Abstract
Objective: To evaluate the efficiency and feasibility of contrast-enhanced ultrasound (CEUS)-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional US.
Materials and methods: From March 2006 to February 2010, 107 patients (93 male, 14 female; mean age 58.9 ± 11.0 years) with 107 hepatocellular carcinoma (HCC) nodules (mean maximum diameter 19.5 ± 8.5 mm) inconspicuous on conventional US underwent microwave (MW) ablation under CEUS guidance in this study. US contrast agent was SonoVue (Bracco, Milan, Italy), a second-generation contrast agent. CEUS was performed first, and then MW ablation was carried out by means of CEUS guidance under unconscious intravenous anaesthesia if the tumours were displayed on CEUS.
Results: 105 tumours were successfully visualised on CEUS by using 1–2 times contrast agent injection and MW ablation was performed under CEUS guidance. The technical success rate was 98.13% (105/107). The number of antenna insertions for each tumour was 1.89 ± 0.92, and the mean session of MW ablation for each tumour was 1.08 ± 0.28. The mean duration of energy application for each tumour was 7.05 ± 4.03 min. The follow-up time was 12–54 months (median 18 months). The technique effectiveness rate was 99.05% (104/105). The local tumour progression rate was 1.9% (2/105). There were no severe complications in any patients.
Conclusion: CEUS-guided MW ablation is an efficient and feasible treatment method for patients with hepatocellular carcinoma inconspicuous on conventional US.
Introduction
Thermal ablation therapy, such as radiofrequency (RF) ablation and microwave (MW) ablation, is a widely used method for solid tumours, especially for liver tumours, which has been a curative method for small liver cancer treatment Citation[1]. Apart from careful pre-procedure planning and elaborate post-procedure evaluation, accurate intra-procedure targeting, monitoring, and controlling play a critical role in the success of the technique Citation[2]. MW ablation is a promising, minimally invasive treatment method for liver tumours, which has been performed by using ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) guidance Citation[3–5]. US is a real-time technique and allows body scan from different positions and angles, so compared with CT or MRI guidance, MW ablation under US guidance is more convenient. However, the target tumours cannot always be visualised on B-mode US, due to the texture of the tumour Citation[6–8]. In addition, residual tumour is difficult to differentiate from the treated area of tumours after transcatheter arterial chemo-embolisation (TACE) or thermal ablation on US. A study Citation[9] reported that tumours could not be visualised on pre-procedural planning B-mode US in 30% of the patients referred for percutaneous RF ablation of hepatocellular carcinoma (HCC). Accurate delineation of the area of tumours is necessary to successfully apply MW ablation.
Contrast-enhanced US (CEUS) allows better visualisation of focal hepatic lesions that cannot be clearly visualised on B-mode US Citation[10], Citation[11]. Specifically, the second generation US contrast agents enable real-time continuous imaging of focal hepatic lesions for several minutes Citation[12]. This technique has been introduced to guide radiofrequency ablation of hepatic tumours Citation[6], Citation[13]. To our knowledge, there has been no report to assess the usefulness of real-time CEUS guidance for MW ablation of HCC inconspicuous on conventional ultrasound.
In this study, we evaluated the efficiency and feasibility of CEUS-guided MW ablation for HCC inconspicuous on conventional US.
Materials and methods
Patients
Inclusion criteria for this study were patients with HCC who refused to undergo surgery, nodules undetectable on conventional US but detectable by intravenous contrast-enhanced CT or MRI, three or fewer hepatic tumours, absence of portal vein thrombosis, prothrombin time of less than 25 s, prothrombin activity higher than 40%, and platelet count higher than 40 cells × 109/L. From March 2006 to February 2010, 107 patients with 107 HCC nodules, consisting of 93 males and 14 females (age range 37–87 years; mean 58.9 ± 11.0 years), were enrolled in this study (). Of all patients, 105 patients had hepatitis B- or C-induced liver cirrhosis, and two patients had biliary cirrhosis (102 Child-Pugh A, 5 Child-Pugh B). HCC was diagnosed using imaging analysis, including dynamic contrast-enhanced CT or MRI. The maximum diameter of the HCC nodules ranged from 8 to 44 mm (mean ± SD, 19.5 ± 8.5 mm). Among the 107 nodules, 21 were recurrent or residual nodules after TACE or MW ablation. This study was approved by our institutional human research review committee. Written informed consent was obtained from all patients.
Table 1. Clinical characteristics of patients included (n = 107)
Preablation imaging work up
All patients underwent US, CEUS, and contrast-enhanced CT or gadolinium-enhanced MRI in order to delineate the target tumour before MW ablation.
US and CEUS were performed using Sequoia 512 system (Acuson, Mountain View, CA) with a multifrequency transducer (4V1, 2.0–4.5 MHz). US contrast agent used was SonoVue (Bracco), a second-generation contrast agent. A vial of the contrast agent was divided into two doses of 2.4 ml each. The first dose was injected intravenously for the pre-procedural planning CEUS and the second dose was used for the CEUS-guided MW ablation. Each dose was immediately followed by a 5 ml normal saline flush. Contrast-specific software was used. The mechanical index was 0.12–0.18. The focus was positioned at the bottom of the screen to minimise microbubble destruction. Field of view and gain were optimised to provide the clearest depiction of the lesion. Immediately after the injection, the operator tried to detect the target tumour by continuously sweeping the transducer across the hepatic region considered to contain the target tumour. If no target tumour was identified within 5 min, the injected microbubbles were disrupted by imaging the liver in high mechanical index mode for several minutes, and then the planning CEUS was repeated with the second dose to search for the predetermined target or any other potential target in the liver. If the second planning CEUS did not reveal any target tumour either, MW ablation was not performed. Such cases were counted as technical failures. However, when a target tumour was displayed on CEUS, the radiologist could measure the tumour diameter and depth, plan a safe needle trajectory (marking on the skin), and rehearse the microwave ablation procedure (including instruct the patient to suspend respiration).
All CT studies were performed with the same multi–detector row CT (Lightspeed 16; GE Medical Systems, Milwaukee, WI) and contrast medium (iopromide, Ultravist 300; Schering, Berlin, Germany). All MRI studies were carried out with the same 1.5-T unit (Signa EchoSpeed, GE Medical Systems, Voorhees, NJ), contrast medium (Magnevist, Bayer Schering, Leverkusen, Germany) (0.1 mmol/kg of body weight) and sequences.
Microwave system
The microwave unit (KY-2000, Kangyou Medical, Nanjing, China) consists of three independent MW generators with frequency of 2450 MHz, three flexible coaxial cables and three water-pumping machines, which can drive three 15-gauge and 18-cm long cooled-shaft antennae (1.1-cm antenna tip) simultaneously. The MW unit generators are capable to produce 1–100 W of power. The cooled-shaft antennae are coated with Teflon which is used to prevent adhesion. Inside the antenna shaft, there are dual channels through which distilled water is circulated by a peristaltic pump, continuously cooling the shaft to prevent overheating.
Microwave ablation procedures under CEUS guidance
All treatments were performed under CEUS guidance in the operating room with the patients under unconscious intravenous anaesthesia (propofol, 6–12 mg/kg/h; ketamine, 1–2 mg/kg). All procedures were performed by two experienced doctors (L.P. and Y.X.L.); both had more than 10 years’ experience in MW ablation of liver tumours. A detailed protocol including the placement of the antennae, power output setting, duration of energy application and appropriate approach was applied to each patient on an individual basis before treatment. If tumours were adjacent to the diaphragm dome MW ablation was performed under CEUS guidance assisted by artificial pleural effusion in order to prevent injury to the lung. Saline, 800 to 1500 ml, was infused in the pleural cavity in Fowler's position, followed by thoracocentesis by a 16-gauge trocar under general anaesthesia using one lung ventilation. In general, MW ablation was performed at 50 W using two to three cooled-shaft antennae simultaneously, the distance between which was less than 2.5 cm. A thermal monitoring system attached to the MW unit was used during ablation. One to two 21-gauge MW thermal monitoring needles were inserted in the margin of the targeted tumour (proximity to the tumour periphery about 5 mm) under CEUS guidance. During MW ablation we monitored the hyperechoic area of ablation using grey-scale sonography and thermal monitoring to decide the endpoint of treatment. The treatment session would be ended if the transient hyperechoic zone between antennae on grey-scale US merged and covered the target region; meanwhile the temperature of the thermal needles achieved target temperature (over 60°C for lesions in safe location and keeping 54°–60°C for more than 3 min for lesions adjacent to the gastrointestinal tract, diaphragm, gallbladder, etc.) according to our previous clinical experiences Citation[3], Citation[14]. When withdrawing the antennae, the needle tracks were routinely cauterised to avoid tumour seeding and bleeding. Ten minutes after ablation every patient received CEUS to evaluate treatment response. Possible residual tumour was identified if abnormal nodular hypervascular region existed in the peripheral ablation region. Additional treatment was performed with one to three cooled-shaft antennae depending on the size of residual tumour. Treatment was not considered complete until the entire tumour showed no enhancement on CEUS. Duration of energy application and power of each generator were recorded. Emission energy was calculated as the sum of each generator's emission energy during the treatment of one patient. Duration of energy application represented the generators’ working time.
Therapeutic efficacy assessment and follow up
Therapeutic efficacy was assessed by the result of contrast-enhanced imaging and serum tumour marker levels after the treatment. The follow-up period was calculated starting from the beginning of MW ablation in all patients. CEUS and contrast-enhanced CT or MRI were repeated at 1-month and at 3-month intervals within 1 year and then at 6-month intervals after MW ablation. If abnormal peripheral nodular enhancement of the ablation area was found during follow up and identified as local tumour progression, further MW ablation was performed under CEUS guidance.
Statistical analysis
Data analysis was performed using SPSS 11.0 for windows (SPSS, Chicago, IL) and the continuous data were expressed as means ± standard deviations (SD). A case was counted as a technical success if the tumour could be visualised after 1–2 SonoVue injections and MW ablation procedure could be performed under CEUS guidance. The technique effectiveness was defined as the ‘complete ablation’ of the macroscopic tumour proved by the contrast enhanced imaging 1 month after ablation. Local tumour progression was defined as incompletely treated viable tumour continuing to grow or a new tumour (or in the case of hepatocellular carcinoma, ‘daughter’ or ‘satellite’ tumours) growing at the original site during follow up. The number of antenna insertions was defined as the total number of antenna placements for each tumour until this tumour was ablated completely.
Results
Outcome of microwave ablation under CEUS guidance
Of all 107 tumours, 105 tumours were successfully visualised on CEUS by using 1–2 times contrast agent injections. Two tumours could not be visualised on CEUS. One was a residual tumour after TACE treatment which could not be visualised on CEUS because the tumour was adjacent to the diaphragm and affected by the attenuation of iodised oil in the treated area. The other was a new tumour after MW ablation, and some abnormal blood supply areas were found in the liver around the tumour, so it was difficult to make sure of the location of tumour blood supply. In vascular phases in contrast enhanced ultrasound of the liver, the arterial phase has been considered as the period from 10–20 s post-injection to 25–35 s, the portal venous phase as the period from 30–45 s post-injection to 120 s, and the late phase as the period from 120 s post-injection to the time of bubble disappearance (approx. 240–360 s) Citation[15]. Of the 105 lesions, 100 lesions were hyper-enhanced in the arterial phase and hypo-enhanced in the portal venous phase and late phase, and five lesions were iso-enhanced in the arterial phase and hypo-enhanced in the portal-venous phase and late phase.
MW ablation was performed under CEUS guidance for the 105 patients whose tumours can be visualised on CEUS ( and ), for which six cases with tumours adjacent to the diaphragm dome MW ablation was performed under CEUS guidance assisted by artificial pleural effusion in order to prevent injury to the lung. The technical success rate was 98.13% (105/107). The number of antenna insertions for each tumour was 1.89 ± 0.92, and the mean session of MW ablation for each tumour was 1.08 ± 0.28. The mean duration of energy application for each tumour was 7.05 ± 4.03 min. The follow-up time was 12–54 months (median, 18 months). 104 cases achieved complete ablation confirmed by the contrast-enhanced imaging 1 month after ablation. One case with the tumour adjacent to the diaphragm was confirmed as a residual tumour by the contrast-enhanced imaging 1 month after ablation. The technique effectiveness rate was 99.05% (104/105).
Figure 1. Images in a 64-year-old man who underwent CEUS guidance MW ablation of HCC in segment V of the liver. (A) Conventional US could not detect the tumour. (B) The CEUS arterial phase image shows a 2.2 × 1.4 cm tumour in the right hepatic lobe (arrows). (C) MW antenna was inserted along the guideline under CEUS guidance (arrows). (D) Arterial phase of CEUS obtained 3 months after MW ablation shows complete necrosis of the tumour (arrows).
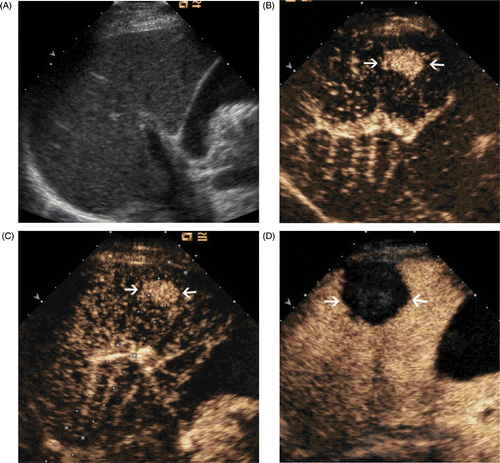
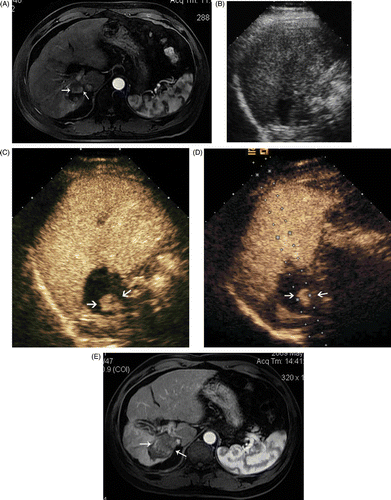
Figure 2. Images in a 52-year-old male with a 1.8 × 1.5 cm local recurrence of HCC after MW ablation in segment VII of the liver. (A) Contrast-enhanced MRI shows a local recurrence (arrows) in the right hepatic lobe. (B) Conventional US could not detect the tumour. (C) CEUS shows arterial enhancement adjacent to the ablation zone indicative of local recurrence (arrows). (D) MW antenna was inserted along the guideline under CEUS guidance (arrows). (E) Arterial phase of contrast-enhanced MRI obtained 3 months after MW ablation shows complete necrosis of the recurrence (arrows).
Complications
Major complication was defined as an event that led to substantial morbidity and disability, increasing the level of care, or resulted in hospital admission or substantially lengthened hospital stay (SIR classifications C–E). This included any case in which a blood transfusion or interventional drainage procedure was required. All other complications were considered minor. No deaths and no thrombosis of major vessels in any patients occurred. In two patients with an ablated lesion adjacent to the surface of the liver a dose of oral analgesics (oxycodone) was administered due to a moderate right upper quadrant pain (grade 2). The symptoms gradually disappeared after 1 week; 32 cases had non-infective high fever, while the temperature reduced to normal within 2 to 7 days without special treatments.
Local tumour progression
Local tumour progression was found in two of 105 tumours (1.9%) on follow-up contrast enhanced images. One case with a tumour adjacent to the diaphragm was confirmed as a residual tumour by the contrast enhanced imaging 1 month after ablation, the other one with a tumour adjacent to the diaphragm dome was confirmed as a recurrent tumour in the periphery of the ablation zone by the contrast enhanced imaging 6 months after ablation. All cases of local tumour progression were confirmed by biopsy performed during re-treatment. All local tumour progressions were completely ablated by further MW ablation.
Discussion
MW ablation is one of the most effective localised thermal ablation methods for liver tumours. Image guidance is essential in liver tumours treatments using percutaneous MW ablation techniques. MW ablation is widely performed by US guidance, in comparison with CT or MRI guidance, due to the real-time imaging characteristics. However, the target tumours cannot be always visualised on B-mode US. Although CEUS using Levovist (Schering, Berlin, Germany), a first-generation intravenous contrast agent, has been used to depict tumour vascularity with high sensitivity and accuracy, this contrast medium does not allow enhanced HCC nodule visualisation in real time, because it could be rapidly destroyed when exposed to high acoustic power insonation Citation[16]. The second generation US contrast agents can provide a stable non-linear oscillation in a low-power acoustic field and produce greater detail imaging through second harmonic signals in real time, such as Sonazoid (GE Healthcare, Oslo, Norway) and SonoVue Citation[17]. Miyamoto et al. Citation[18] reported that CEUS guidance using Sonazoid could be useful for RF ablation therapy of local recurrence of previously treated HCC undetected by conventional sonography. To our knowledge, this is the first report concerning the usefulness of real-time CEUS guidance using SonoVue for MW ablation of HCC inconspicuous on conventional ultrasound.
SonoVue is a second-generation contrast agent, which has been widely used in the diagnosis of liver lesions, especially in the diagnosis of HCC Citation[19]. After injection of 2.4 ml SonoVue, HCC lesions can be examined continuously for up to 5 min using low mechanical index sonography and the lesions can be visualised according to the difference of microcirculation between lesions and liver parenchyma. Yoon et al. Citation[20] reported that real-time CEUS-guided biopsy using SonoVue was technically feasible for hepatic focal lesions not confidently localised on B-mode US, and the technical success rate was 86% (38/44). In our study, of all 107 lesions undetectable on conventional US, 105 lesions were successfully visualised on CEUS by using 1–2 contrast agent injections and they received MW ablation under CEUS guidance. The technical success rate was 98.13% (105/107). There may be two reasons why we got a higher technical success rate in our study. Firstly, immediately after the injection the doctor detected the target by continuously sweeping the transducer across the hepatic region, where the lesion was considered to be located (according to the location displayed on MRI or CT). The second reason may be related to the experience of the doctors themselves. The doctor who performed the CEUS in our study has more than 5 years’ experience (more than 2000 cases performed with CEUS). The two patients whose tumours could not be visualised on CEUS had been previously treated. One patient had a residual tumour after TACE treatment which could not be visualised on CEUS because the tumour was adjacent to the diaphragm and affected by the attenuation of iodised oil in the treated area. The other patient had previously undergone MW ablation, and some abnormal blood supply areas were found in the liver around the tumour, so it was difficult to be sure of the location of the tumour blood supply.
The 105 patients whose tumours could be visualised on CEUS underwent MW ablation under CEUS guidance. In six cases, with lesions adjacent to diaphragm dome, MW ablation under CEUS guidance assisted by artificial pleural effusion was needed in order to obtain a safe puncture tract. Artificial pleural effusion has been used, not only in order to obtain clear images of the whole tumour, but also to select a puncture tract. This provides protection against serious complications such as hepatic infarction, intraperitoneal haemorrhage, and lung injury Citation[21]. In addition, artificial pleural effusion did not adversely affect respiratory function of the patients in our study.
In our study, local tumour progression was found only in two of 105 tumours (1.9%) on follow-up contrast-enhanced imaging, which was lower than previous reports Citation[3], Citation[22]. Although there could be many risk factors for local tumour progression, the insufficient safety margin and the size of the tumour were the two main ones for local tumour progression Citation[23]. The margin of the tumour could be more clearly displayed on CEUS than on conventional US, so a sufficient safety margin could be obtained more easily under CEUS guidance as well. In addition, the small dimension of HCC nodules (mean diameter 19.5 ± 8.5 mm) may be one reason for the lower local tumour progression rate encountered in our study. Moreover, because the examinations were performed by experts in US and CEUS, the results could be different with trainees or non-expert radiologists.
This study had two main limitations. First, the present study was a retrospective study without a control group. Second, this was only a single centre study; a multi-centre study would be more representative.
Conclusion
CEUS-guided microwave ablation is an efficient and feasible treatment method for patients with HCC inconspicuous on conventional US.
Acknowledgements
The authors would like to acknowledge Alex Dell’ Era for his help in the manuscript revision.
Declaration of interest: This study was supported by two grants, respectively, from the National Scientific Foundation Committee of China (30825010) and the Ministry of Health of the People's Republic of China (2008ZX10002026). The authors alone are responsible for the content and writing of the paper.
References
- Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 2006; 25: 3848–3856
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Jr, et al. Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005; 235: 728–739
- Dong BW, Liang P, Yu XL, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180: 1547–1555
- Sato M, Watanabe Y, Tokui K, Kawachi K, Sugata S, Ikezoe J. CT-guided treatment of ultrasonically invisible hepatocellular carcinoma. Am J Gastroenterol 2000; 95: 2102–2106
- Kurumi Y, Tani T, Naka S, Shiomi H, Abe H, Endo Y, Morikawa S. MR-guided microwave ablation for malignancies. Int J Clin Oncol 2007; 12: 85–93
- Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: Usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. Am J Roentgenol 2004; 183: 153–156
- Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, Pallavicini D, Campani R. Hepatocellular carcinoma treated with radiofrequency ablation: Comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. Am J Roentgenol 2001; 177: 375–380
- Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, Ducerf C. Small nodule detection in cirrhotic livers: Evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr 2001; 25: 327–336
- Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. Am J Roentgenol 2008; 190: 1324–1330
- Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: Multicenter study. Radiology 2003; 227: 361–370
- Chami L, Lassau N, Malka D, Ducreux M, Bidault S, Roche A, Elias D. Benefits of contrast-enhanced sonography for the detection of liver lesions: Comparison with histologic findings. Am J Roentgenol 2008; 190: 683–690
- Catalano O, Nunziata A, Lobianco R, Siani A. Real-time harmonic contrast material-specific US of focal liver lesions. Radiographics 2005; 25: 333–349
- Minami Y, Kudo M, Chung H, Kawasaki T, Yagyu Y, Shimono T, Shiozaki H. Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: Prospective randomized controlled trial. Am J Roentgenol 2007; 188: 489–494
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Grastrointest Surg 2009; 13: 318–324
- Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) – update 2008. Ultraschall Med 2008; 29: 28–44
- Numata K, Tanaka K, Kiba T, Saito S, Isozaki T, Hara K, Morimoto M, Sekihara H, Yonezawa H, Kubota T. Using contrast-enhanced sonography to assess the effectiveness of transcatheter arterial embolization for hepatocellular carcinoma. Am J Roentgenol 2001; 176: 1199–1205
- Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, Takahashi S, Inoue T, Hagiwara S, Chung H, et al. Differential diagnosis of hepatic tumors: Value of contrast enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology 2008; 51: S61–69
- Miyamoto N, Hiramatsu K, Tsuchiya K, Sato Y, Contrast-enhanced sonography-guided radiofrequency ablation for the local recurrence of previously treated hepatocellular carcinoma undetected by B-mode sonography. J Clin Ultrasound 2010. Sep;38(7):339-45
- von Herbay A, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: Differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound 2010; 38: 1–9
- Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH, Kim TK. Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 2010; 20: 2047–2056
- Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. Am J Roentgenol 2004; 183: 583–588
- Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, Xu ZF, Zheng YL, Liang JY. Liver cancer: Increased microwave delivery to ablation zone with cooled-shaft antenna –Experimental and clinical studies. Radiology 2007; 242: 914–924
- Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur J Radiol 2006; 59: 432–441