Abstract
Purpose: The purpose of this study was to quantify hypoxia changes in viable tumour volumes after thermal ablation of a murine breast carcinoma.
Methods: Murine breast 4T1 tumours were grown in the rear leg of BALB/c mice to an average diameter of 10–12 mm. Tumours were treated with conductive interstitial thermal therapy (CITT) at a peak temperature of 80–90°C for 10 min. The animals were euthanised 72 h later, and the tumours were removed for immunohistochemical staining with pimonidazole – a marker of partial pressure of oxygen. The levels of pimonidazole staining intensity were used to quantify changes in hypoxia gradients in terms of strong, medium and weak positive pixel fractions.
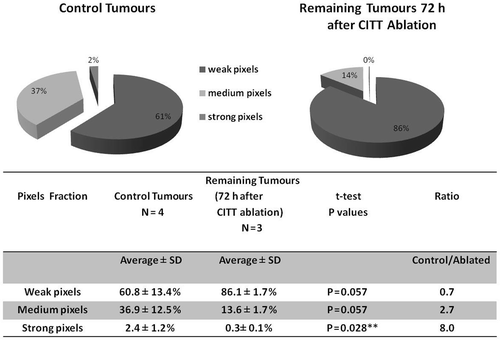
Results: The pimonidazole staining ratio of viable control tumour tissue to viable tissue in tumours that were ablated was 0.7 for weak staining, 2.7 for medium staining and 8.0 (p < 0.03) for strong pimonidazole staining.
Conclusion: This shift of pimonidazole staining toward lower intensity pixels in the remaining tumour indicates that tumour ablation with CITT may increase radiosensitivity of the remaining tumour tissue and presents a rationale for combination therapy.
Introduction
Conventional hyperthermia (40–45°C) has been shown to reoxygenate hypoxic tumour tissue in animal models, spontaneous tumours in dogs as well as in human tumours Citation[1–4]. Clinical data demonstrated that the addition of hyperthermia to radiotherapy improves treatment response of solid tumours Citation[5–8]. In animal models of solid tumours it was previously observed that thermal therapies in the range of 40–43°C increase sensitivity of tumour tissue to ionising radiation via an increase in oxygenation Citation[9–12]. Tumour ablation therapies utilising temperatures >45° are becoming widely used for the treatment of solid tumours. A variety of heating techniques including radiofrequency Citation[13–14], high-intensity focused ultrasound Citation[15–16], laser Citation[17] and interstitial conductive probes Citation[18–19] are being utilised to thermally ablate tumours. In ablative temperatures cells undergo necrotic death. The post-ablative necrotic tumour area is surrounded by cells exposed briefly to a gradient of hyperthermic temperatures. Very little is known about how this type of exposure to hyperthermia (originating from tumour ablation) may affect cells in the tumour periphery. In the current study we evaluated the effect of thermal ablation on hypoxia in tumour tissue remaining viable outside of the ablation zone. Oxygen partial pressure (pO2) may vary in a tumour from 0.5 mmHg to 20 mmHg or above Citation[20], Citation[21]. The lowest values of pO2 correspond to the most radiation resistant fractions in tumour and the highest values – to the most radiosensitive fractions. We used our conductive interstitial thermal therapy (CITT) device to ablate a portion of locally advanced 4T1 murine breast carcinomas. In the process of ablation, the remaining tumour is exposed to a gradient of conventional, non-ablative, hyperthermia temperatures. The changes in hypoxia due to CITT induced hyperthermia were quantified with pimonidazole staining, 72 h after ablation.
Material and methods
Mice and tumour cells
BALB/c female mice were obtained from Jackson Laboratories (Bar Harbor, ME). They were maintained in our institutional animal facility where temperature and light were controlled. Food and water were provided ad libitum. The experiments were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. We purchased 4T1 tumour cells from the American Type Culture Collection (ATCC), number CRL-2539 (Rockville, MD). Cells were grown to 80% confluency in T75 flasks in RPMI 1640 medium (Mamassas, VA) supplemented with 10% foetal bovine serum (Tissue Culture Biologicals, Tulare, CA) and antibiotics: 100 U/mL penicillin and 100 ug/mL streptomycin (Thermo Scientific, HyClone, Logan, UT). Trypsin, mix 1:1 of 0.25% and 0.05% in 1 mM EDTA (Mediatech, Manassas, VA), was used for cell harvesting. Cells were washed and diluted in serum-free media to a density of 4 × 106 cells/mL. Thereafter, 2 × 105 cells in 50 µL of serum-free media were injected subcutaneously into the right rear leg of each mouse. Tumour growth was monitored by measuring length and width with a caliper and tumour volumes were calculated according to the formula: volume (mm3) = 0.5(length × width2). Five tumours were ablated when they reached an average size of diameter 10–12 mm. Anaesthesia with 1–2% isofluorane was applied during all animal treatments. Four tumours were used as untreated controls.
Tumour ablation
CITT was applied as described Citation[18], Citation[19] with the following modifications. Previously, the CITT device deployed pins to increase the conductance and spread of the thermal ablation lesion in pig and rabbit models. For application in mice, the CITT device and method were modified by the use of an internally placed laser fibre to heat the tip of the metal probe. Briefly, a customised thermal ablation probe (hollow, closed end needle) of 1.5 mm in outside diameter was constructed. The miniature probe was used to avoid skin damage during intra-tumour ablation. The probe was made from stainless steel and silicon rubber insulation was applied to the sheath to protect skin and overlying tissue from thermal damage. Heating was applied by inserting a 600-µ laser fibre inside the lumen to heat the end of the probe. The 4T1 tumour grown subcutaneously in the rear leg had approximately an ellipsoid shape with the longer axis aligned parallel to the femur. The probe was inserted to the approximate centre of the solid tumour. Two thermocouples were used to measure temperatures. One was adhered to the probe so that the thermocouple tip was within 1–2 mm of the probe tip (T1, ). The other was placed on the distal side of the tumour, approximately 5–7 mm from the probe tip when inserted to the approximate midline of the tumour (T2, ).
Figure 1. Conductive interstitial thermal therapy (CITT) of 4T1 murine tumor: ablation characteristics and tumour damage. (A) CITT treatment and tumour sample preparation. The CITT ablation probe (P) was inserted perpendicularly to the skin surface. Two thermocouples, T1 and T2. measured temperatures in the centre of the ablation and on the surface of the tumour, respectively. The dotted line designates the plane of the tumour cut after necropsy. One half of the tumour was used for TTC staining and another half was used to prepare tumour sections for hypoxia analysis.(B) Typical temperature profiles obtained during 4T1 murine breast tumour ablation with CITT probe. Temperature recordings were done with a thermocouple in two spatial points: centre (red) and peripheral (green) edge of each tumour (T1 and T2 on ). (C) Typical ablation zone determined with 2, 3, 5-triphenyltetrazolium chloride (TTC). Mice were sacrificed and tumours were isolated and bisected 72 h after CITT ablation. The cross sectional area of the tumour was stained with TTC and photographed. Viable areas of tumour stain red and necrotic areas remain white.
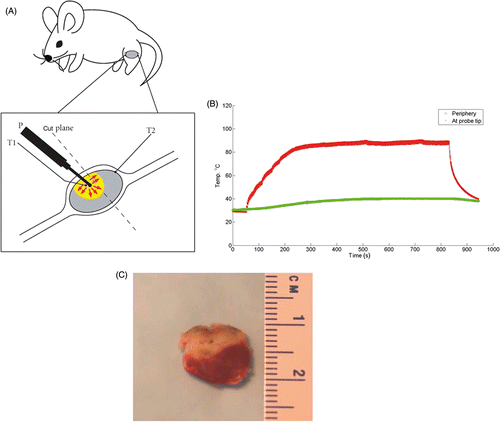
The tumours were ablated with a tip, T1, temperature ranging from 80–90°C. When the temperature reached 80°C inside the tumour the ablation was continued for 10 min. In general, the ablated tumour volume was closer to the skin surface, and the remaining viable tumour was more distant from the skin. After necropsy, the tumour was cut in half perpendicularly to the skin surface and along the track from the probe insertion ().
Triphenyltetrazolium chloride viability staining
Triphenyltetrazolium chloride (TTC) staining is commonly used to visualise viable tissue. In viable tissue, TTC is reduced intracellularly by the mitochondrial enzyme succinate dehydrogenase to formazan, a water-insoluble red compound that remains inside the cell. This reaction does not occur in non-viable tissue, which remains unstained (white to tan) Citation[22]. The TTC (Fisher Scientific, Fair Lawn, NJ) was dissolved in sterile PBS. Tumour sections were incubated in 2% TTC solution for 30 min at 37°C.
Immunohistochemistry
Pimonidazole hydrochloride was used as a hypoxia marker. Pimonidazole is activated in hypoxic cells to form stable adducts with thiol groups. The pimonidazole probe was injected intraperitoneally (i.p.) at a dosage of 60 mg/kg body weight to all of the animals 60 min before the animals were euthanised. The tumours were removed, fixed in 10% neutral buffered formalin (NBF), embedded in paraffin and sectioned. Five-µm sections were immunostained with Hypoxyprobe-1 kit according to manufacturer recommendations and as described Citation[23]. In brief, peroxidase quench with 3% H2O2 was followed by antigen retrieval at 90°C for 20 min in antigen unmasking fluid (ABD Serotec, Raleigh, NC), blocking with Dako blocking solution (Carpinteria, CA) for 10 min, application of primary antibody 1:50 (HPI, Burlington, MA) for 60 min followed by secondary antibody 1:100 (HPI, Burlington, MA), for 20 min, diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA) for 10 min and counterstained with Gill's haematoxylin for 30 s. In a negative control sample, antibody diluent (Millipore, Billerica, MA) was used in place of primary antibody. Tissue imaging was performed using an Aperio ScanScope (Vista, CA) at 20% magnification and analysed using ImageScope software (Aperio). Necrotic areas, tissue folds and borders were excluded from analyses and remaining viable tissue was analysed for positive DAB signals using a colour deconvolution algorithm (Aperio). The algorithm allows the categorising of each positive pixel as strong, medium and weak. The output of the program contains the following characteristics of pixels: percentage weak positive, percentage medium positive, percentage strong positive and percentage negative. The summary of pixel intensity data includes average values of weak, medium and strong pimonidazole pixels as percentages of total positive pixels.
Statistical analyses
To test differences in strong, medium and weak pixel fractions between control and ablated groups a t-test was applied using SigmaPlot 11.2 (Systat, San Jose, CA).
Results
Characteristics of CITT ablation
The representative temperature profiles, measured with a thermocouple placed in the centre (red) and edge (green) of the tumour are shown in . It took 250 s to heat the tumour to maximum temperature, and then the temperature of the probe was held constant for 550 s followed by temperature drop for 150 s to the level measured on the periphery. Thus, a thermal dose that far exceeds the critical threshold for cell death, generally regarded as 240 equivalent min at 43°C, was delivered to the central portion of the tumour Citation[24]. The TTC staining visualises the extent of tissue death caused by the ablation ().
Decrease of strong pimonidazole signals and changes in staining pattern in remaining tumour after ablation
To test the effect of ablation on hypoxia in the remaining tumour volume, staining for pimonidazole hydrochloride (Hypoxyprobe-1) was performed on sections prepared from 4T1 breast carcinoma after thermal ablation with CITT. Our previous experience with ablated tumours has indicated that 72 h after ablation is an adequate time point to assess the final pathological outcomes of thermal ablation Citation[19]. Therefore, we harvested all tumours in the current study at 72 h post ablation. We observed that the distribution of pimonidazole staining underwent distinct changes (). In samples of ablated tumours the strongest pimonidazole signals were mainly observed in the very thin margin, the transition zone (TZ), between the ablation zone and the remaining viable tumour. There was also a noticeable decrease in intensity of the staining pattern in the remaining viable tumour tissue (). In control tumours hypoxia was distributed throughout the entire tumour area (brown) showing globular pattern typical for hypoxic breast tumour (). A colour deconvolution algorithm was applied to pimonidazole signals and hypoxia staining was pseudocoloured as red (strong pixels), orange (medium pixels) and yellow (weak pixels); pimonidazole negative pixels are marked blue (). An example of negative control where no primary antibody was added () shows that background staining is negligible. and C show examples of pimonidazole staining intensity distributions in control tumours. In tumour tissue remaining after ablation (–F) there was a decrease in strong (red) and orange (medium) pixels, whereas in control tumours (), there was more strong and medium staining observed. Weakly stained pixels (yellow) showed an opposite relationship – there was a trend of more yellow (weak) staining in tumour remaining after ablation than in control tumours. The pixel fractions calculation with colour deconvolution algorithm is summarised in . The average percentage of weak pixels was lower in the control group than in the ablated group (60.8% versus 86.1%; ratio = 0.7). The average percentages of medium and strong pixels showed an opposite trend. There were 2.7 times more medium pixels (36.9% versus 13.6%) and 8 times more strong pixels (2.4% versus 0.3%; P = 0.028) in the control group than in the ablated group.
Figure 2. Patterns of pimonidazole staining in an ablated 4T1 tumour (A) and untreated tumour (B). Pimonidazole was injected 1 h before necropsy and detected by immunohistochemistry using a primary antibody to pimonidazole adducts formed and detected by DAB substrate (brown). ‘Ablation zone’ denotes non-viable area of tumour 72 h post ablation. ‘TZ’ denotes transition zone observed as the intense staining of the margin between the ablated zone and remaining tumour. ‘Necrotic zone’ denotes the natural tumour necrotic zone in control tumours.
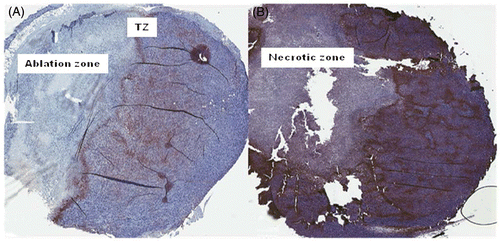
Figure 3. Pseudocolour images of representative tumours showing three levels of pimonidazole staining. Pimonidazole stained sections were scanned and analysed using an Aperio ImageScope. A colour deconvolution algorithm converted brown pimonidazole staining into red, orange and yellow pseudocolours corresponding to high, medium and weak intensities of pixels. Blue colour denotes lack of positive pixels. The negative control (without primary antibody) is mostly blue (A). Examples of control tumours are shown in panels B and C. Examples of tumour remaining after ablation are shown in panels D, E and F.
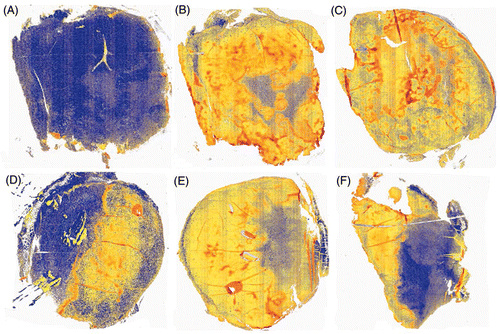
Figure 4. Quantification of pimonidazole staining: percentage of weak, medium and strong pimonidazole signal in control tumours and tumours remaining after ablation. Tumour sections stained for pimonidazole were scanned and the percentages of red, orange and yellow pixels corresponding to weak, medium and strong pimonidazole intensity staining were calculated (pie charts).
Discussion
This study is the first, to our knowledge, to demonstrate a significant decrease of severe hypoxia within viable tumour tissue that survives after thermal ablation. Importantly, this may provide a way to sensitise residual tumour with radiation or drugs leading to an improvement in the recurrence rates that are observed after many ablative approaches. Hypoxia varies within and between different tumours and a stratification of pimonidazole staining performed in this study was aimed at detecting changes in hypoxia distribution within tumours. Tumours with high levels of hypoxia have been associated with high levels of angiogenesis and metastases in some studies Citation[25], Citation[26]. Clonogenic tumour cells that exist in severe hypoxia (below 5 mmHg) are 2–3-fold more resistant to radiation-induced cell death than cells at pO2 above 10 mmHg Citation[27]. Since hyperthermia has been found to alleviate tumour hypoxia it can be used as an adjuvant therapy to reoxygenate, and consequently to radiosensitise tumours Citation[28]. In thermal ablation, due to dissipation of thermal energy, the central ablated zone of the tumour is surrounded by cells exposed to a gradient of temperatures. We sought to investigate whether thermal ablation affects hypoxia in remaining tumour due to the possible direct effect of hyperthermic exposure of the tumour periphery and the expected inflammatory response generated by ablation. We used a murine model of breast carcinoma (4T1) to define changes in tumour hypoxia in remaining tumour 72 h after thermal ablation using conductive heating method.
We have found that strong pimonidazole staining decreased significantly outside of the ablation zone. Therefore, we interpret this to mean that the area outside the ablated tumour underwent a redistribution of partial oxygen pressure towards the higher values (lower hypoxia) on the scale from 0 to 10 mmHg as the tumour responded to the ablation and associated thermal history. Comparison of temperatures in the centre and the periphery of tumour during ablation indicate that the non-ablated area was exposed to a marked temperature gradient (∼from 90–40°C across nearly 10 mm of tissue in some cases). Generation of temperature gradients is typically observed when a focused thermal energy is applied to the centre of a tumour Citation[18]. Our finding that a tumour may become less hypoxic in the non-lethal portion of the temperature gradient up to three days after ablation suggests that combination of the CITT ablation therapy with radiation therapy may further improve solid tumour treatment and possibly reduce the recurrence rates that hamper either modality alone. Our finding is also important for further dissecting the alterations in tumour microenvironment induced by thermal ablation methods. The elimination of the most hypoxic tumour areas may change levels of heat shock proteins, cytokines and angiogenic factors which play important role in supporting surviving cells to eventually result in tumour recurrence. In our previous work we demonstrated that hyperthermia may cause vascular thermotolerance, permeability increases and improved blood flow Citation[9] and these phenomena may contribute to the observed re-oxygenation in the temperature gradient generated by ablation of 4T1 tumours.
On the other hand, we also observed that the narrow transition zone (TZ), between ablated and remaining tumour areas had intense pimonidazole staining indicating high micro-regional levels of hypoxia in this zone (). This is typical of what we know to occur at the TZ of necrotic tissue. We included these regions in our analyses which actually decreased the overall difference between control and ablated tumours in strong hypoxia staining. However, a significant difference was still found in strong pixel intensity between the two groups. This acute area (TZ) is a potential site of initiation of tumour re-growth. Indeed, the accelerated perinecrotic outgrowth of colorectal liver metastases was observed after radiofrequency ablation (RFA) Citation[27]. Interestingly, in that study it was revealed that hypoxia inducible factor (HIF-1α) staining was very intense in the transition zone and that tumour recurrence is associated with tumour microvascular abnormalities but not with hypoxia-driven angiogenesis. The narrow hypoxic zone between ablated and remaining tumour may mediate recruitment of progenitor cells supporting tumour re-growth and also may be a site of increased immune cell activity. Molecular analyses of this zone, as well as analyses of systemic changes in blood and bone marrow are warranted to help answer these questions.
In addition, it should be remembered that the ablation zone itself is not necessarily 100% non-viable. There can be some small percentage of tumour cells or stroma that remain alive and could fuel the re-growth of the tumour. This was suggested by a recent study showing that vascularity began to emerge within the necrotic zone after ablation using RFA Citation[29]. We have observed that vessels within the CITT ablation zone have increased expression of damage and repair markers such as von Willebrand factor (data not shown) suggesting that they did not die immediately after ablation. Therefore, we are currently working to understand the importance of biological events occurring in the ablation zone, transition zone and viable remaining tumour. Finally, our approach to analyse heterogeneity of pimonidazole staining may be applied to define more precisely the overall tumour hypoxia by considering different levels of hypoxia within the tumour area.
Conclusion
In conclusion, the decrease of the most hypoxic areas in remaining tumour observed here suggests that thermal ablation may improve radiosensitivity and ultimately local regional control of solid tumours upon combination therapy approaches. Micro-regional acute hypoxia changes after ablation may be linked to aspects of local recurrence. The possibility of completing tumour eradication with a targeted radiation treatment using a smaller or custom intensity modulated doses according to the size of the ablation zone may be another advantage of combining ablation and radiation therapy.
Declaration of interest: This work was supported, in part, by a grant from the Fashion Footwear Association of New York (FFANY/QVC) to G.S. and R.J.G. and by National Institutes of Health grant number CA44114 (RJG). The authors alone are responsible for the content and writing of the paper.
References
- Nishimura Y, Urano M. The effect of hyperthermia on reoxygenation during the fractionated radiotherapy of two murine tumors, FSa-II and MCa. Int J Radiat Oncol Biol Phys 1994; 29: 141–148
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, Samulski TV, Prosnitz LR, Dewhirst MW. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996; 56: 5347–5350
- Vujaskovic Z, Rosen EL, Blackwell KL, Jones EL, Brizel DM, Prosnitz LR, Samulski TV, Dewhirst MW. Ultrasound guided pO2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int J Hyperthermia 2003; 19: 498–506
- Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM, Vujaskovic Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 2004; 10: 4287–4293
- Zagar TM, Higgins KA, Miles EF, Vujaskovic Z, Dewhirst MW, Clough RW, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomized data. Int J Hyperthermia 2010; 26: 612–617
- Huilgol NG, Gupta S, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia 2010; 26: 21–25
- Kang MK, Kim MS, Kim JH. Clinical outcome of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia 2011; 27: 482–490
- Griffin RJ, Dings RP, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia 2011; 26: 256–263
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 2009; 25: 91–95
- Shakil A, Ogawa A, Griffin RJ, Song CW. Reduction of tumour blood flow with KB-R8498 potentiates the response of tumours to hyperthermia. Int J Hyperthermia 1999; 15: 1–6
- Griffin RJ, Okajima K, Barrios B, Song CW. Mild temperature hyperthermia combined with carbogen breathing increases tumor partial pressure of oxygen (pO2) and radiosensitivity. Cancer Res 1996; 56: 5590–5593
- Izzo F, Barnett CC, Jr, Curley SA. Radiofrequency ablation of primary and metastatic malignant liver tumors. Adv Surg, 35: 225–250
- Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, Golberg SN. Radiofrequency ablation: Effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. JVIR 2004; 15: 269–274
- Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. Am J Roentgenol 2010; 195: W245–252
- Crouzet S, Murat FJ, Pasticier G, Cassier P, Chapelon JY, Gelet A, et al. High intensity focused ultrasound (HIFU) for prostate cancer: Current clinical status, outcomes and future perspectives. Int J Hyperthermia 2010; 26: 796–803
- Elliott AM, Shetty AM, Wang J, Hazie JD, Stafford JR. Use of gold nanoshells to constrain and enhance laser thermal therapy of metastatic liver tumors. Int J Hyperthermia 2010; 26: 434–440
- Shafirstein G, Hennings L, Kaufmann Y, Novak P, Moros EG, Ferguson S, Siegel E, Klimberg SV, Waner M, Spring P. Conductive interstitial thermal therapy device for surgical margin ablation: In vivo verification of a theoretical model. Int J Hyperthermia 2007; 23: 477–492
- Shafirstein G, Kaufmann J, Hennings L, Siegel E, Griffin RJ, Novak P, Ferguson S, Moros EG. Conductive interstitial thermal therapy (CITT) inhibits recurrence and metastasis in rabbit VX2 carcinoma model. Int J Hyperthermia 2009; 25: 446–454
- Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the ‘hypoxic fraction’ in determining tumor response to fractionated radiotherapy. Radiat Res 1997; 147: 541–550
- Koch CJ, Oprysko PR, Shuman AL, Jenkins WT, Branddt G, Evans SM. Radiosensitization of hypoxic tumor cells by dodecafluoropentanene: A gas-phase perfluorochemical emulsion. Cancer Res 2002; 62: 3626–3629
- Lippold HJ. Quantitative succinic dehydrogenases histochemistry. A comparison of different tetrazolium salts. Histochem 1982; 76: 381–405
- Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, et al. Comparison between pimonidazole binding, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix. Cytometry B, Clinical Cytometry 2006; 70: 45–55
- Saparato SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol 1984; 10: 787–800
- Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 2007; 26: 319–331
- Zou Y, Cheng C, Omura-Minamisawa M, Kang Y, Hara T, Guan X, Inoue T. The suppression of hypoxia-inducible factor and vascular endothelial growth factor by siRNA does not affect the radiation sensitivity of multicellular tumor spheroids. J Radiat Res 2010; 51: 47–55
- Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006; 82: 699–757
- Sun X, Xing L, Ling CC, Li GC. The effect of mild temperature hyperthermia on tumor hypoxia and blood perfusion: Relevance for radiotherapy, vascular targeting and imaging. Int J Hyperthermia 2010; 26: 224–231
- Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg 2009; 249: 814–823