Abstract
Purpose: This study used a dog model to determine the optimal temperature of percutaneous microwave ablation that causes complete necrosis of liver but not the adjacent bowel, supporting the use of this method to specifically and effectively treat liver tumour abutting the bowel.
Materials and methods: Ultrasound-guided percutaneous microwave ablation of liver abutting the bowel was performed on healthy adult dogs. Temperature of the ablation margin was monitored and controlled through inserted thermal monitoring needles. Dogs were divided into three groups and received microwave ablation at 75–95°C, 65–75°C, or 55–65°C. Imaging and histological examination were used to evaluate the damage of the bowel adjacent to the ablated liver.
Results: Within one hour of treatment, the bowel adjacent to the ablated liver was seriously burned in the group receiving 75–95°C microwave ablation. Inflammation and congestion were found in the submucosa of the bowel in the group receiving 65–75°C microwave ablation. Minor inflammation was found in the mucosa of the bowel in the group receiving 55–65°C microwave ablation. Moreover, in the group receiving 55–65°C microwave ablation, ablated liver areas were covered with omenta, and histological examination revealed inflammatory reaction of the omenta 28 days after ablation.
Conclusions: Microwave ablation at 55–65°C for 6 min is preferred for ablation of liver tissue abutting the bowel in dogs. These findings may provide some valuable reference for percutaneous microwave ablation of human liver tumour adjacent to the bowel.
Introduction
Surgical treatment is a standard therapy for malignant liver tumour. However, most patients are not surgical candidates due to poor liver function or other reasons Citation[1], Citation[2]. Imaging-guided thermal ablation using radiofrequency, microwave, or laser continues to gain favour as a minimally invasive technique for the treatment of primary and metastatic hepatic malignancies Citation[3–13]. Microwave ablation has been reported as a safe and efficient treatment with minimal mortality rates for hepatic neoplasms Citation[14–17] and is currently the most commonly used thermal ablation method for treatment of hepatic malignancy in China. The advantages of microwave therapy mainly include low cost compared with radiofrequency ablation in China, being able to be performed on an outpatient basis, and the implementability of repeatable operations in case of tumour recurrence. Although percutaneous microwave ablation is considered safe, complications are possible due to injury of adjacent normal tissues caused by excessive heat delivered by microwave Citation[18–21], especially when the tumours are abutting other organs such as the bowel. A balance must be made between keeping the integrity of the bowel and killing the tumour effectively. If heat generated by microwave is excessive, the bowel might be injured, which leads to potential complications. On the other hand, if the heat generated by microwave is too mild to protect the bowel, the tumour would survive from being removed completely, which results in recurrence. Fortunately, the temperature of the ablation margin can be measured through inserted thermal monitoring needles during the performance of the microwave ablation, which provides a valuable parameter for monitoring the treatment. However, little is known on the feasible temperature of ablation for treating liver tumour effectively with minimal bowel damage.
In this study we used the dog as an animal model to determine the safe temperature range for treating liver tumour adjacent to the bowel with microwave ablation. We ablated the dog's liver abutting the bowel with microwave ablation at different temperatures which were measured at the ablated margin. Our aim was to find the feasible temperature at which the liver can achieve complete necrosis with minimal injury to the adjacent bowel.
Materials and methods
Animals
Twelve adult dogs with weight between 18–25 kg were used in this study. The dogs were provided by the Animal Centre of the General Hospital of the People's Liberation Army. All animals were treated according to the standards set in the Guide for the Care and Use of Laboratory Animals, NIH Publication No. 86-23.
Microwave ablation
Animal preparation
The dogs were fasted for 12–24 h before they were anaesthetised with sodium phenobarbital injection (30 mg per kg weight). An intravenous infusion of 0.9% sodium chloride at initial rate of 120 mL/h was given to each dog. Parts of the hair on the abdomen and rear legs were shaved and then the surfaces were etched with 8% sodium disulphide so that percutaneous ultrasound scan as well as vein puncture and contrast-enhanced ultrasound could be performed.
Microwave ablation system
The microwave ablation system (KY-2000, Kangyou Medical, NanJing, China) consists of two independent microwave generators, two flexible coaxial cables and two water-pumping machines, which drive two cool-tip needle antennae. The generator is capable of producing 0–100 W of power with a frequency of 2450 MHz.
The thermal monitor instrument was custom made. A thermocouple (Omega Engineering, Stanford, CT) was made with a pair of copper and constantan wires which was 0.1 mm in diameter. One end of the thermocouple passed through a 21-gauge thermal sensor needle catheter and was fixed to the needle tip and another end was dipped into the ice-water mixture. The conductor of the thermocouple was connected to a data acquisition unit (34970A-16, HP, USA) and the temperature data were displayed on the screen of the machine. The accuracy of these thermocouples was correct to less than 0.1°C.
Microwave ablation procedure and thermal monitoring
The dogs were divided into three groups based on the temperature to be tested at the liver ablation area margin abutting the bowel. The temperatures of group A (three dogs), group B (three dogs) and group C (six dogs) were controlled in the range of 75–95°C, 65–75°C and 55–65°C, respectively for 6 min. Groups A and B and three dogs from group C were sacrificed with sodium phenobarbital injection within one hour of microwave ablation, and subsequent imaging and histological examination were carried out to determine the damage of liver and bowel. The other three dogs from group C were fed for 28 days for further observation.
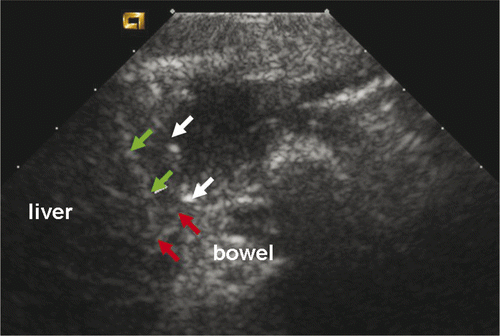
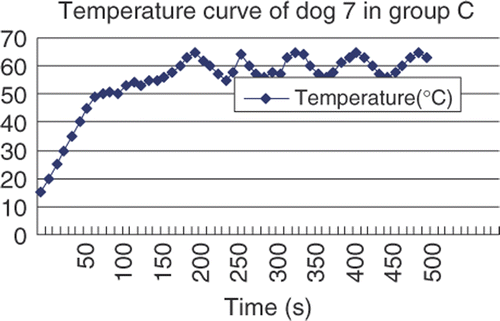
Microwave ablation was performed at 2450 MHz, 50 W, for 370 s to 600 s. For each ablation, the microwave needle was first planted in the correct site under the capsule abutting the bowel. The needle tip was placed 0–7 mm from the liver capsule: 0–3 mm for group A dogs, 3–5 mm for group B dogs, 5–7 mm for group C dogs. The microwave needles were basically perpendicular to the long axis of the bowel and the microwave needle tips were 2–5 mm from the bowel. The thermal needle was placed at the margin under liver capsule and we could observe its tip directly through real-time ultrasound (). Both the microwave needle and thermal needle were fixed by haemostats. The microwave machine was then turned on. The temperature range was controlled effectively through the power switch of the system. Microwave emission would stop immediately as temperature reached the peak of each range, such as 65°C in group C, and would be re-activated when temperature dropped to range bottom, such as 55°C in group C (). Temperature levels were recorded once every 10 seconds and corresponding curves were plotted accordingly. Microwave and thermal needles were removed after ablations.
Contrast-enhanced ultrasonography examination
Contrast-enhanced ultrasonography was performed in all dogs immediately after the ablation in order to confirm the ablated liver areas were adjacent to the bowels. An Aucson Sequoia 512 ultrasound machine with imaging technique of CPS, low MI (0.18) and a microbubble US contrast agent, SonoVue (Bracco, Milan, Italy) were used. SonoVue was administered as an IV fast bolus of 2 mL and followed by 5 mL of saline flush. Images of different phases were saved during contrast-enhanced ultrasound. The liver ablation zones were not enhanced in arterial, arterial portal and parenchymal phase. For the two dogs in group A, parts of the bowel wall abutting the ablation area were not enhanced.
Contrast-enhanced CT
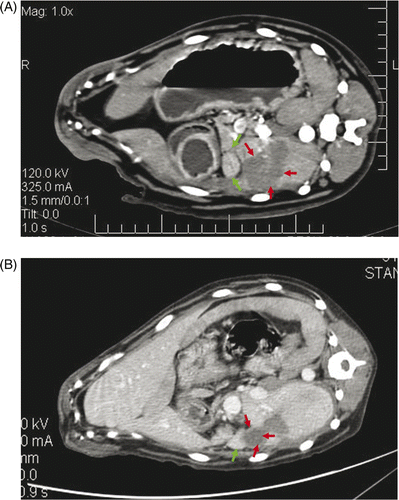
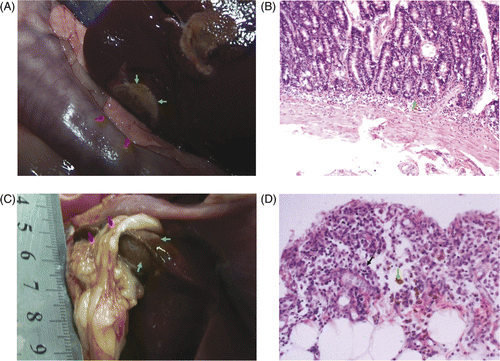
Contrast-enhanced CT was performed to examine the bowel wall injury for the three dogs from group C before, immediately after, and 28 days after microwave ablation. According to the contrast-enhanced CT scans for group C samples, the bowel walls adjacent to the ablated liver had not changed at the time slots that were prior to, immediately after and 28 days after microwave ablation. Also, after 28 days there would be no enhancement inside the lesions. The lesions were covered with omenta. (). The abutting bowel walls were no different from 28 days before on CT.
Figure 3. (A) Immediately after microwave ablation, contrast-enhanced CT showed the ablated lesions were located at the liver margin and were not enhanced (red arrow). The adjacent bowel walls were normal (green arrow). (B) Twenty-eight days after microwave ablation, the contrast-enhanced CT revealed there was no enhancement inside the lesion (red arrow) and the omentum covering the ablated liver was enhanced (green arrow).
Histological examination
Immediately after the dogs were sacrificed, the liver and bowel were examined and the tissue samples including the ablated liver tissue and adjacent bowel were fixed in formalin for light microscopic evaluation. The specimens were stained with hematoxylin and eosin (H&E) for assessment of standard histology. In all cases, all slides were reviewed by pathologists.
Results
All the ablation areas located at the liver margin and were directly adjacent to the bowel, which were confirmed by contrast-enhanced ultrasonography examination and gross examination immediately after microwave ablation. Among the twelve ablation liver zones, four were irregular with the maximal longitude of 40–55 mm, and eight were round or round-like with diameter of 30–35 mm (). The bowel showed quick peristaltic movement in real time ultrasound during microwave ablation.
Table I. Results of twelve ablations.
Histological findings
Bowel wall injuries could be categorised into mild, moderate and severe levels. Mild level includes inflammation, oedema, and congestion on the wall; moderate level contains haemorrhage in addition to more obvious inflammation, oedema, and congestion; whilst severe injury refers to coagulation necrosis with more serious haemorrhage than moderate injuries.
Group A: severe injury. Gross and histological examinations showed bowel walls abutting the ablated liver received severe and full-thickness injury as microwave ablation commenced. Discoloured, thickened pale areas were seen with diameters of 30–51 mm in maximum. The injured adventitia appeared white due to ischaemia while the mucosa turned red as congestion took place (). Microscopically, adventitia structures had been destructed, at the meantime, coagulation necrosis was shown on the muscular layers as cell structure vanished due to nucleus pyknosis, and even karyolysis. Congestion, haemorrhage, cellular degeneration and oedema were noted in submucosa and mucosa (). Additionally, in one case, pancreatic tissue adjacent to the ablated liver was wrecked, with the consequences of cell degeneration, necrosis, haemorrhage and congestion.
Figure 4. (A) The bowel was severely damaged in group A within one hour of microwave ablation. The damaged bowel wall (yellow arrow), the ablation liver (red arrow). (B) Histological examination showed tissue congestion (red arrow) and oedema (black arrow) in submucosa and mucosa in large bowel in group A within one hour of microwave ablation (HE × 200).
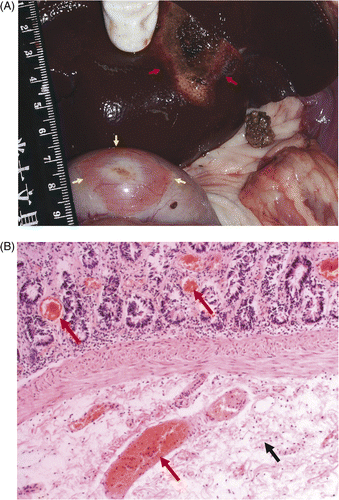
Group B: moderate injury. Gross examination showed the bowel walls abutting the ablated livers were a little thicker and the colour had not changed much. However, histological examinations manifested abnormal phenomena on the submucosa layer of bowel walls, such as oedema, congestion, haemorrhage and inflammatory cell infiltration. Also, two sample dogs were found to have injured pancreatic tissue that abutted ablated livers. Histological examinations demonstrated that pancreatic cells had degenerated and the pancreas had been necrotic with congestion and haemorrhage ().
Figure 5. (A) Gross examination showed the colour of the pancreas (black arrow) abutting the ablated liver (white arrow) turned red in group B within one hour of microwave ablation. (B) Histological examination showed pancreatic cell degeneration (black arrow) and vessel haemorrhage (white arrow) in group B within one hour of microwave ablation (HE × 200).
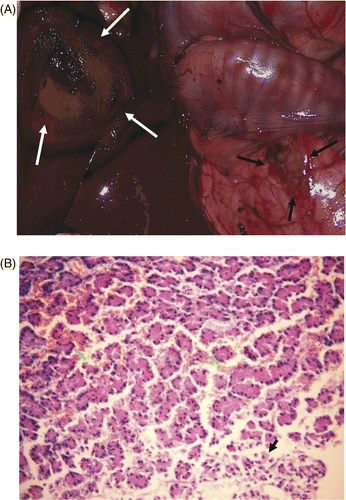
Group C: mild injury. Although gross examination indicated that the colour of the bowel walls had not obviously changed, histological examinations showed the emergence of universal inflammation, oedema and congestion on the mucosa of bowel wall and a single event of pancreas congestion (). Three dogs were sacrificed 28 days after microwave ablation and were examined for changes in bowels. Our findings showed there was no apparent colour distinction between normal bowels and the bowels abutted to ablated livers. The surface of the ablated liver turned brown with edges covered by omentua, so that it could be dissected without hesitation from the liver. Histological examination revealed inflammatory reaction in omenta, including in-grown capillaries, mononuclear cells, and macrophages containing haemosiderin ().
Figure 6. (A) Gross examination showed the colour of the bowel walls and pancreas (red) abutting the ablated liver (green arrow) were not changed obviously in group C within one hour of microwave ablation. (B) Histological examinations showed inflammatory cell infiltration (green arrow) in mucosa of small intestine wall (HE × 200). (C) On day 28 after microwave ablation, the surface of the ablated liver (green arrow) was brown and the edges were covered with omenta (red arrow). (D) On day 28 after microwave ablation, histological examination showed inflammatory reaction in omentum, including inflammatory cells (black arrow), and macrophages containing haemosiderin (green arrow) (HE × 200).
Discussion
Most thermal ablations were performed percutaneously with imaging guidance. Various complications occurred occasionally, such as intraperitoneal haemorrhage, haemobilia, liver abscess, and pleural effusion, which were usually resolved with conservative treatment Citation[17–21]. Perforation of the gastrointestinal tract has been reported as a serious complication of thermal ablation with an overall incidence of 0.1–0.3% Citation[22–23]. Some authors have recommended that percutaneous thermal ablation should be avoided or carefully considered when treating liver tumours in so-called high-risk locations Citation[23–25]. However, others have maintained that radiofrequency is safe and effective when tumours abut the gallbladder, diaphragm or gastrointestinal tract Citation[26–28]. In an animal study, full-thickness burns of the adjacent stomach and colon were found when the boundary of the thermal lesion was less than 10 mm from the surface of the liver Citation[29]. Temperature is a valuable parameter to monitor the efficacy and safety of thermal ablation. Thermal injury may be prevented by strict temperature monitoring of hepatic marginal tissue adjacent to the gastrointestinal tract. However, to our knowledge, no studies have determined the feasible temperature and the pathological changes of the bowel after microwave ablation of adjacent liver.
Our results determined the feasible temperature for microwave ablation of liver abutting the bowel and the resulting pathological changes of the bowel. When temperature of the liver edge was controlled between 55°C to 65°C for 6 min, bowel walls abutting the ablated liver of the tested dogs showed mild injury with inflammation in the wall immediately after microwave ablation. Twenty-eight days after microwave ablation, the bowel returned to normal and the omenta covered the ablated liver. When temperature of the liver edge was higher than 65°C, bowel and pancreas abutting the ablated liver were damaged to different extents, including congestion, haemorrhage, and inflammation, even coagulation necrosis. In addition, because of the quick peristalsis of the bowel, the maximum diameter in bowel was larger than in the liver in group A. Therefore, microwave ablation treatment of liver at 55°C to 65°C for 6 min could effectively destroy the liver tumour tissue but at the same time keep the integrity of adjacent normal tissues in dogs. Viability staining is a better marker of the actual zone of ablation than H&E staining which was not used in the study Citation[30–32]. But, the purpose of the study was to find the feasible temperature of percutaneous microwave ablation causing complete necrosis of liver but not adjacent bowel, through contrast enhanced CT and histologic finding 28 days after ablation; this goal can be achieved.
Although we have not measured the temperature on the surface of the bowel abutting the ablated liver, we reasoned that it should be lower than that of the liver margin for the following reasons. First, heat can be transmitted from one place to another in the peritoneal cavity. Second, the regular movement of the bowel increases when the bowel is irritated by heat, which could prevent prolonged heating of a single spot. Third, the rich blood vessels in mesentery and bowel walls could transfer heat and decrease the temperature of the bowel. It was reported that major blood vessels may influence temperature in the bowel wall over a distance of approximately 2 mm Citation[33].
The clinical efficacy of microwave ablation of liver tumour abutting the gastrointestinal tract has been studied Citation[34], which showed that microwave ablation at 54°C in patients without laparotomy history or 50°C in patients with laparotomy history were tolerable, and under those conditions the tumours could achieve complete necrosis without complications. In that study the temperature of marginal tissue of the tumour or liver proximal to the gastrointestinal tract was measured and for lesions protruding or in contact with the gastrointestinal tract a small dose of ethanol injection was used. Ethanol injection could have a direct effect on the tumour or could lower the temperature. The safe temperature determined by the above study Citation[33] was lower than that determined by our animal study.
The anatomical difference of liver and adjacent organs between dogs and humans should be noted when applying our animal study results to clinical test. The location of the dog's pancreas is different from that of human. The human pancreas lies just under the stomach and along the duodenum, while the dog's pancreas is located in the retroperitoneal space, which resulted in pancreas burn in some dogs during microwave ablation when the ablation area was adjacent to the pancreas. Moreover, dog's liver is divided into seven lobes and is much thinner than human's, which should also be considered when choosing the temperature for microwave ablation on human liver tumour.
Our results showed a more detailed animal study and indicated future clinical applications. The use of a three-dimensional guided system and development of a multiple-point temperature monitoring system should improve the monitoring of microwave ablation in the future so that liver tumour abutting the bowel could be specifically and effectively treated.
Conclusion
In this study we showed that when temperature for microwave ablation of dog liver abutting the bowel was controlled between 55°C and 65°C for 6 min, bowel walls adjacent to the ablated liver sustained only mild injury. This temperature range struck a balance between ablation of the liver target and minimising damage to the adjacent bowel, thus providing a valuable reference for treatment of human tumour abutting the bowel.
Ackowledgement
Project was supported by the National Natural Science Foundation of China (30825010).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Zeng ZC, Tang ZY, Liu KD, Lu JZ, Xie H, Yao Z. Improved long-term survival for unresectable hepatocellular carcinoma (HCC) with a combination of surgery and intrahepatic arterial infusion of 131I-anti-HCC mAb. Phase I/II clinical trials. J Cancer Res Clin Oncol 1998; 124: 275–280
- Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg 2008; 12: 1521–1526
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994; 74: 817–825
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions. Radiology 2000; 214: 761–768
- Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol 1996; 167: 759–767
- Lencioni R, Cioni D, Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: Techniques, indications, imaging findings, and clinical results. Abdom Imaging 2001; 26: 345–360
- Buscarini L, Buscarini E, Stasi MD, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: Long-term results. Eur Radiol 2001; 11: 914–921
- Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: Experience with complications in 899 patients (2520 lesions). Radiology 2002; 225: 367–377
- Murakami R, Yoshinatse S, Yamashita Y, Matsukawa T, Takahashi M, Sagara K. Treatment of hepatocellular carcinoma: Value of percutaneous microwave coagulation. Am J Roentgenol 1995; 164: 1159–1164
- Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: Comparison with percutaneous ethanol injection therapy. Cancer 1999; 85: 1694–1702
- Matsukawa T, Yamashita Y, Arakawa A, Nishiharu T, Urata J, Murakami R, Takahashi M, Yoshimatsu S. Percutaneous microwave coagulation therapy in liver tumors: A 3-year experience. Acta Radiol 1997; 38: 410–415
- Andromanakos N, Filippou D, Papadopoulos V, Kouraklis G, Christianakis E, Kostakis A. New concepts in the therapeutic options of liver metastases from colorectal cancer. Journal of the Balkan Union of Oncology 2007; 12: 445–452
- Mazzaglia PJ, Berber E, Siperstein AE. Radiofrequency thermal ablation of metastatic neuroendocrine tumors in the liver. Curr Treat Options Oncol 2007; 8: 322–330
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: An experimental and clinical study. Am J Roentgenol 1998; 171: 449–454
- Yu Z, Liu W, Fan L, Shao J, Huang Y, Si X. The efficacy and safety of percutaneous microwave coagulation by a new microwave delivery system in large hepatocellular carcinomas: Four case studies. Int J Hyperthermia 2009; 25: 392–3928
- Zhai W, Xu J, Zhao Y, Song Y, Sheng L, Jia P. Preoperative surgery planning for percutaneous hepatic microwave ablation. Med Image Comput Comput Assist Interv 2008; 11: 569–577
- Mitsuzaki K, Yamashita Y, Nishiharu T, Sumi S, Matsukawa T, Takahashi M, Beppu T, Ogawa M. CT appearance of hepatic tumors after microwave coagulation therapy. Am J Roentgenol 1998; 171: 1397–1403
- Ohmoto K, Yoshioka N, Tomiyama Y. Thermal ablation therapy for hepatocellular carcinoma: Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology 2006; 53: 651–654
- Lu MD, Chen JW, Xie XY, Liu L, Huang XQ, Liang LJ, Huang JF. Hepatocellular carcinoma: Sonography-guided percutaneous microwave coagulation therapy. Radiology 2001; 221: 167–172
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180: 1547–1555
- Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals, revised. Washington, DC: National Institutes of Health, 1996.
- Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002; 89: 1206–1222
- Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma an analysis of 1000 cases. Cancer 2005; 103: 1201–1209
- Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006; 43: 1101–1108
- Chopra S, Dodd GD, III, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: Feasibility and safety. Am J Roentgenol. 2003; 180: 697–701
- Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: Assessment of safety and therapeutic efficacy. Korean J Radiol 2009; 10: 34–42
- Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, Lim JH, Paik SW, Koh KC, Yoo BC. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. Am J Roentgenol 2004; 183: 1417–1424
- Hansen PD, Rogers S, Corless CL, Schafmayer C, Fändrich F, Tepel J. Radiofrequency ablation lesions in a pig liver model. J Surg Res 1999; 87: 114–121
- Hennings L, Kaufmann Y, Griffin R, Siegel E, Novak P, Corry P, Moros EG, Shafirstein G. Dead or alive? Autofluorescence distinguishes heat-fixed from viable cells. Int J Hyperthermia 2009; 25: 355–363
- Yamashiki N, Kato T, Bejarano PA, Berho M, Montalvo B, Shebert RT, Goodman ZD, Seki T, Schiff ER, Tzakis AG. Histopathological changes after microwave coagulation therapy for patients with hepatocellular carcinoma: Review of 15 explanted livers. Am J Gastroenterol 2003; 98: 2052–2059
- Ozaki T, Tabuse K, Tsuji T, Nakamura Y, Kakudo K, Mori I. Microwave cell death: Enzyme histochemical evaluation for metastatic carcinoma of the liver. Pathol Int 2003; 53: 837–845
- Hume SP, Robinson JE, Hand JW. The influence of blood flow on temperature distribution in the exteriorized mouse bowel during treatment by hyperthermia. Br J Radiol 1979; 52: 219–222
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009; 13: 318–324