, Maftah A, Ratinaud MH, Julien R. 10N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur J Biochem 1992; 209: 267–273
- Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ 2002; 13: 449–455
- Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA 2005; 102: 17975–17980
- Zhao QL, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 2006; 40: 1131–1143
- Fukami T, Nakasu S, Baba K, Nakajima M, Matsuda M. Hyperthermia induces translocation of apoptosis-inducing factor (AIF) and apoptosis in human glioma cell lines. J Neurooncol 2004; 70: 319–331
- Chen F, Rezavi R, Wang CC, Harrison LE. Proteasome inhibition potentiates the cytotoxic effects of hyperthermia in HT-29 colon cancer cells through inhibition of heat shock protein 27. Oncology 2007; 73: 98–103
- Majumder PK, Mishra NC, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D. Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ 2001; 12: 465–470
- Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, Muthusamy N, Byrd JC, Cheng AL, Chen CS. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res 2008; 68: 1204–1212
- Wang QJ, Lu G, Schlapkohl WA, Goerke A, Larsson C, Mischak H, Blumberg PM, Mushinski JF. The V5 domain of protein kinase C plays a critical role in determining the isoform-specific localization, translocation, and biological function of protein kinase C-delta and -epsilon. Mol Cancer Res 2004; 2: 129–140