Abstract
Purpose: Tumour cell-derived exosomes may represent a novel type of cancer vaccine. However, the immunogenicity of exosomes derived from tumour cells has been shown to be poor. Therefore, in this study, exosome immunogenicity following heat treatment of exosomes from malignant ascites obtained from gastric cancer patients was evaluated.
Materials and methods: Tumour-derived exosomes were isolated from heat-treated and untreated malignant ascites of gastric cancer patients using serial centrifugation and sucrose gradient ultracentrifugation. Next, in vitro experiments were performed to investigate the influence of heat treatment on exosome immunogenicity.
Results: Exosomes from heat-treated malignant ascites of gastric cancer patients (HS exosomes) were found to contain higher concentrations of heat shock proteins, Hsp70 and Hsp60, than exosomes derived from untreated malignant ascites obtained from gastric cancer patients. Additional in vitro studies suggest that exosomes derived from heat-treated malignant ascites are able to promote dendritic cell (DC) maturation and induce a tumour-specific cytotoxic T lymphocyte (CTL) response.
Conclusions: Overall, these results demonstrate that exposure to heat stress can improve the immunogenicity of exosomes obtained from malignant ascites of gastric cancer patients.
Introduction
Exosomes are small membrane vesicles secreted by many types of cells Citation[1], including tumour cells. In the latter case, these have been proposed to have potential antitumour efficacy for cancer vaccines Citation[2]. For example, exosomes derived from malignant ascites of tumour patients have been shown to induce a cytotoxic T lymphocyte (CTL) response in vitro by delivering tumour antigens to autologous dendritic cells (DCs) Citation[3]. In addition, exosomes derived from colorectal cancer ascites in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) have been shown to induce a tumour-specific antitumour CTL response Citation[4]. However, the antitumour effects induced by exosomes derived from tumour cells and ascites are very weak. Therefore, it is necessary to enhance the immunogenicity of exosomes derived from tumour cells and tumour ascites for possible clinical applications.
Heat stress can significantly affect both innate and adaptive immunity Citation[5]. For example, heat stress can induce the release of heat shock protein 70 (Hsp70) from tumour cells, which activates tumour cells to produce chemokines for the chemoattraction of DCs and T cells Citation[6]. Heat stress has also been shown to trigger a marked increase in the levels of heat shock proteins expressed in exosomes secreted from B cells Citation[7]. Exosomes prepared from heat-stressed tumour cells enhance tumour-specific CTL responses both in vitro and in vivo Citation[8–10], and have been associated with an enhanced immunogenicity of tumour cells and exosomes secreted by tumour cells. Also, recent research found that exosomes derived from heat-stressed tumour cells contain chemokines (CCL2, CCL3, CCL4, CCL5, and CCL20) via a lipid raft-dependent pathway, which can chemoattract and activate DC and T cells, inducing a potent antitumour immune response Citation[11]. Moreover, until now, exosomes secreted by heat-stressed malignant ascites of gastric cancer patients have not been investigated.
Therefore, in this study the capacity for heat stress to improve the immunogenicity of exosomes secreted from malignant ascites of gastric cancer patients was investigated. Our preliminary research identified the ability of these exosomes to promote DC maturation and to induce a tumour-specific CTL response in vitro. As a result, exosomes derived from heat-stressed malignant ascites of gastric cancer patients may represent an effective tumour vaccine.
Materials and methods
Patients
A total of 18 patients with pathologically confirmed gastric adenocarcinoma treated at the Zhejiang Cancer Hospital (Hangzhou, China) were enrolled in this study. The experiments were conducted in all 18 patients and each experiment was repeated at least three times. In this study, none of the patients had surgery, chemo- or radiotherapy before ascite collection. Patient age for these cases ranged from 41–70 years (mean 59.5), and high levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were detected in all serum samples collected. The clinical characteristics are summarised in . In addition, four patients received two to four cycles of oral Xeloda 1700 mg m−2 on days 1–14, and oxaliplatin 130 mg m−2 intravenously on day 1, followed by the collection of ascites. Cycles were repeated every 3 weeks. The other 14 patients refused further chemotherapy.
Table I. Patient characteristics.
Preparation of ascites cells
Specimens of ascites were obtained from the patients. Ascites were then centrifuged at 400 × g for 10 min at room temperature to sediment cells. The cells were washed three times with PBS and counted by trypan blue exclusion. Tumour cells were grown in Roswell Park Memorial Institute (RPMI 1640) medium supplemented with 10% heat-inactivated foetal bovine serum (FBS) (Hyclone, Logan, UT), penicillin (100 units/mL), and streptomycin (100 mg/mL).
Isolation of exosomes from heat-treated malignant ascites of gastric cancer patients
Malignant ascites (approximately 9 × 109 cells/L) were incubated in a water bath at 42.8°C. After 4 h, the supernatant was collected and the exosomes were isolated by sequential centrifugation (4°C) at 800 × g for 10 min, 1200 × g for 30 min, then 10,000 × g for 30 min. The resulting pellets were resuspended in 0.25 M sucrose, and floated into a discontinuous density cushion containing 20 mM Tris/30% sucrose/45% sucrose (pH 7.2), and centrifuged at 100,000 × g for 2 h. The protein concentrations of the isolated exosome samples were determined using Bradford assay reagents (Pierce Biotechnology, Rockford, IL) Citation[12]. Exosomes and cell lysate prepared from heat-treated malignant ascites of gastric cancer patients were labelled ‘HS-exosomes’ and ‘HS-lysate’, respectively, while untreated malignant ascites of gastric cancer patients are referred to as “exosomes”.
Electron microscopy
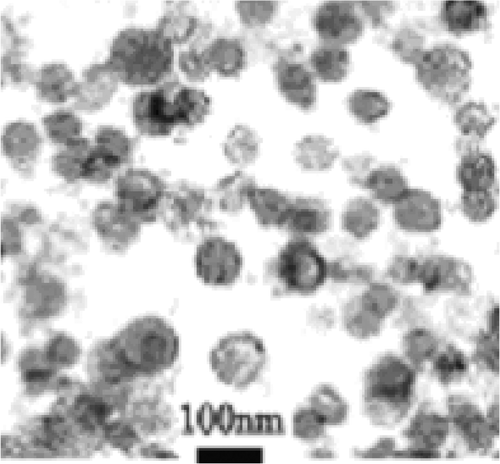
Exosome pellets were fixed in 4% paraformaldehyde before being loaded onto electron microscopy grids coated with Formvar carbon. Exosomes were contrasted and embedded in a mixture of uranyl acetate and methylcellulose before being imaged using a Philips Tecnai-10 transmission electron microscope operating at 80 kV (Phillips Electronic Instruments, Mahway, NJ).
Western blot analysis of exosomes
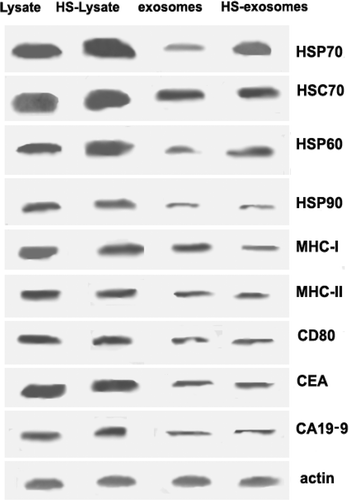
Ascite cell or exosomal lysate were prepared from three freeze-thawed cycles and the quantity of both proteins was detected by BCA protein assay Kit (Santa Cruz, CA). The same amount (8 µL) of exosome or cell lysate, at a concentration of 4 µg/µL, was separated using 10% SDS-PAGE, then transferred to polyvinylidene difluoride membranes. Primary antibodies were diluted according to the manufacturer's recommendations, and included: anti-Hsp70, anti-Hsc70, anti-Hsp60, anti-Hsp90, anti-MHC-I, anti-MHC-II, anti-CD80, anti-CEA, anti-CA19-9, and anti-actin antibodies (Chemicon International, Temecula, CA, USA; Santa Cruz Biotechnology, Santa Cruz, CA; and Sigma, St Louis, MO). Membranes were then incubated with the appropriate secondary antibodies, and bound antibodies were visualised using enhanced chemiluminescence reagents (Pierce Biotech, Rockford, IL) and the gel documentation system, UVP c-80 (UVP Inc., Upland, USA).
Generation and phenotypic analysis of DCs
Human peripheral blood monocytes (PBMC) were obtained from gastric cancer patients by Ficoll-Hypaque (Amersham Pharmacia Biotech, Arlington Heights, IL) density gradient centrifugation. Cells were cultured in 24-well plates at 5% CO2 and 37°C. Adherent cells were cultured by adding GM-CSF and IL-4 (Sigma), and DCs were collected 5 days later Citation[13].
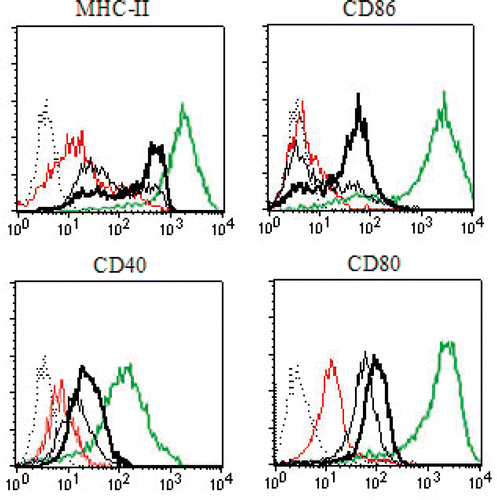
Immature human DCs (4 × 105/mL) were stimulated with 20 µg exosomes, 20 µg HS-exosomes, 20 µg HS-lysate, or 1 µg LPS Lipopolysaccharide (LPS). After 24 h, immature DCs were incubated with phycoerthrin (PE)-labelled anti-MHC-II, anti-CD86, anti-CD80, and anti-CD40 antibodies (BD Biosciences) for 50 min at 4°C. Labelled DCs were then analysed by flow cytometry.
Cytokine assays
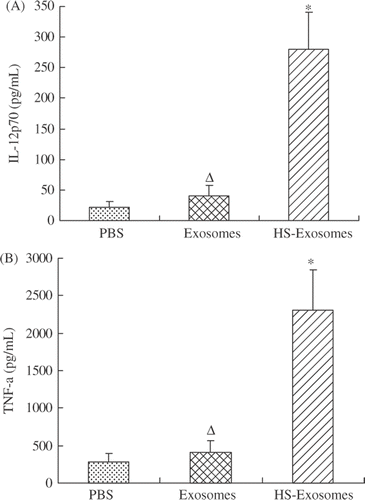
Immature DCs (4 × 105/mL) were stimulated with 20 µg exosomes, 20 µg HS-exosomes, or PBS for 24 h. Supernatants were then collected and levels of interleukin-12p70 (IL-12p70) and tumour necrosis factor-α (TNF-α) were detected using peroxidase-conjugated antibodies recognising digoxigenin in enzyme-linked immunosorbent assays (ELISAs) (Endogen, Cambridge, MA). Optical density (OD) values for each well were measured at 450 nm and recorded using an ELISA reader (Bioteck EL-340, Pegasus Scientific, Rockville, MD).
Mixed lymphocyte reaction
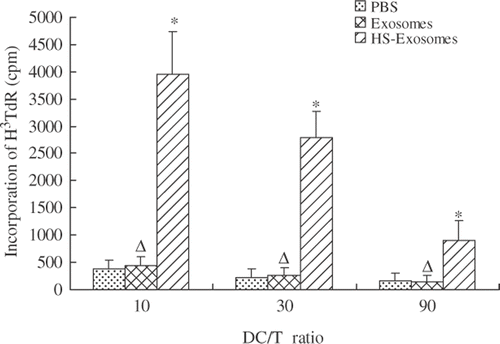
Immature DCs (4 × 105/mL) were incubated with 20 µg exosomes, 20 µg HS-exosomes, or PBS. After 24 h, DCs were collected and irradiated (3000 rad) for use as stimulator cells. Responder T cells were isolated from allogeneic PBMC of healthy donors. CD3 is expressed on all T cells and is associated with the T cell receptor. To obtain purified T cells, PBMC of a different (allogeneic) donor was positively sorted by anti-CD3 magnetic beads (Miltenyi Biotec, Auburn, CA). Stimulator cells were co-cultured with responder cells (5 × 105/mL) at various ratios for 3 days. After 72 h, 0.5 µCi 3HTdR was added to each reaction. After another 18 h, incorporation of 3HTdR was assayed by liquid scintillation counting.
CTL induction in vitro
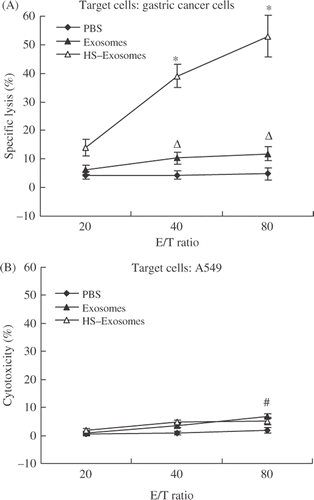
Cytotoxic activity was analysed using a lactate dehydrogenase (LDH) cytotoxicity assay (Mountain View, CA). Briefly, immature DCs were pulsed with 20 µg exosomes, 20 µg HS-exosomes, or PBS for 10 h, then co-cultured with autologous T cells in the presence of IL-7 (Sigma). Seven days after the final stimulation step, the stimulated T cells were used as effector cells. Targets for CTL activity were autologous gastric cells and a human lung cancer cell line, A549. Target cells were mixed with different ratios of effector cells and incubated for 6 h at 37°C. Cytotoxicity was subsequently calculated as follows: percentage of specific lysis = 100 × (experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous).
Statistical analysis
Statistical analyses were performed using Student's t-test. A P value <0.05 was considered to be statistically significant.
Results
Identification of exosomes derived from malignant ascites of gastric cancer patients
Exosomes of malignant ascites of gastric cancer patients which were exposed to heat stress or were not, were isolated and purified using serial centrifugation and sucrose gradient ultracentrifugation. The exosomes isolated had a bi-layer membrane and their diameters ranged from 40–90 nm (). The preparation of 300–500 mL of ascites resulted in 200 µg of exosomes. When exosomes were prepared from heat-treated malignant ascites, a 1.5-fold increase in the amount of exosomes isolated was observed.
Increased levels of Hsp70 and Hsp60 proteins in HS-exosomes
To evaluate the influence of heat stress on exosomal production from malignant ascites of gastric cancer patients, protein levels of Hsp70, Hsc70, Hsp60, Hsp90, MHC-I, MHC-II, CD80, CEA, and CA19-9 were assayed by western blot. Both HS-exosomes and HS-lysate samples were found to contain higher levels of Hsp70 and Hsp60 compared with exosome and cell lysate samples derived from untreated ascites ().
Figure 2. Western blot analysis of exosome preparations and cell lysate before and after heat treatment. Cell lysate and exosome samples with and without heat treatment (HS) were prepared as described in the Materials and methods. The primary antibodies used for detection are indicated in the figure, with detection of actin used as a loading control. Data from a representative sample (from patient 7) is shown.
Promotion of DC maturation by HS-exosomes
To investigate whether HS-exosomes can induce the maturation of DCs, immature human DCs were stimulated with 20 µg exosomes, 20 µg HS-exosomes, 20 µg HS-lysate, or 1 µg LPS (positive control). After 24 h of incubation, the cells were collected and stained for the DC activation markers MHC class II, CD86, CD40, and CD80. All of these activation markers were up-regulated by the positive control, LPS. Compared to untreated exosomes, HS-exosomes produced a significant increase in all of the DC activation markers ().
Figure 3. Characterisation of DC phenotype. Immature DCs pulsed with HS-exosomes, untreated exosomes, HS-lysate, or LPS were stained with antibodies recognising MHC-II, CD86, CD40, or CD80, and analysed by flow cytometry. Positive control (LPS) for DC maturation was used. The dashed line represents the isotype control, the fine black line represents exosomes, the bold black line represents HS-exosomes, the red thin line represents HS-lysate, and the green thin line represents LPS. Data from a representative sample (from patient 14) is shown.
The supernatants of immature DCs stimulated with 20 µg exosomes, 20 µg HS-exosomes, or PBS were also assayed after 24 h for levels of IL-12p70 and TNF-α by ELISA. In these assays, no significant differences were found between DCs pulsed with exosomes and DCs pulsed with PBS (P > 0.05, and ). For DCs pulsed with HS-exosomes, higher levels of IL-12p70 and TNF-α were detected in the supernatant compared with DCs pulsed with exosomes or PBS (P < 0.01, ).
Figure 4. Cytokine secretion. IL-12p70 (A) and TNF-α (B) levels ± standard deviation (SD) are shown for supernatants of immature DCs pulsed with HS-exosomes or untreated exosomes detected by ELISA. ΔP > 0.05, for the exosome samples versus the PBS samples; ☆P < 0.01, for HS-exosomes versus untreated exosomes or PBS treatment group. Results are presented as the mean levels ± standard deviation (SD) of 18 patient samples.
In mixed lymphocyte reactions, DCs pulsed with exosomes or PBS were not able to stimulate T cell proliferation (P > 0.05). In contrast, DCs pulsed with HS-exosomes stimulated T cell proliferation compared with DCs pulsed with exosomes or PBS (P < 0.01, ). Taken together, these results demonstrate that HS-exosomes are able to promote DC maturation.
Figure 5. Mixed lymphocyte reaction assays. Proliferation of T cells was assayed in mixed lymphocyte reactions containing various ratios of DCs and T cells as indicated. ΔP > 0.05, for exosomes versus PBS treatment group; ☆P < 0.01, for HS-exosomes versus untreated exosomes or PBS treatment group. Results are presented as the mean proliferation ± standard deviation (SD) of 18 patient samples.
Induction of tumour-specific CTL response by HS-exosomes in vitro
The ability of HS and untreated exosomes to induce a CTL response in vitro was also evaluated. In CTL assays, DCs pulsed with HS-exosomes increased CTL activity compared to DCs pulsed with untreated exosomes or PBS (P < 0.01, ). However, the cytotoxicity induced by DCs pulsed with HS-exosomes was not able to kill A549 cells (P > 0.05, ). These results demonstrate that DCs pulsed with HS-exosomes can induce a tumour-specific CTL response in vitro.
Figure 6. CTL activity assay. Specific cytotoxicity was evaluated in LDH assays containing autologous gastric cancer cells (A) or A549 cells (B) as the target cells. ΔP > 0.05, for the exosome group compared with the PBS group; ☆P < 0.01 for the HS-exosome group compared with the exosome or PBS group; #P > 0.05 for the HS-exosome group compared with the exosome or PBS group. Results are presented as the mean percentage of specific lysis ± standard deviation (SD) of 18 patient samples.
Discussion
Gastric cancer is the second leading cause of death due to cancer worldwide Citation[14]. Moreover, when gastric cancer cells metastasise, malignant ascites can be seeded in the peritoneal cavity and play a key role in disease progression. Patients diagnosed with malignant ascites are typically associated with a poor prognosis and a reduced quality of life, and currently, there are no effective treatment methods available. However, in this study, heat treatment of malignant ascites obtained from patients with gastric cancer was shown to enhance the immunogenicity of the exosomes isolated from the heat-treated ascites.
Regarding the biological characteristics of exosomes derived from tumour cells, two opinions have been dominant. One is based on the hypothesis that tumour-derived exosomes represent a new kind of cancer vaccine Citation[2], while the other hypothesis is that tumour-derived exosomes may represent a mechanism involving tumour tolerance Citation[15]. Moreover, exosomes present in tumour ascites can contain tumour cells, DCs, B lymphocytes, and T lymphocytes Citation[16]. Correspondingly, tumour ascites are associated with a strong inflammatory response involving both cellular and humoral immunity. Therefore, exosomes secreted from tumour ascites contained many proteins which were secreted by tumour cells, DCs, and lymphocytes. For example, exosomes from tumour cells have been found to contain MHC class I molecules and HSPs, both of which have roles in antigen presentation Citation[2]. Similarly, exosomes secreted by DCs and B cells contain high levels of MHC class II and CD86 proteins, which are involved in antigen presentation and the stimulation of T cells Citation[17–20]. Thus, exosomes from malignant ascites represent an efficient tool for antigen delivery and presentation.
Tumour-derived exosomes contain tumour rejection antigens and have the capacity to induce T cell dependent immunity. However, immunogenicity associated with tumour-derived exosomes has been shown to be poor Citation[21]. However, we and other laboratories have found that the in vivo anti-tumour immune responses induced by exosomes are enhanced when tumour-derived exosomes are prepared from heat- stressed tumour cells, gene modified (i.e. IL-2, IL-18) tumour cells Citation[22], Citation[23], or when superantigens are anchored to the surface of exosomes Citation[24]. In a Phase I clinical trial using exosomes derived from ascites of patients with colorectal cancer, exosomes plus GM-CSF were shown to induce a tumour-specific cytotoxic T cell response Citation[4]. Correspondingly, in the current study, exosomes derived from heat-stressed malignant ascites of gastric cancer patients were shown to promote DC maturation and induce a tumour-specific CTL response in vitro. Based on the assays performed, these processes are associated with higher levels of Hsp70 and Hsp60 present in exosomes compared with exosomes prepared from ascites of gastric cancer patients that were not treated with heat.
As molecular chaperones and potent adjuvants, HSPs have important roles in the cross-presentation of tumour-derived antigenic peptides. For example, HSPs can deliver antigenic peptides to MHC class I molecules of DCs and stimulate DCs to secrete pro-inflammatory cytokines, thus creating an immunogenic environment that is required for the induction of CD8 + T cell responses Citation[25–29]. Correspondingly, it is noteworthy that DCs pulsed with HS-exosomes produce higher levels of IL-12p70 and TNF-α, which are two important cytokines involved in the stimulation of an inflammatory reaction. In another study, the release of Hsp70 from heat-shocked tumours was shown to initiate an effective anti-tumour immune response via the chemoattraction and activation of DCs Citation[6], while expression of Hsp70 on heat-stressed DCs was shown to promote the proliferation of CD4+/CD45/RA− memory T cells Citation[30], Citation[31]. These results, in combination with the data from the current study, suggest that heat-induced increases in Hsp70 and Hsp60 in tumour-derived exosomes play a vital role in inducing a CTL immune response in vitro, which involves the promotion of DC maturation.
As illustrated by , the variability between patient samples regarding exosome function is low. This minimal variability could be attributed to the fact that the 18 patients included in the study had similar baseline characteristics, as summarised in .
There are many reports that describe the spontaneous remission of cancer patients following an infection accompanied by a fever. In fact, hyperthermia has also been shown to improve treatment effects in combination with chemotherapy or radiotherapy in the clinic Citation[32], Citation[33]. Therefore, the capacity for heat-treated exosomes to facilitate the elimination of tumour cells is consistent with these clinical data. In the current study, an improved immune response was associated with heat-treated exosomes derived from malignant ascites. Therefore, heat treatment may represent an important consideration in optimising the effectiveness of immunotherapy treatments received by patients with advanced gastric cancer.
Declaration of interest: This work was supported by grants from the Medical Scientific Research Foundation of Zhejiang Province, China (2008 B023). Haijun Zhong and Yunshan Yang contributed equally to this work. The authors alone are responsible for the content and writing of the paper.
References
- Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7: 297–303
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360: 295–305
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 2008; 16: 782–790
- Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunol Immunother 2006; 55: 312–319
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol 2009; 182: 1449–1459
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci 2005; 118: 3631–3638
- Haijun Zhong, yunshan Yang, Shenlin Ma, Weimin Mao, Yiping Zhang, Fangming Xiu, Zhijian Cai, Weilin Chen, Qingqing Wang. Enhanced antitumor effects of exosomes derived from heat-shocked E.G7-OVA tumor cells. Chin J Microbiol Immunol 2010; 30: 164–168
- Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 2006; 36: 1598–1607
- Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res 2005; 11: 7554–7563
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 2011; 186: 2219–2228
- Xiu F, Yang Y, Cai Z, Wang J, Cao X. Isolation and characterization of exosomes derived from tumor cells genetically expressing model antigen. Chin J Microbiol Immunol 2004; 2: 278–285
- Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol 2001; 166: 5407–5415
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009; 374: 477–490
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009; 124: 2621–2633
- Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol 2004; 31: 114–121
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat Med 1998; 4: 594–600
- Théry C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 2002; 3: 1156–1162
- Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: What's next?. Cancer Res 2010; 70: 1281–1285
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183: 1161–1172
- Xiu F, Cao X. Exosomes in the immune response and tolerance. Chin J Microbiol Immunol 2004; 2: 231–236
- Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, Cao X. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol 2007; 133: 389–399
- Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med 2006; 84: 1067–1076
- Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med 2007; 85: 511–521
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002; 20: 395–425
- Udono H, Ichiyanagi T, Mizukami S, Imai T. Heat shock proteins in antigen trafficking – Implications on antigen presentation to T cells. Int J Hyperthermia 2009; 25: 617–625
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int J Hyperthermia 2009; 25: 610–616
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol 2002; 169: 5424–5432
- Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 2007; 282: 31688–31702
- Chen T, Cao X. Stress for maintaining memory: Hsp70 as mobile messenger for innate immunity and adaptive immunity. Eur J Immunol 2010; 40: 1541–1544
- Wang Y, Seidl T, Whittall T, Babaahmady K, Lehner T. Stress-activated dendritic cells interact with CD4(+) T cells to elicit homeostatic memory. Eur J Immunol 2010; 40: 1628–1638
- Schlemmer M, Wendtner CM, Lindner L, Abdel-Rahman S, Hiddemann W, Issels RD. Thermochemotherapy in patients with extremity high-risk soft tissue sarcomas (HR-STS). Int J Hyperthermia 2010; 26: 127–135
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia 2011; 27: 180–186