Abstract
Hyperthermia is known to serve as a powerful tool in the treatment of prostate cancer which is commonly diagnosed in men. Quercetin and KNK437, Hsp70 inhibitors, play an important role in blocking thermotolerance in some cancer cells. In the present study we investigated the effects of KNK437 and quercetin on the acquisition of thermotolerance and heat-induced apoptosis. Also, it was examined whether the possible mechanism triggering apoptotic pathway included caspase-3 activation in prostate cancer cells.
For this purpose, PC-3 and LNCaP cells were treated with hyperthermia following pretreatment with or without KNK437 or quercetin. Thermotolerance was investigated by colony formation assay in these cells, while Hsp70 mRNA levels were measured by real time RT-PCR. Sandwich ELISA was used for detection of Hsp70 protein levels. Apoptosis was detected by flow cytometric annexin V binding assay and by western blot analysis of procaspase-3 and cleaved poly (ADP-ribose) polymerase levels.
In our study, KNK437 and quercetin inhibited thermotolerance in a dose-dependent manner in PC-3 cells. KNK437 and quercetin decreased heat-induced accumulation of Hsp70 mRNA and protein in PC-3 and LNCaP cells. KNK437 and quercetin pretreatment enhanced the apoptotic effect of hyperthermia in both cells. We found that KNK437 was more effective than quercetin in inducing apoptotic cell death, activation of caspase-3, and cleavage of PARP in prostate cancer cells. We suggest that KNK437 is a useful agent for enhancing the efficiency of hyperthermic therapy which has less toxic side-effects in prostate cancer.
Introduction
Hyperthermia is an ideal therapy that has low toxicity, mild side-effects, and has been shown to provide synergies with many of the traditional treatment modalities Citation[1]. In vitro experiments and in vivo animal studies and clinical trials have revealed that hyperthermia may serve as a powerful tool in the treatment of prostate cancer Citation[2].
The most important problem in hyperthermia treatment is the acquisition of thermotolerance Citation[3]. Heat shock induces the expression of heat shock proteins (HSPs), such as Hsp27, Hsp70, and Hsp90, and these protein chaperones often times render cells thermotolerant and resistant to subsequent stressful stimuli, including chemotherapeutic agents Citation[4]. In general, cancer cells exposed to temperatures >42°C will undergo cell death, but as the temperature rises, the percentage of cells undergoing apoptosis decreases with a concomitant increase in necrosis Citation[5]. Elevated expression of Hsp70 has been reported in high-grade malignant tumours. In breast cancers, overexpression of Hsp70 is associated with short-term disease-free survival, metastasis and poor prognosis Citation[6]. Hsp70 which protects cells from heat damage plays a key role in the modulation of cell growth and differentiation Citation[7]. Hsp70 has been shown to act as an inhibitor of apoptosis in prostate cancer Citation[8].
Approaches against the formation of thermotolerance may help to improve the curative effects of hyperthermia. Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a flavonoid with a wide variety of biological activities, is known to act as an Hsp70 and thermotolerance inhibitor and a heat sensitiser in various cells Citation[9]. It is reported that quercetin may enhance heat-induced cytotoxicity in prostate cancer cell lines through the inhibition of Hsp70 production Citation[10]. Quercetin has also been shown to augment the thermosensitivity of ovarian carcinoma Citation[11], melanoma Citation[12], cholangiocellular carcinoma Citation[13] and leukaemia Citation[9] cells during thermotherapy.
KNK437 (N-formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam), a benzylidene lactam compound, was also found to inhibit the synthesis of HSPs and acquisition of thermotolerance in vitro Citation[14] and in vivo Citation[15].
No study investigating the effect of KNK437 on the acquisition of thermotolerance, Hsp70 expression and apoptosis in prostate cancer cells exposed to hyperthermia has been found in the literature. Our study is aimed to investigate the effect of KNK437 and quercetin on the acquisition of thermotolerance and to compare their effects. The present study examined the possible effect of KNK437 or quercetin as a sensitiser on heat-induced cell death in prostate cancer cells. In addition we wanted to find out whether the mechanism of apoptosis is related to activation of caspase-3 or not in hyperthermia and/or KNK437-treated prostate cancer cells.
Materials and methods
Cells and culture conditions
PC-3 and LNCaP prostate carcinoma cell lines were provided by Salih Şanlıoğlu (Akdeniz University, Antalya, Turkey). Cells were cultured in RPMI-1640 (Roswell Park Memorial Institute) supplemented with 10% foetal bovine serum (FBS) in a humidified 5% CO2 atmosphere at 37°C.
KNK437 (N-formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam; synthesised by Calbiochem) and quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, Sigma) were dissolved in dimethyl sulphoxide (DMSO) for use at the indicated concentrations. Cells were seeded in 6-well plates. When the cells reached 70–80% confluency they were treated with drugs and hyperthermia. The final concentration of DMSO in each culture medium was 0.25% (v/v), irrespective of the concentrations of the drugs. The same concentration of DMSO was used as a control. °
Hyperthermia and drug treatments
All heating procedures were applied as described previously Citation[14]. Briefly, heat and drug treatments used in this study are indicated as following:
Experimental groups for colony formation assay
Group 1. Hyperthermia group (H): cells were incubated at 37°C for 5 h with DMSO and then heated at 45°C for 1 h.
Group 2. KNK437 + hyperthermia group (KNK437 + H): cells were incubated at 37°C for 5 h with 100 µM KNK437 and then heated at 45°C for 1 h.
Group 3. Repeated hyperthermia group (RH): cells were incubated at 37°C for 1 h with DMSO followed by heating at 45°C for 10 min and then recovered at 37°C for 4 h followed by heating at 45°C for 1 h.
Group 4. KNK437/quercetin + repeated hyperthermia group (KNK437/quercetin + RH): cells were incubated at 37°C for 1 h with 100 µM KNK437/quercetin followed by heating at 45°C for 10 min and then recovered at 37°C for 4 h followed by heating at 45°C for 1 h.
Experimental groups for other assays
Group 1. Control (C): cells were incubated at 37°C for 150 min with DMSO.
Group 2. Hyperthermia group (H): cells were incubated at 37°C for 60 min with DMSO and then heated at 43°C for 90 min.
Group 3. KNK437 and hyperthermia group (KNK437 + H): cells were incubated at 37°C for 60 min with 100 µM KNK437 and then heated at 43°C for 90 min.
Group 4. Quercetin and hyperthermia group (quercetin + H): Cells were incubated at 37°C for 60 min with 100 µM quercetin and then heated at 43°C for 90 min.
Colony-formation assay
After drug and hyperthermia treatments, cells were trypsinised, counted, and re-plated at the appropriate dilutions. Cultures were incubated for 10 to 12 days at 37°C until visible colonies formed. Colonies were fixed in absolute methanol and stained with 1% crystal violet. The stained colonies were counted and colonies containing at least 50 cells were scored. Plating efficiencies were routinely about 80–90%. All clonogenic experiments were done at least twice in independent procedures.
Real time RT-PCR assay
Cells were harvested immediately after drug and hyperthermia applications and total RNA was purified by using a commercial total RNA isolation kit (Biological Industries, Israel) according to the instructions from the manufacturer. Reverse transcription of 2 µg of total RNA was performed using TaqMan Reverse Transcription Reagents (Applied Biosystems, Cat. No. 8080234). Sequences for Hsp70 primers and probe were provided commercially (MWG Biotech). Following is the sequence information for the Hsp70 sets:
Forward Primer: 5′-GGA GGC GGA GAA GTA CA-3′,
Reverse Primer: 5′-GCT GAT GAT GGG GTT ACA-3′,
Probe: 5′-FAM- AGA TCA GCG AGG CGG ACA A-TAMRA-3′.
rRNA was amplified as an internal control in the same reaction. Both the rRNA primers and probes were obtained from Applied Biosystems. The TaqMan PCR reaction was carried out as described in the manufacturer's protocols. ΔΔCt method was used as described by Applied Biosystems to calculate the relative quantities of Hsp70 mRNA.
Sandwich enzyme-linked immunosorbent assay (ELISA)
Cells were washed with PBS three times 24 h after drug and hyperthermia applications. After trypsinisation, cells were centrifuged at 150 g for 5 min and the supernatant was removed. Cell pellet was resuspended with extraction buffer which was included in Hsp70 sandwich enzyme-linked immunosorbent assay (ELISA) kit (StressXpress, USA, Ann Arbor, MI). Cell suspension was centrifuged at 21,000 g and 4°C for 10 min. Total protein concentrations were measured in the supernatant by the method of Bradford (Coomassie Plus protein assay kit, Pierce Chemical Co, Rockford, IL) and samples were saved at −80°C for ELISA assays. A 100 µL sample was used for analysis of Hsp70 protein levels. The results were calculated using an Hsp70 standard graph and expressed as ng/mL.
Apoptosis detection
An apoptosis detection kit (Biosource International, USA Camarillo, CA) was used to determine the extent of apoptosis 24 h after treatments. The ApoTarget annexin V-FITC kit employs fluorescein isothiocyanate (FITC)-conjugated annexin-V in concert with propidium iodide (PI). Hyperthermia and drug-treated cells were trypsinised and centrifuged at 1200 g for 5 min at 4°C. Cells removed from supernatant were washed with PBS twice and resuspended (2–3 × 106 cells/mL) in 1 × annexin-V binding buffer. Cells were aliquoted at 100 µL/tube. Then, 5 µL of annexin-V FITC and 10 µL PI buffer were added to each tube and incubated at room temperature for 15 min in the dark. 400 µL 1 × annexin-V binding buffer was added to each tube. Cells were analysed by flow cytometer within 1 h of staining. A minimum of 10,000 cells were collected for each sample.
Western blot analysis
The cells were washed with cold PBS and disrupted by the addition of lysis buffer (10 mM Tris (pH:7.4), 100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM NaF, 20 mM sodium pyrophosphate, 2 mM sodium ortho-vanadate, 0.1% sodium dodecyl sulphate (SDS), 1% triton X-100, 10% glycerol and 1 mM PMSF) containing protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany) at 4°C and 24 h after hyperthermia and drug treatments of cells. They were then centrifuged at 10,000 g for 30 min at 4°C. The protein concentrations were analysed by the method of Bradford (Coomassie Plus Protein Assay Kit, Pierce).
Proteins were separated in a 10% (for poly (ADP-ribose) polymerase (PARP)) and 15% (for procaspase-3) SDS-polyacrylamide gel and electroblotted to nitrocellulose membrane (UK, Little Chalfont, Buckinghamshire). The immunoblot was performed with procaspase-3 ((Chemicon International, USA, Tamecula, CA)) and PARP (BioSource International) antibodies (each 1 : 1000 diluted in tris buffered saline (TBS) + 0.1% Tween-20 (TBST)), followed by horse-radish peroxidase (HRP)-conjugated secondary antibody, and revealed following the enhanced chemiluminescence (ECL) (Pierce) western blotting analysis procedure. We used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody to assure equal loading of samples. All the western analyses were repeated three times. Quantitative analysis was performed from the density of the bands by using Scion Image software.
Statistical analysis
Data were expressed as means ± standard deviation (SD). Comparison of groups according to the parameters was performed using the student t-test and test of one-way ANOVA (Tukey's multiple comparison) in Prism from GraphPad Software (San Diego, CA). P values less than 0.05 were considered statistically significant.
Results
Effects of hyperthermia and drugs on the acquisition of thermotolerance
As shown in , exposure of cells to 100 µM KNK437 (KNK437 + H) significantly decreased the colony number of cells compared to H group (p < 0.01). Both of these groups were treated with a single heating process.
Figure 1. (A) Colony numbers of PC-3 cells treated with hyperthermia and KNK437 (100 µM). Cells were preincubated with DMSO (H) or DMSO + KNK437 (KNK437 + H) at 37°C for 5 h before heat shock at 45°C for 1 h. Additionally, cells were preincubated with DMSO (RH) or DMSO + KNK437 (KNK437 + RH) at 37°C for 1 h followed by repeated heating process (first heating at 45°C for 10 min → recovered at 37°C for 4 h → second heating at 45°C for 1 h). Values represent means ± SD of duplicate samples per group; *p < 0.05; **p < 0.01; ***p < 0.001. (B) Comparison of the inhibitory effects of KNK437 (100 µM) and quercetin (100 µM) on the acquisition of thermotolerance in PC-3 cells. Cells were preincubated at 37°C with DMSO (RH), DMSO + KNK437 (KNK437 + RH) and DMSO + quercetin (quercetin + RH) for 1 h followed by repeated heating process as described above. Values represent means ± SD of duplicate samples per group. H, hyperthermia; RH, repeated hyperthermia; *p < 0.05; **p < 0.01; ***p < 0.001.
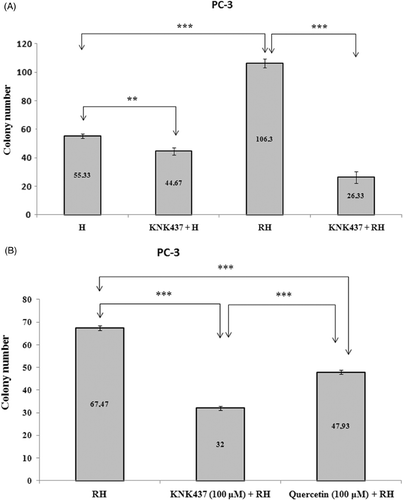
Thermotolerance was observed by the repeated heating process in PC-3 cells (). That a significant difference in colony numbers was found between H and RH groups (p < 0.001) has shown the acquisition of thermotolarance in PC-3 cells (). The colony number of PC-3 cells which were pretreated with 100 µM KNK437 (KNK437 + RH) was found to be lower than that of cells which were exposed to repeated heating process (RH) (, p < 0.001). This result shows that KNK437 is effective for inhibition of thermotolerance in PC-3 cells.
To examine the dose-dependent effect of KNK437 on thermotolerance, cells were incubated with different doses of KNK437 (0, 5, 25, 50, 100, 200 µM) at 37°C for 1 h followed by heating at 43°C for 90 min and then recovered at 37°C for 2 h followed by heating at 45°C for 1 h. The effective dose for inhibition of thermotolerance was calculated by using nonlinear regression analysis in GraphPad Prism statistical program and expressed as EC50. EC50 value for inhibition of thermotolerance was found to be 95 µM in PC-3 cells (data not shown). We also compared the inhibitory effects of 100 µM KNK437 and 100 µM quercetin on thermotolerance. The colony numbers of both KNK437 + RH and quercetin + RH groups were found to be lower than RH group (, p < 0.001). KNK437 was found to be significantly more effective than quercetin in inhibition of thermotolerance (, p < 0.001).
There was no difference between the colony numbers of H (26.33 ± 0.58) and RH (26 ± 1) groups in LNCaP cells. This result signifies that thermotolerance was not observed in LNCaP cells. However, pretreatment of cells with 100 µM KNK437 significantly (p < 0.001) decreased the colony numbers of both single (KNK437 + H, 20 ± 1) and repeated (KNK437 + RH, 18 ± 1) heat treated cells (data not shown). We evaluated the dose-dependent changes in thermal response of LNCaP cells by using five different concentrations of KNK437 (0, 5, 25, 50, 100 and 200 µM). KNK437 lowered the colony numbers in a dose-dependent manner. The effective dose that decreased colony number was found as 69.6 µM for LNCaP cells (data not shown). We also compared the effectiveness of KNK437 with that of quercetin at concentrations of 100 µM in LNCaP cells. The colony numbers in KNK437 + RH (16.67 ± 1.53) and quercetin + RH (13 ± 1) groups were found to be significantly lower than in RH group (34 ± 1) (p < 0.001). The number of colonies in quercetin + RH group was found to be lower than KNK437 + RH group (p < 0.05) in LNCaP cells (data not shown).
Effects of hyperthermia and drugs on Hsp70 mRNA levels
As shown in , the Hsp70 mRNA levels of both PC-3 and LNCaP cells increased significantly after heat shock compared to control (p < 0.001). However, exposure of cells to 100 µM KNK437 or 100 µM quercetin significantly decreased the heat shock-induced accumulation of Hsp70 mRNA compared to DMSO alone (, p < 0.001).
Figure 2. Synthesised levels of Hsp70 mRNA of PC-3 (A) and LNCaP (B) cells exposed to hyperthermia with or without KNK437 (100 µM)/quercetin (100 µM). Cells were preincubated with DMSO at 37°C for 150 min in control group (C). Cells were preincubated at 37°C with DMSO (H), DMSO + KNK437 (KNK437 + H), and DMSO + quercetin (quercetin + H) for 60 min and then heated at 43°C for 90 min. After these processes, cells were harvested immediately and total RNA was isolated. The TaqMan PCR reaction was carried out as described in the manufacturer's protocols. Values represent means ± SD of triplicate samples per group; *p < 0.05; **p < 0.01; ***p < 0.001.
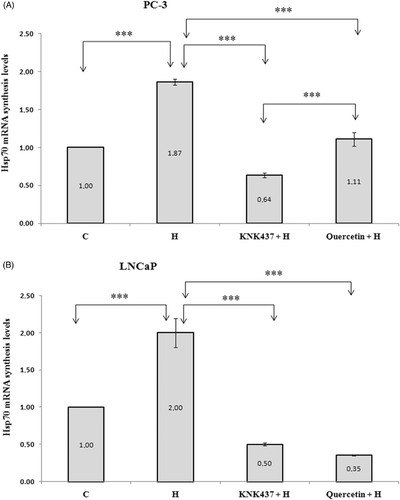
It was found that KNK437 was more effective in inhibition of Hsp70 expression than quercetin (p < 0.001) in PC-3 cells (). On the other hand, we did not find a significant difference between Hsp70 mRNA levels of KNK437 + H and quercetin + H groups () in LNCaP cells.
Effects of hyperthermia and drugs on Hsp70 protein levels
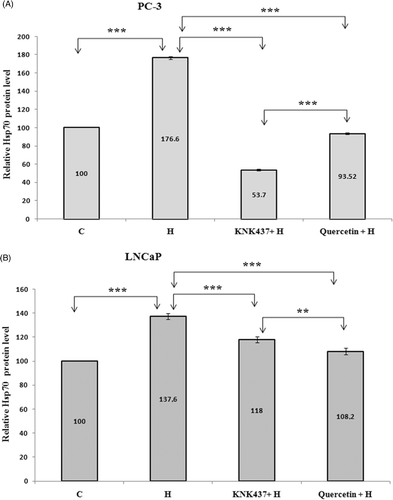
Hsp70 protein levels of both PC-3 and LNCaP cells were found to be significantly increased by hyperthermia compared to control. Both KNK437 and quercetin pretreatment decreased Hsp70 protein levels in these cells (, p < 0.001). Besides, Hsp70 protein levels in PC-3 cells pretreated with KNK437 were found to be lower than in cells pretreated with quercetin (, p < 0.001). However, quercetin was found to be more effective in decreasing Hsp70 protein levels in LNCaP cells, in contrast to PC-3 cells (, p < 0.01).
Figure 3. Hsp70 protein levels of PC-3 (A) and LNCaP (B) cells exposed to hyperthermia with or without KNK437 (100 µM)/quercetin (100 µM). Cells were preincubated and treated with drugs and hyperthermia as described in . After treated with drugs and hyperthermia, cells were incubated at 37°C for 24 h and washed with PBS three times, trypsinised and centrifuged. Cell pellet was resuspended with extraction buffer in Hsp70 ELISA kit. Cell suspension was centrifuged at 21,000 g for 10 min at 4°C. Supernatants of samples, 100 µL, were used for analysis of Hsp70 protein levels. The results were expressed as ng/mL. Values represent means ± SD of triplicate samples per group; *p < 0.05; **p < 0.01; ***p < 0.001.
Effects of hyperthermia and drugs on apoptosis determined by annexin-V binding method
Heat treatment induced the increases in early and late apoptotic cells in both PC-3 and LNCaP cell lines after 24 h of exposure (, and ). The percentages of early apoptotic cells increased significantly by pretreatment with either KNK437 or quercetin (, and , p <0.001). Early apoptotic cells in KNK437 + Hyperthermia group was higher than in quercetin + H group in PC-3 (p < 0.01) and LNCaP (p < 0.001) cells. However, late apoptotic or necrotic cells were observed to be higher in quercetin + H group than in the KNK437 + H group in PC-3 cells (p < 0.001)
Figure 4. Effects of hyperthermia, KNK437 and quercetin on the induction of apoptosis in PC-3 (A) and LNCaP (B) cells. Cells were preincubated and treated with drugs and hyperthermia as described in . Cells treated with hyperthermia and drugs were trypsinised and centrifuged at 1200 g for 5 min at 4°C. An apoptosis detection kit (Biosource International) was used to determine the extent of apoptosis 24 h after treatments. Cell pellet was incubated with annexin-V FITC and PI and incubated at room temperature for 15 min in the dark. Cells were analysed by flow cytometer within 1 h of staining. A minimum of 10,000 cells were collected for each sample. Flow cytometric analysis of the apoptotic cells is shown. The vertical scale represents PI and the horizontal scale represents annexin-V conjugated by FITC. Viable cells, annexin-V-/PI-; dead cells, annexin-V-/PI + ; early apoptotic cells, annexin-V + /PI-; late apoptotic cells, annexin-V + /PI + . Results are representative.
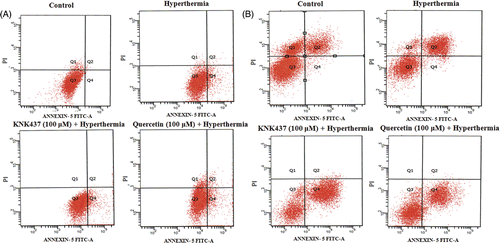
Table I. Apoptosis index of PC-3 cells exposed to hyperthermia, KNK437 and quercetin.
Table II. Apoptosis index of LNCaP cells exposed to hyperthermia, KNK437 and quercetin.
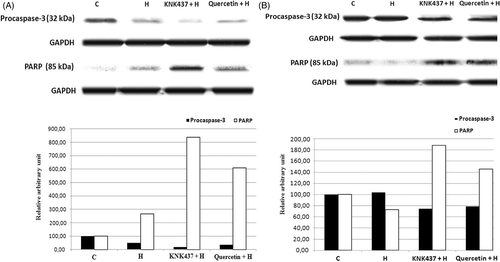
Effects of hyperthermia and drugs on procaspase-3 and PARP levels
Treatment of PC-3 cells with hyperthermia resulted in caspase-3 activation with a resultant degradation of PARP and apoptosis as shown in . With the treatment of hyperthermia at 43°C for 90 min. we could not observe any change in caspase-3 activation and PARP cleavage in LNCaP cells (). Hyperthermia-induced apoptotic cell death was enhanced by KNK437 or quercetin in prostate cancer cells (). Western blot analysis shows that procaspase-3 (32 kDa) and PARP cleavage were enhanced with 100 µM KNK437 or quercetin at 24 h. The band intensities showing caspase-3 activation and PARP cleavage in KNK437 pretreated hyperthermic cells were higher than in quercetin pretreated cells.
Figure 5. The effects of hyperthermia, KNK437 and quercetin on proteolytic cleavage of PARP and activation of caspase-3 in PC-3 (A) and LNCaP (B) cells determined by western blot. Cells were preincubated and treated with drugs and hyperthermia as described in . The cells were washed and disrupted by the addition of lysis buffer containing protease inhibitor cocktail tablet at 4°C and 24 h after hyperthermia and drug treatments of cells. They were then centrifuged at 10,000 g for 30 min at 4°C. Proteins were separated in a 10% (for cleaved-PARP) and 15% (for procaspase-3) SDS-polyacrylamide gel and electroblotted to nitrocellulose membrane. The immunoblotting of cleaved PARP (85 kDa apoptosis related cleavage fragment) and procaspase-3 (32 kDa anticaspase-3 antibody detects inactive form) is shown. Blots are representative. Graph shows the mean ± SD of band intensities (relative arbitrary unit) of three experiments.
Discussion
Hyperthermia is reported to be an influential therapeutic procedure which has mild side-effects, and effective cell-killing and apoptosis inducing properties Citation[16], Citation[17]. The major problem in hyperthermia is the ‘thermotolerance’ phenomenon. In our study, repeated hyperthermia increased the number of colonies compared to single heat treatment group in PC-3 cells (). This result is evidence of acquisition of thermotolerance for PC-3 cells and is consistent with the former studies carried out by Yokota et al. Citation[14] and Roigas et al. Citation[2] On the other hand, we could not find thermotolerance in LNCaP cells. This finding called our attention to the fact that LNCaP cells are less aggresive than other prostate cancer cells. A similar result was shown in a study by Lloyd et al. Citation[18] who found LNCaP cells to be non-thermotolerant, whereas DU145 was thermotolerant.
HSPs act as the main mediators of thermotolerance to protect cells against hyperthermia-induced damage. Karlseder et al. Citation[19] showed that murine fibrosarcoma cells overexpressing Hsp70 showed resistance against adriamycin, a chemotherapeutic agent, and this resistance was not related to multi-drug resistance.
HSP inhibitors, such as KNK437 and quercetin, are useful agents for the inhibition of thermotolerance Citation[20]. Although the effects of quercetin have commonly been studied in a number of experiments, little is known about the effects of KNK437. In our study, both KNK437 and quercetin were shown to impede hyperthermia-induced thermotolerance in PC-3 cells. Also, it was seen that KNK437 (52.57%) was more effective than quercetin (28.96%) in inhibition of thermotolerance in PC-3 cells, supporting other related studies Citation[3], Citation[14]. Although thermotolerance was not observed in LNCaP cells, KNK437 (50%) and quercetin (61.76%) were found to decrease the colony numbers compared to the repeated hyperthermia treatment group. These results suggest that LNCaP cells are more prone to hyperthermia/repeated hyperthermia and agents’ effects than PC-3 cells. In LNCaP cells, quercetin was found to be more effective than KNK437 in reducing the capability of colony formation. This finding may be due to the fact that quercetin may use different pathways in inhibiting cell survival.
Heating to 43°C is reported to increase the expression of Hsp70 in prostate cancer cells and to lead to cell death through apoptosis Citation[10]. In the present study, Hsp70 mRNA and protein levels were found to be increased by hyperthermia at 43°C for 90 min in both PC-3 and LNCaP cells. Hsp70 has been reported to prevent cell death initiated by various apoptotic stresses, such as heat shock, ceramide, ionising radiation, tumour necrosis factor-alpha and ischaemia Citation[21]. Also, Hsp70 has been shown to inhibit mitochondria-mediated apoptosis by physically interacting with and inhibiting APAF-1 and apoptosis-inducing factor (AIF), resulting in suppression of caspase-dependent and -independent apoptosis, respectively Citation[22], Citation[23]. Therefore, Hsp70 inhibitors increase the cell death effect caused by hyperthermia. Indeed, both quercetin and KNK437 have been shown to inhibit the accumulation of Hsp70 mRNA and protein levels of Hsp70 and the formation of heat shock-induced thermotolerance in HeLa, colon cancer, squamous carcinoma and glioblastoma cells Citation[14], Citation[24–26]. In this study, both KNK437 and quercetin decreased Hsp70 mRNA and protein levels in PC-3 and LNCaP cells. Hsp70 mRNA level in LNCaP cells was decreased less than that in control cells by KNK437 and quercetin treatment (), but Hsp70 protein level was not decreased by these treatments (). This may be explained by possible post-transcriptional mechanisms or different factors that regulate mRNA stability in cells.
Because there are only limited studies explaining the effects of KNK437 Citation[25], Citation[27] and quercetin Citation[28], Citation[29] on heat-induced cell death in some cell types, we focused on the relationships between KNK437 and apoptosis in prostate cancer cells. Nimmanapalli et al. Citation[27] reported that KNK437 caused more apoptosis in melphalan resistant 8226/LR5 cells and sensitised them to melphalan. Also, Fujita et al. Citation[29] reported that certain leukaemias were sensitive to heat, that whole body hyperthermia provided cell death successfully, and that quercetin (50 µM) enhanced heat-induced apoptosis in leukaemia cells. Apoptosis was induced by hyperthermia in both PC-3 and LNCaP cells. Both KNK437 and quercetin enhanced hyperthermia-induced apoptosis in PC-3 and LNCaP cells. In early apoptosis, KNK437 was found to be more effective than quercetin in both cells. This result may be due to its more effective function on the inhibition of Hsp70. Compared the apoptotic index of the cells exposed to hyperthermia, it may be suggested that PC-3 cells are more thermosensitive than LNCaP cells. However, hyperthermia combined with drugs is seen to enhance the apoptotic cell death in both cell types.
It is well known that caspase-3 plays a central role among apoptotic pathways. In our study, hyperthermia at 43°C for 90 min decreased the levels of procaspase-3 and increased the levels of cleaved-PARP in PC-3 cells. A similar result was reported by Adachi et al. Citation[30] who found decreased procaspase-3 levels by heat treatment combined with gemcitabine in pancreatic cancer cell lines. KNK437 and quercetin pretreatments increased the effect of hyperthermia on inducing caspase-3 activity and cleavage of PARP in these cells. KNK437 was found to be more effective than quercetin in inducing caspase-3 activity and cleavage of PARP in these cells. Present data constitutes the most original part of this study, since there is no study investigating the effect of KNK437 on caspase-3 and PARP levels in prostate cancer cells. These results suggest that the inhibition of Hsp70 which can suppress the caspase cascade derived from mitochondria Citation[29] sensitises prostate cancer cells to heat-induced apoptosis.
In conclusion, KNK437 pretreatment appears to play an important role in the regulation of heat-induced changes. KNK437 is more effective at inhibiting thermotolerance and increasing heat-induced apoptosis than quercetin. It may be suggested KNK437 can shift the balance between proliferation and cell death in favour of apoptosis caused by hyperthermia. In addition, this study highlights the emphasis of hyperthermia by using KNK437 as a sensitiser of apoptosis for prostate cancer.
Declaration of interest
Funding for this study was provided by grants from the Akdeniz University Scientific Research Project Unit (2005.03.0122.009). Also, this study was supported by Institute of Health Science, Akdeniz University. We would like to thank Salih Şanlioğlu, who provided the cell lines, for valuable advice. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Szasz A, Szasz O, Szasz N. Physical background and technical realization of hyperthermia. Hyperthermia in cancer treatment: A primer, GF Baronzio, ED Hager. Springer, New York 2006; 25–57
- Roigas J, Wallen ES, Loening SA, Moseley PL. Effects of combined treatment of chemotherapeutics and hyperthermia on survival and the regulation of heat shock proteins in Dunning R3327 prostate carcinoma cells. Prostate 1998; 34: 195–202
- Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res 2001; 7: 215–219
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 2000; 92: 1564–1572
- Samali A, Holmberg CI, Sistonen L, Orrenius S. Thermotolerance and cell death are distinct cellular responses to stress: Dependence on heat shock proteins. FEBS Lett 1999; 461: 306–310
- Jakubowicz-Gil J, Rzymowska J, Gawron A. Quercetin, apoptosis, heat shock. Biochem Pharmacol 2002; 64: 1591–1595
- Elia G, Santoro MG. Regulation of heat shock protein synthesis by quercetin in human erythroleukaemia cells. Biochem J 1994; 300: 201–209
- Jones EL, Zhao MJ, Stevenson MA, Calderwood SK. The 70 kilodalton heat shock protein is an inhibitor of apoptosis in prostate cancer. Int J Hyperthermia 2004; 20: 835–849
- Larocca LM, Ranelletti FO, Maggiano N, Rutella S, La Barbera EO, Rumi C, Serra F, Voso MT, Piantelli M, Teofili L, et al. Differential sensitivity of leukemic and normal hematopoietic progenitors to the killing effect of hyperthermia and quercetin used in combination: Role of heat-shock protein-70. Int J Cancer 1997; 73: 75–83
- Nakanoma T, Ueno M, Iida M, Hirata R, Deguchi N. Effects of quercetin on the heat-induced cytotoxicity of prostate cancer cells. Int J Urol 2001; 8: 623–630
- Wachsberger PR, Burd R, Bhala A, Bobyock SB, Wahl ML, Owen CS, Rifat SB, Leeper DB. Quercetin sensitizes cells in a tumour-like low pH environment to hyperthermia. Int J Hyperthermia 2003; 19: 507–519
- Piantelli M, Tatone D, Castrilli G, Savini F, Maggiano N, Larocca LM, Ranelletti FO, Natali PG. Quercetin and tamoxifen sensitize human melanoma cells to hyperthermia. Melanoma Res 2001; 11: 469–476
- Kudo M, Naito Z, Yokoyama M, Asano G. Effects of quercetin and sunphenon on responses of cancer cells to heat shock damage. Exp Mol Pathol 1999; 66: 66–75
- Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res 2000; 60: 2942–2948
- Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 2003; 304: 505–512
- Fuller KJ, Issels RD, Slosman DO, Guillet JG, Soussi T, Polla BS. Cancer and the heat shock response. Eur J Cancer 1994; 30A: 1884–1891
- Milani V, Lorenz M, Weinkauf M, Rieken M, Pastore A, Dreyling M, Issels R. Combination of hyperthermia and bortezomib results in additive killing in mantle cell lymphoma cells. Int J Hyperthermia 2009; 25: 262–272
- Lloyd SN, Chalmers D, Leake RE, Kirk D. Local hyperthermia for prostatic disease: In vitro studies on human prostatic cancer cell lines. Br J Urol 1992; 70: 529–533
- Karlseder J, Wissing D, Holzer G, Orel L, Sliutz G, Auer H, Jaattela M, Simon MM. HSP70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. Biochem Biophys Res Commun 1996; 220: 153–159
- Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol 1992; 12: 3490–3498
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. Embo J 1998; 17: 6124–6134
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the APAF-1 apoptosome. Nat Cell Biol 2000; 2: 469–475
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3: 839–843
- Nonaka T, Akimoto T, Mitsuhashi N, Tamaki Y, Yokota S, Nakano T. Changes in the localization of heat shock protein 72 correlated with development of thermotolerance in human esophageal cancer cell line. Anticancer Res 2003; 23: 4677–4687
- Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol 2004; 80: 607–614
- Hansen RK, Oesterreich S, Lemieux P, Sarge KD, Fuqua SA. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem Biophys Res Commun 1997; 239: 851–856
- Nimmanapalli R, Gerbino E, Dalton WS, Gandhi V, Alsina M. Hsp70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br J Haematol 2008; 142: 551–561
- Wei YQ, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of apoptosis by quercetin: Involvement of heat shock protein. Cancer Res 1994; 54: 4952–4957
- Fujita M, Nagai M, Murata M, Kawakami K, Irino S, Takahara J. Synergistic cytotoxic effect of quercetin and heat treatment in a lymphoid cell line (OZ) with low HSP70 expression. Leuk Res 1997; 21: 139–145
- Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, Naito Y, Yoshikawa T. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia 2009; 25: 210–219