Abstract
Purpose: To evaluate the feasibility, safety and efficacy of ultrasound-guided percutaneous microwave ablation combined with percutaneous ethanol injection in the treatment of liver tumours adjacent to the hepatic hilum.
Materials and methods: From December 2005 to April 2008, 18 consecutive patients with 18 pathologically proven or clinically diagnosed liver tumours (15 HCCs, three metastatic tumours) adjacent to the hepatic hilum underwent ultrasound-guided percutaneous microwave ablation combined with percutaneous ethanol injection. One or two microwave antennae were inserted and placed at designated places in the tumour. One or two ethanol needles were placed at the tumour periphery near the hepatic hilum. An aliquot of 2–10 mL of absolute ethanol was injected into the tumour at the same time as microwave emission. A thermocouple was placed directly abutting the bile ducts of the hepatic hilum to monitor temperature in real time during ablation to avoid thermal injury.
Results: No more than two sessions were performed to complete the treatment (one session for 10 patients, two sessions for eight, mean 1.4 sessions per patient). Complete ablation was achieved in 94.4% (17 out of 18 cases). In a median follow up of 15 months (range 4 to 27 months, mean 13.5 months), no mortality or complications occurred. Local tumour progression was noted in one patient 12 months after treatment.
Conclusion: A combination of ultrasound-guided percutaneous microwave ablation and percutaneous ethanol injection assisted with real-time temperature monitoring appears to be feasible and effective in the treatment of liver tumours adjacent to the hepatic hilum, and initial experience with safety is promising.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, with an incidence of more than 500,000 new cases a year Citation[1]. The liver is also the most common organ involved in metastatic disease Citation[2]. Surgical resection has been considered the first choice treatment of liver tumours Citation[3–5]. However, curative surgical resection is contraindicated in the majority of patients with liver tumours, due to cirrhosis, impaired liver function, or multiplicity of lesions Citation[4], Citation[6]. Thermal ablation methods such as microwave (MW) and radiofrequency (RF) have been widely used in the treatment of liver tumours with good efficacy and low complication rates Citation[7–21]. However, tumours adjacent to the hepatic hilum have been reported to be unsuitable for thermal ablation because of likelihood of incomplete necrosis caused by the heat sink effect and major complications such as bile duct stricture and biloma [11, 17, 18, 22]. Recently, RF ablations combined with percutaneous ethanol injection (PEI) have been reported to be more effective than RF ablation alone, with a higher complete necrosis rate and similar complication rate, for tumours in high-risk locations Citation[23], Citation[24]. Zhou et al. concluded in their study that combined therapy of PEI with MW ablation could coagulate significantly larger volumes of tumour and improve the rate of complete necrosis Citation[25]. Thus, the purpose of this study is to evaluate the feasibility, safety and efficacy of ultrasound-guided (US-guided) percutaneous MW ablation combined with PEI in treatment of liver tumours adjacent to the hepatic hilum.
Materials and methods
Patients
The study was performed in accordance with the Society of Interventional Radiology (SIR) reporting standards Citation[20]. From December 2005 to April 2008, 18 consecutive patients with 18 pathologically proven or clinically diagnosed liver tumours adjacent to the hepatic hilum received US-guided percutaneous MW ablation combined with PEI. Pathology was proven by biopsy in 10 cases and by clinical and imaging features in eight cases. The clinical and imaging diagnostic criteria for HCC included hepatitis B cirrhosis and contrast-enhanced computerised tomography (CT) or magnetic resonance imaging (MRI) demonstrating arterial phase enhancement and parenchymal phase wash-out. All the 18 patients were not surgical candidates. There were no more than three lesions per patient and those lesions which were not adjacent to the hepatic hilum were ablated at the same time. All patients were treated as inpatients. Institutional review board approval was obtained and all patients gave written informed consent. The inclusion criteria were as follows: 1) tumour nodules were 5 cm or smaller in diameter and located adjacent to the hepatic hilum (no more than 5 mm from the main portal vein and its major branches); 2) no extrahepatic metastasis or vascular tumour invasion was observed; 3) an appropriate puncture route was present on US; 4) Child-Pugh classification of liver function was A or B, with prothrombin time <30 s, prothrombin activity >30%, and platelet count >30,000/uL. There were 12 men and 6 women, aged 36–79 years (mean age, 59 years).
Equipment
The commercially available MW ablation system (KY2000, Kangyou Medical, China) consists of a microwave generator, a flexible coaxial cable and a 20-cm long, 15-gauge cooled-shaft antenna. The generator is capable of producing 1–100 W of power at 2450 MHz, which can drive up to two antennae simultaneously. The antenna can be easily seen on US and has a shaft coated with Teflon to prevent adhesion. The antenna was designed to minimise power feedback and provide optimal energy deposition into the tissue. Inside the antenna shaft, there are dual channels through which distilled water is circulated by a peristaltic pump, continuously cooling the shaft to prevent shaft overheating. The microwave machine is also equipped with a thermal monitoring system which can measure temperature in real time during ablation. A percutaneous transhepatic cholangiography (PTC) needle was used to inject absolute ethanol into the tumour periphery.
Technique
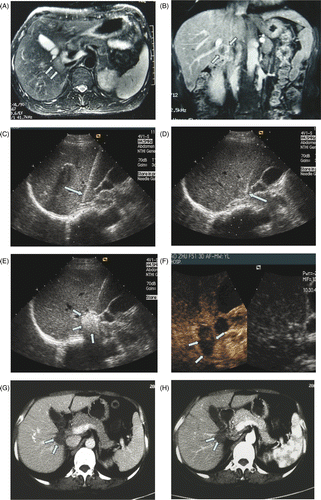
Before treatment, all patients received an US examination and contrast-enhanced CT scan or MR imaging (, B), and an appropriate puncture route was chosen on US. After local anaesthesia with 1% lidocaine, the microwave antenna was percutaneously inserted and placed at the designated places of the tumour under US guidance: for tumours less than 2 cm, one antenna was inserted to the centre of the tumour; for tumours measuring 2 cm or greater, two antennae were inserted into the tumour with an inter-antenna distance of no more than 1.8 cm, and were activated simultaneously during ablation to obtain a larger ablation zone (). The antenna was at least 5 mm away from the hepatic hilum. One or two 20-gauge ethanol needles were inserted and placed at the tumour periphery close to the hepatic hilum (). A 20-gauge thermocouple was inserted adjacent to the hilar bile duct closest to the tumour, allowing real-time temperature monitoring during MW ablation and prevention of thermal-mediated bile duct injury. All insertions were performed by either of the two experienced radiologists who had cooperated for more than ten years in MW ablation. After all insertions, intravenous anaesthesia was administered by a combination of propofol and ketamine via a peripheral vein. A power output of 45 to 50 Wfor 200 to 700 s was used during MW ablation. If the temperature measured by the thermocouple reached 54°C, MW emission was stopped immediately and was restarted when the temperature became lower than 45°C. This continued until the entire tumour was completely covered by the hyperechoic micro-bubbles on grey-scale US () Citation[26]. Absolute ethanol was injected into the tumour very slowly (approximate 1 mL per min) by assistants at exactly the same time as microwave emission to enlarge the coagulation zone by diffusion of hot ethanol. The amount of absolute ethanol injected was determined according to the size and location of the tumour empirically. The larger the tumour was, the more ethanol was injected. For tumours less than 3 cm, no more than 5 mL ethanol was injected; for tumours larger than 3 cm, more than 5 mL ethanol was injected (maximum 10 mL). While withdrawing the antenna, the needle track was coagulated to prevent tumour cell seeding. All patients received contrast-enhanced US examination 1 day after MW ablation to assess the immediate treatment efficacy. If residual tumour was noted, a further session was performed to ablate the entire tumour. No tumour enhancement was detected on contrast-enhanced US in all patients before discharge ().
Figure 1. MW ablation in a 51-year-old woman with liver tumor adjacent to hepatic hilum. (A), (B) MRI before MW ablation showed tumor adjacent to hepatic hilum. (C) The place of antenna during MW ablation. (D) The place of absolute ethanol needle during MW ablation. (E) Tumor was covered by the hyperechoic microbubbles on gray-scale US. (F) CEUS showed no enhancement of the ablation zone at 1 day after treatment. Contrast-enhanced CT showed no enhancement of the ablation zone at six months (G) and twelve months (H) after treatment.
Post-procedure observation and imaging follow-up
After treatment, all patients were closely monitored for possible complications such as bile duct stricture, biloma and skin burn. Side effects such as fever, pleural effusion and pain were also documented. The follow-up period was calculated starting from the day the last MW ablation was performed and the follow-up protocol consisted of contrast-enhanced US and contrast-enhanced CT and/or MRI at 1, 3 and 6 months after the last MW ablation and every 6 months thereafter. Ablation was considered complete when contrast-enhanced imaging findings at 1 month showed no enhancement in the index tumour. Local tumour progression was defined as the appearance of viable tumour during follow-up that was contiguous with the zone that had been considered completely ablated Citation[18].
Results
In this study, there were 15 HCCs and three liver metastases (two from colon, one from ovary). Tumour size in maximum diameter ranged from 1.8 to 4.1 cm (mean size, 2.8 cm). All patients with HCC were infected by Hepatitis B and the severity of liver dysfunction was classified as Child class A in 13 patients and Child class B in two patients. No more than two sessions were performed to complete the treatment (one session for 10, two sessions for 8, mean 1.4 sessions per patient). 2–10 mL absolute ethanol (mean 6.1 mL, up to two needles per session and up to 5 mL per needle) was injected into the tumours. Complete ablation was achieved in 94.4%. All patients were followed up regularly according to the protocol. During a median follow up of 15 months (range 4 to 27 months, mean 13.5 months), there was no mortality. Residual tumour was detected in the contrast-enhanced US and MRI 1 month after treatment in one patient. Local tumour progression was noted in one patient 12 months after treatment and partial hepatectomy was performed. After treatment, 12 patients experienced grade 1 pain at the puncture site according to the standardization of terms and reporting criteria for image-guided tumour ablation Citation[20]. Fourteen patients had a fever of 37.2–39.7°C which persisted for 1–5 days. Thrombosis was found in the right portal vein and the umbilical part of the left portal vein by contrast-enhanced US in one patient 1 month after treatment, which disappeared 3 months later without any management. The ablation zone was well defined on contrast-enhanced CT/MRI and contrast-enhanced US and shrank gradually over time (, 1H).
Discussion
Treatment for patients with tumours adjacent to the hepatic hilum is difficult. Traditional open surgery has the disadvantages of high morbidity and invasiveness. In the past decade, local thermal ablation as a minimally invasive yet effective treatment option has gained popularity in the treatment of liver tumours. An increasing number of patients with liver tumours undergo treatment with a local thermal ablation procedure to avoid risks related to surgical or transcatheter management. However, patients with liver tumours adjacent to the hepatic hilum have been considered not suitable for thermal ablation Citation[11], Citation[17], Citation[18], Citation[22]. Recently, more investigators have begun to investigate the feasibility and safety of local ablation of liver tumours adjacent to the hepatic hilum. Takuma Teratani Citation[27] ablated 79 tumour nodules less than 5 mm away from a first or second branch of the portal vein by RF; bile duct injury occurred in six (7.6%). In a study of Elias Citation[28], RF ablation was performed for 13 patients with liver tumours less than 6 mm away from the central bile duct. Thermal mediated injury of bile ducts was avoided by cooling the main bile ducts (right, left, or both) with a 4°C saline solution quickly infused by a catheter introduced inside the bile duct through an intraoperative choledochotomy. Although the clinical outcome was fairly good, this technique is difficult to perform for percutaneous thermal ablations.
Combinations of chemical and thermal ablation have also been reported. The combination of RF ablation and PEI in the management of HCC in high-risk locations was more effective than RF ablation alone Citation[24]. In a study by Zhou et al. Citation[25], combining PEI and MW ablation achieved a significantly wider maximum diameter of coagulation and more complete necrosis of the treated tumours. This was thought to be due to increased thermal conduction and diffusivity through previously coagulated tissue. In our study, percutaneous MW ablation combined with PEI was performed. The relatively high rate of complete ablation (17/18, 94.4%) and low rate of complication achieved may be attributable to the following reasons: 1) Our accumulated experience in percutaneous microwave ablation procedures amounting to a total of 1136 cases; 2) Long-duration, low-power (45–50 W) ablation may cause gradual ablation of the tumour nodule and avoid thermal injury; 3) We placed no restrictions on the number of treatment sessions. If contrast enhanced US 1 day after treatment showed enhancement, a repeated MW ablation combined with PEI was performed under contrast enhanced US guidance until complete necrosis of the entire tumour was confirmed; 4) In our study, PEI was performed simultaneously with MW emission to augment the effect of MW ablation. The risk of thermal injury to hilar bile ducts may preclude complete MW ablation of the tumour periphery close to the hepatic hilum, but the tumour periphery could be coagulated by the chemical ablation effect of the absolute ethanol injected at the same time as MW emission; 5) Real-time peritumoural temperature monitoring was used as an indicator for avoiding bile duct injury.
This study has some limitations. First, these data were obtained in a single centre with extensive experience in microwave ablation for liver tumours, which may lead to lower rates of residual tumour and local tumour progression. A multicentre study is required. Second, only 18 patients were included in this study. More patients should be recruited for better assessment of treatment safety and efficacy. Third, our study didn’t have a comparative group. A comparative study is required for further assessment of treatment safety and efficacy.
Conclusion
A combination of US-guided percutaneous microwave ablation and ethanol injection, assisted with real-time temperature monitoring, appears to be feasible and effective in the treatment of liver tumours adjacent to the hepatic hilum, and the initial experience with safety is promising. This may potentially expand indications of MW ablation.
Declaration of interest: The study was supported by two grants respectively from the National Scientific Foundation Committee of China (30825010) and from the Ministry of Health of the Peoples Republic of China (2008ZX1000026). The authors alone are responsible for the content and writing of the paper.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362: 1907–1917
- Vogl TJ, Müller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. Liver metastases: Interventional therapeutic techniques and results, state of the art. Eur Radiol 1999; 9: 675–684
- Liver Cancer Study Group of Japan. Primary liver cancers in Japan. Cancer 1980; 45: 2263–2269
- Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995; 221: 291–298
- Lee CS, Sheu JC, Wang M, Hsu HC. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br J Surg 1996; 83: 330–333
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994; 74: 817–825
- Matsukawa T, Yamashita Y, Arakawa A, Nishiharu T, Urata J, Murakami R, Takahashi M, Yoshimatsu S. Percutaneous microwave coagulation therapy in liver tumors: A 3-year experience. Acta Radiol 1997; 38: 410–415
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223: 331–337
- Dong BW, Liang P, Yu XL, Su L, Yu DJ, Cheng ZG, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180: 1547–1555
- Liang P, Dong BW, Yu XL, Yu DJ, Wang Y, Feng L, Xiao QJ. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Liang P, Dong BW, Yu XL, Yang Y, Yu DJ, Su L, Xiao QJ, Sheng L. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. Am J Roentgenol 2003; 181: 1319–1325
- Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, Xu ZF, Liu GJ, Zheng YL. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol 2005; 40: 1054–1060
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg 1999; 230: 1–8
- Dodd GD III, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: Postablation syndrome. Am J Roentgenol 2005; 185: 51–57
- Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology 2000; 217: 633–646
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complication encountered in a multicenter study. Radiology 2003; 226: 441–451
- McGahan JP, Dodd GD III. Radiofrequency ablation of the liver. Current status. Am J Roentgenol 2000; 176: 3–16
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001; 221: 159–166
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD III, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Jr, , Society of Interventional Radiology Technology Assessment Committee, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005; 16: 765–778
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010; 26: 448–455
- Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: State-of-the-art. Liver Transpl 2004; 10: S91–97
- Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, Yoshida S, Nakai S, Murota M, Miyauchi Y, et al. Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol 2002; 21: 611–615
- Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. Am J Roentgenol 2008; 190: W187–195
- Zhou P, Liu X, Li R, Nie W. Percutaneous coagulation therapy of hepatocellular carcinoma by combining microwave coagulation therapy and ethanol injection. Eur J Radiol 2009; 71: 338–342
- Liang P, Wang Y, Yu XL, Dong BW. Malignant liver tumors: Treatment with percutaneous microwave ablation – Complications among a cohort of 1136 patients. Radiology 2009; 251: 933–940
- Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006; 43: 1101–1108
- Elias D, Sideris L, Pocard M, Dromain C, De Baere T. Intraductal cooling of the main bile ducts during radiofrequency ablation prevents biliary stenosis. J Am Coll Surg 2004; 198: 717–721