Abstract
Purpose: To calculate a modified heat capacity (mHC) of small hepatocellular carcinomas (HCCs) in vivo during radio frequency ablation (RFA) and to determine if mHC correlates with tumour vascularity, adjacent vessels or local recurrence.
Patients and methods: This study was IRB approved and informed consent was obtained from all patients. Before formal RFA, ambient HCC temperature and temperature 1 min after heating at constant wattage were measured in 29 patients. From temperature change and wattage, individual mHCs (joules required to increase tumour temperature by 1° Celsius) were calculated. Pre‐RFA, three‐phase computerised tomography (CT) scans were reviewed blindly for hepatic arteries, hepatic veins and portal veins abutting or within 3 mm of tumour edge from which twelve vascular parameters were quantified. Tumour enhancement (homogeneous or heterogeneous on arterial phase) was also assessed. Multiple regression was used to correlate mHC with vascular parameters and tumour enhancement. Cox proportional hazard model was used to examine the relationship of mHC to local recurrence.
Results: There was significant correlation of mHC with lesion enhancement (P = 0.0018), length of hepatic arteries (P < 0.0001) and total hepatic vein volume in contact with tumour (P = 0.016). No correlation was found with any non‐abutting vessel or portal vein parameter. The chance of local recurrence increased with increasing mHC.
Conclusion: Because the modified heat capacity of small HCCs in our study population correlated with HCC enhancement, abutting hepatic arteries, the volume of abutting hepatic veins and local recurrence, it may be an indicator of the heat sink effect (HSE) and supports the HSE as a risk factor for local recurrence.
Introduction
Radio frequency ablation (RFA) has become well established in the treatment of unresectable hepatocellular carcinoma (HCC) and in bridging patients with HCC prior to transplantation. The current intrinsic limitations of RFA are well known and include tissue impedance which inhibits energy deposition into tumour Citation[1], a relatively small zone of active heating Citation[2], and tumour size. Additionally, vessels adjacent to tumour can conduct heat and are believed to limit the ability of RFA to cause complete tumour ablation Citation[3] and to achieve an adequate ablative margin Citation[4]. This heat sink effect (HSE) as formally defined by Goldberg et al. Citation[5] is tissue cooling by adjacent vessels above 1 mm in diameter causing a thermal lesion whose shape results from deflection away from these vessels.
Attempts to quantify the HSE during hepatic RFA have been made by multiple authors. Patterson et al. Citation[6] found the number of central blood vessels on histological examination predicted minimum ablation diameter and volume of RFA lesions created in normal domestic swine liver in vivo. In their work, however, the separate effects of different vessel types (hepatic artery, portal and hepatic veins) were not discerned. Lu et al. Citation[7] also performed RFA on normal pig liver and on histology, found invagination of normal liver between an RFA lesion and corresponding vessels when vessel diameter exceeded 3 mm. The heat sink effect in HCC however, may not be well‐represented from applying RFA to normal liver in these animal models. Such models neglect the relative hypervascularity of HCC compared with adjacent liver Citation[8], and the often altered surrounding hepatic parenchyma resulting from underlying liver disease. Additionally, a histological approach to quantification of the HSE is not readily applicable in the clinical setting as no tissue is usually available for histological analysis immediately after RFA of HCC.
The perceived importance of the HSE is that it theoretically places a patient at risk for local tumour recurrence. Kim et al. Citation[9] evaluated the effect of HCC recurrence when tumour was in contact with the inferior vena cava or portal or hepatic veins, and identified local tumour recurrence more frequently when lesions were in contact with vessels above 3 mm. In this work, the extent of vessel contact with tumour was determined by contrast enhanced CT performed within 24 h after RFA. An additional retrospective clinical study Citation[10] lends credence to the HSE, again identifying abutting vessels above 3 mm as a risk factor for recurrence.
A starting point to quantitatively examine vessel proximity to tumour and thus the heat sink effect, is to assess a tumour's overall ability to tolerate heating, i.e. to determine the amount of energy required to increase a tumour's temperature. Heat capacity of tissue is formally defined as the amount of energy required to increase the tissue by 1°C per gram of tissue. A modified definition of heat capacity, the amount of energy required to increase the temperature of tissue directly adjacent to an RFA electrode, can be assessed immediately prior to RFA by employing the RFA electrode's thermocouple. Local temperatures obtained within tumour have been used by other investigators as an indicator for the extent of overall tumour heating Citation[11]. Thus, this modified heat capacity (mHC) could potentially be a general indicator of a tumour's ability to tolerate thermal energy which logically depends on a multitude of factors including intrinsic tumour vascularity and any heat sink effects from adjacent vessels. Thus, we sought to calculate the mHC of small (<3 cm in diameter) HCCs in patients undergoing RFA and to determine whether these values correlate with tumour vascularity, vessels adjacent to or abutting tumour as identified on pre‐RFA, contrast enhanced CT scans, and local recurrence after RFA.
Patients and methods
Study population
This study was IRB approved, and informed consent was obtained from all patients. Patients were identified by a multidisciplinary team consisting of transplant surgeons, hepatologists and interventional radiologists with RFA used primarily on tumours below 3 cm to bridge to liver transplantation. Study entrance also required pathological confirmation of HCC, meeting standard criteria for RFA ablation Citation[12] and lack of prior ablation (radio frequency, microwave, and ethanol) or chemoembolisation, either of which may alter peri‐tumoural vascularity. From October 2008 to October 2010, 29 consecutive patients with 34 HCCs met these criteria. There were 25 men and four women, median age 58.2 years (range 29–84). Patient demographics are shown in . Of the 29 study patients, 25 had documented cirrhosis (12 Child Class A, 13 Child Class B). Fifteen had HCV, nine had HBV, one had alcoholic cirrhosis, one had both HBV and HCV, and three had non‐alcoholic steatohepatitis (NASH).
Table I. Patient demographics (N = 29).
Radio frequency ablation and calculation of mHC:
Radio frequency ablation was performed using a single, internally‐cooled (Cool‐tip®, Valleylab, Covidien, Boulder, CO) radio frequency electrode with a 3‐cm exposed tip, a 200 W generator (Covidien) and carried out by one of two interventional radiologists with a minimum of six years of experience in RFA. To avoid any cluster effects, when a patient had more than one lesion, only one lesion was included in our analysis, this chosen by the interventional radiologist as the first lesion to undergo ablation and based on ease of accessibility. Electrode placement through the tumour epicentre was carried out under CT guidance with adjuvant grey‐scale and colour Doppler ultrasound as needed. The tapered portion of the electrode's tip was placed just beyond the tumour's edge to standardise the position of the electrode's thermocouple within and just proximal to the distal margin of the tumour. After desired electrode placement was confirmed by CT, initial baseline tumour temperature was measured prior to iced saline infusion. The RFA electrode was then used for tumour heating with power maintained constant for each patient for 1 min, after which the generator was turned off and maximum tumour temperature recorded with the electrode's thermocouple. Electrode tip infusion with cold saline, though used, was stopped at approximately 40 s (20 s before the generator) to exclude any effects of the saline on final maximum tumour temperature. Constant power range varied between patients but was between 130 and 150 W in all cases. Knowing ablation time of 1 min, constant generator power and initial and final temperatures as indicated by the RFA electrode, the number of joules required to increase the adjacent tumour temperature by 1°C could be calculated, this value termed the mHC with dimensions of J/°C. Simply put, wattage is joules per second, generator time was 60 s and temperature rise from baseline are all known variables. Multiplying wattage by 60 gives the total number of joules delivered to the tumour adjacent to the probe with the resulting temperature caused by this energy deposition being known. Following the above, all patients underwent standard RFA for 12 min using a pulsed RFA technique Citation[13] as recommended by the manufacturer. In tumours exceeding 2 cm in diameter, overlapping ablations were routinely performed. Immediate post‐ablation probe thermocouple temperature was measured to ensure tumouricidal temperatures over 60°C. An immediate triple‐phase scan was performed employing the same protocol as for post‐ablation follow‐up (see CT protocol below) to assess for any persistent tumour and determine the need for electrode repositioning and additional ablation. Ablation was considered complete when a coagulation zone encompassed the entire tumour with an ablation margin of at least 5 mm.
Imaging assessment of peri‐tumoural hepatic vasculature
Pre‐RFA contrast enhanced CT images of all tumours were reviewed by two abdominal radiologists in consensus with a minimum of five years experience, uninvolved in the actual ablation procedures and using an image archival system (Centricity, General Electric, Milwaukee, WI). Images were assessed blinded to patient information and knowledge of mHC values and at a minimum of 4 weeks after mHC calculation (one abdominal radiologist performed the calculations). Our hepatic multi‐detector CT protocol consisted of injection of 100 mL of non‐ionic contrast (Optiray 350, Mallinckrodt, Hazelwood, MO) via an antecubital vein at 5 mL/s during which bolus tracking was used to acquire an arterial phase CT (triggered at 200 HU). This was followed by a portal venous phase 45 s later. CT parameters included a pitch of 0.984, table increment of 2.5 mm, 40.00 mm/rotation and at 120 kVp with milliampere-second (mAs) tailored using autosmart software. All CT imaging was performed on a 64 detector spiral scanner (Discovery, General Electric) with maximum pixel size of 0.5–0.7 mm. Axial and multi‐planar reformatted images were assessed by consensus for hepatic and portal veins and hepatic arteries above 1 mm in diameter and within 3 mm of the tumour's edge as well as vessels abutting tumour. When abutting tumour, vessel type (artery, hepatic vein, or portal vein), diameter and the length (arc) of contact with tumour were independently measured three times by each reviewer (total of six) and the average used. Tumour dimensions were also measured in a similar fashion and their average documented.
Because HCC enhancement is known to correlate with perfusion and overall vascularity Citation[14], Citation[15], tumours were defined as homogeneous in enhancement if they showed uniform enhancement exceeding that of adjacent liver during the arterial phase or heterogeneous if they had mixed areas of hyper‐, iso‐ and/or hypodensity relative to adjacent liver during the arterial phase.
Vascular parameters correlated to mHC
Quantification of adjacent tumour vascularity, and in theory the heat sink effect, was carried out in the following manner. For each type of vessel less than or equal to 3 mm away from and not abutting tumour, vessel diameters were summated to yield the total diameter of all hepatic arteries (HATD ≤ 3 mm), hepatic veins (HVTD ≤ 3 mm) and portal veins (PVTD ≤ 3 mm). For vessels in contact with tumour, length (arc) of contact was summated for each type of vessel to yield total length (arc) of hepatic artery (HATC), hepatic vein (HVTC) and portal vein (PVTC) contact with tumour. Additionally, to normalise vessel contact for lesion size, the ratio of total length of contact of each type of vessel was divided by lesion volume to yield contact per mm3 of tumour, this defined as HATCn, HVTCn and PVTCn. Tumour volume was determined using the formula for an ellipse as has been done previously Citation[16]. Since the heat sink effect should be related to the volume of blood flow in each adjacent vessel, for all vessels in contact with tumour, their length of contact was multiplied by their cross‐sectional area (assuming a circular shape). These values were summated for each vessel type to approximate vessel volume by type that was in contact with tumour.
Post radio frequency ablation follow‐up
Follow up multi‐detector CT imaging using identical protocol to that described above, was performed immediately after ablation and at 1 month, 3 months and then every 6 months. For patients who did not undergo liver transplantation over the time of this study, local disease‐free follow‐up was considered the time from RFA to the time of their last CT scan, and for those with local recurrence, the time from RFA to the first CT confirming recurrence. Criteria for CT recurrence consisted of abnormally enhancing tissue either within or along any portion of the original ablation zone on arterial phase images. For those who were transplanted, local diseasefree follow‐up or time to local tumour recurrence was based on the date and findings at explant pathology.
Statistical analysis
The overall goal was to examine if any of these 12 defined vascular parameters obtained from pre‐RFA CT and presumed to be an indicator of the heat sink effect, correlated with the mHC. Stepwise multi‐regression analysis was carried out to assess for any true independent association of any vascular parameter with mHC. Tumour enhancement pattern and tumour volume were also added to our analysis to look for any relationship with mHC. A non‐parametric t‐test was used to assess for any differences in follow‐up time or mHC values between patients with and without local recurrence and for differences in mHC between patients with HCV, HBV, cirrhosis and non‐alcoholic steatohepatitis. These analyses were performed using the SAS system, version 9.1 (SAS Institute, Cary, NC). Finally, a Cox proportional hazard model was used to assess for any relationship between mHC and local recurrence using Matlab software version R2007a (Mathworks, Natick, MA).
Results
Vascular parameters correlated to mHC
Mean lesion size (maximum dimension) was 18.6 ± 5.6 mm (± SD) and lesion volume was 4.21 ± 3.58 cm3. The mean number of ablations per lesion was 1.7 (range 1–3) Modified heat capacity values ranged from 96 to 1354.29 J/°C with a mean of 366.34 ± 279.70 J/°C. Twelve tumours were in contact with hepatic arteries (), 13 with portal veins and 13 with hepatic veins. These included four with both hepatic artery and hepatic vein contact, two with artery and portal vein contact and three with contact by both portal and hepatic veins. Two tumours were in contact with all three types of vessels and four had no contact with any vessel. The distribution of vessels in contact with each individual HCC as related to their mHC values is summarised in . For vessels in contact with tumour, mean hepatic artery diameter was 1.4 mm ± 0.7 mm, mean hepatic vein diameter was 2.8 mm ± 1.5 mm and mean portal vein diameter was 2.8 mm ± 1.1 mm.
Figure 1 Each bar represents a single HCC with the type of abutting vessels and associated mHC value indicated on the X and Y axes respectively. Less energy (lower mHC) was required to heat tumour adjacent to the RFA electrode when there were no abutting hepatic arteries or veins or only abutting portal veins. mHC values significantly increased with the extent of contact with hepatic arteries and hepatic veins. mHC, modified heat capacity in J/°C. HA, hepatic artery; HV, hepatic vein; PV, portal vein.
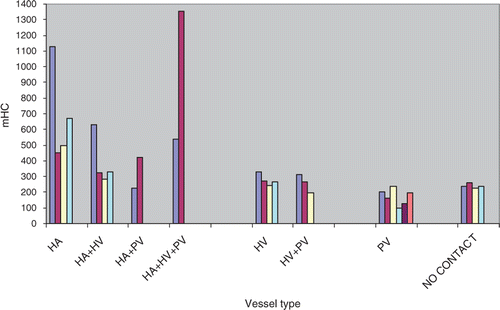
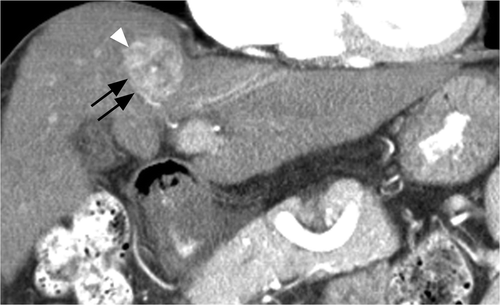
Figure 2 Coronal arterial phase CT image of a 55-year-old man with HBV cirrhosis. Note artery (black arrows) abutting the inferolateral margin of a heterogeneously enhancing HCC (white arrowhead). Patients’ modified heat capacity was 1128 J/°C.
Vascular parameter values and statistical results are summarised in . Multiple regression indicated significant correlation of mHC with lesion enhancement (P < 0.0018), HATC (P < 0.0001), and HATCn (P = 0.0077) but not hepatic artery volume. Hepatic vein and portal vein arc of contact (HVTC, PVTC) did not correlate with the modified heat capacity either alone or when normalised for tumour size (HVTCn and PVTCn). However, total hepatic vein volume in contact with tumour () did correlate with mHC (P = 0.016) while portal vein volume did not ().
Figure 3 Axial portal venous phase CT image in an 84-year-old woman with Nash and an HCC (black arrow) involving segment VIII of the liver. A hepatic vein (white arrowheads) is seen abutting tumour along its posterolateral aspect. Before performing blinded CT assessment, the modified heat capacity was determined to be 670.91 J/°C.
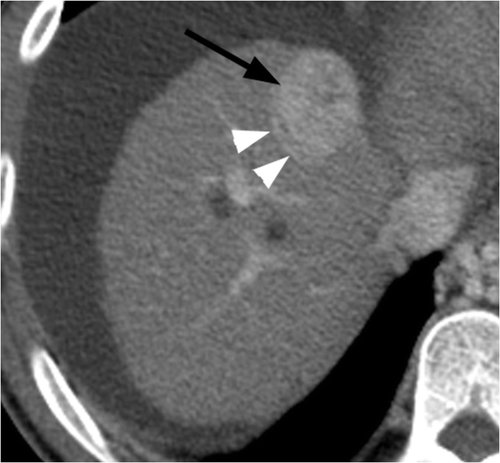
Figure 4 Man, 57 years old, with HCV cirrhosis. Sagittal CT image during the portal venous phase shows a portal vein (black arrow) along the posterior aspect of a pathologically confirmed HCC (white arrow). No other vessels were noted to be abutting tumour. Modified heat capacity was found to be 262.22 J/°C, well below the study population mean of 366.34 J/°C.
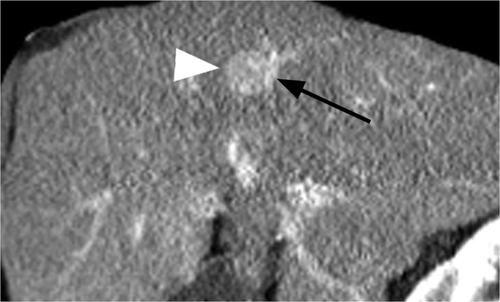
Table II. Correlation of vascular parameters and tumour enhancement to mHC.
No patients had hepatic veins within 3 mm of tumour edge, so the relationship between HVTD ≤ 3 mm and mHC could not be explored. Eight had arteries and 12 had portal veins within 3 mm of tumour edge. In these cases mean arterial diameter was 1.2 mm ± 0.1 mm and for portal veins, 3.2 mm ± 0.1 mm. No correlation between mHC and PVTD ≤ 3 mm or HATD ≤ 3 mm was found. Finally, there was no relationship between mHC and tumour volume, nor significant differences in mHC between patients with HCV, HBV, cirrhosis or NASH.
Post radio frequency ablation follow‐up
Patient follow‐up is summarised in . Mean follow‐up time was 309 days ± 95 days for all 29 patients. Based on CT follow‐up in patients who did not undergo transplantation, seventeen remained free from local recurrence at a mean follow‐up of 310 ± 89 days while four showed local recurrence at 362 ± 47days after RFA.
Table III. Patient follow-up from time of RFA and corresponding mHC.
Eight patients underwent transplantation, none showed local tumour recurrence based on CT, however three had recurrence at 211 ± 123 days based on viable tumour at the periphery of the RFA site seen on explant pathology. The remaining five patients were free of local recurrence based on explant pathology at 322 ± 114 days follow‐up.
Overall, recurrence was seen in seven of 29 patients at a mean follow‐up of 297 ± 112 days, which did not significantly differ from follow‐up in those without recurrence at a mean of 313 ± 92 days. The mean mHC value in those with recurrence was 691.71 ± 393.2 J/°C which significantly differed from the mean mHC value of 267 ± 291.92 J/°C seen in those with recurrence (P < 0.01). Cox proportional hazard model demonstrated a significant relationship between mHC and local recurrence (P = 0.017); and indicated the chance of local recurrence increased 2.03‐fold (95%CI 1.14–3.63) for every increase in mHC of 100 J/°C. All seven patients with local recurrence had a mHC above 315 J/°C compared with only four of 22 without recurrence.
Discussion
The heat sink effect is generally considered a limitation of the effectiveness of RFA in general and when compared to other ablation techniques Citation[17]. Histological confirmation of the HSE has been performed in animal studies in vivo using normal lung and hepatic tissue. Lu et al. Citation[7] carried out RFA on normal swine liver and found viable hepatocytes juxtaposed between the radio frequency lesion and vessel wall in 12 of 24 veins greater than 3 mm in diameter, seven of seven veins greater than 5 mm but in zero of 96 vessels less than 2 mm in diameter. These investigators concluded that the HSE should be expected in hepatic tissue undergoing RFA when adjacent to vessels above 2–4 mm. Similarly, on histological assessment, Steinke et al. Citation[18] found an inverse relationship between the extent of vessel wall damage caused by RFA (their definition of the HSE) and vessel diameter in normal sheep lung. They identified a 3‐mm diameter threshold for vessels above which the HSE was consistently seen. Although such results seem to confirm the concept of the HSE, the clinical applicability of results relying on normal tissue is questionable due to neglecting the effects of tumour perfusion and altered surrounding tissue composition, both of which are known to have a major impact on RFA volume based on tumour and mathematical models Citation[19], Citation[20].
Within the literature there also appears to be controversy concerning the validity of the HSE as a true risk factor for local HCC recurrence after RFA. Kim et al. Citation[9] found that nine of 19 HCCs with abutting vessels above 3 mm had local recurrence after RFA compared with only 10 of 53 with abutting vessels below 3 mm. Although this appeared to be a significant difference on univariate analysis, it was not maintained on multivariate analysis. Additionally, a follow‐up study by these same investigators Citation[21] found only that an ablative margin <3 mm was associated with local HCC progression after RFA. The importance of the ablation margin rather than the HSE was also identified by others Citation[22], Citation[23] who found no correlation between local HCC recurrence and either tumour contact with major (major not defined) vessels or vessels within 5 mm of a tumour margin. On the other hand, Lu et al. Citation[10] performed a retrospective review of 47 HCCs treated with RFA and found local recurrence was significantly higher for lesions adjacent to vessels above 3 mm (8/15) compared to lesions without abutting vessels (28/32). Although these authors also did not discern between vessel types, their findings support the HSE as a risk factor for local recurrence in clinical practice.
Our results were obtained in vivo from pathologically confirmed, small HCCs (<3 cm) within predominantly diseased liver, and appear to support the existence of the HSE during RFA of HCC. The mHC of an HCC, by definition, is a general indicator of the ability of tumour adjacent to the electrode to tolerate heating. Although it does not localise the points of contact of vessel with tumour or necessarily uniformly apply to all of the tumour, because the mHC directly correlated with the volume of abutting hepatic veins and extent (arc) of hepatic arterial contact with tumour, it follows that these abutting vessels affected the ability of RFA to heat the small HCCs in our study population. We believe that the mHC could represent an easily obtainable and clinically realistic parameter which reflects the HSE experienced by small HCCs during RFA.
As important, on initial assessment the mHC may allow insight into the risk of local tumour recurrence after RFA, a finding that further supports the concept of the HSE. Local recurrence was seen in seven of 11 patients with a mHC above 315 J/°C and in none below this value. The risk of local recurrence was found to double for every 100 J/°C increment in mHC. We point out that this is a cautious conclusion given that CT is less sensitive for local tumour evaluation than explant pathology Citation[10] and recurrence based on both were combined to achieve sufficient power for statistical analysis. Because of the limited number of HCC ablations performed at our institution and its use for bridging to transplantation, it is difficult for us to accrue patients who develop local recurrence based on imaging after RFA. The potential that the 19 patients without local recurrence on follow‐up CT could have histological recurrence does exist. We do note that the mean mHC of patients with CT‐confirmed recurrence was 477.07 ± 137.2 versus 207.95 ± 160.83 for those without recurrence on follow‐up CT.
We also found that the mHC of small HCCs was significantly higher in homogeneously hyper‐enhancing tumours during arterial‐phase CT compared to those with heterogeneous enhancement. With increasing tissue enhancement and thus perfusion Citation[14], Citation[15], there is a decrease in thermal resistance and increase in heat flow to adjacent vessels Citation[24]. This would help explain why homogeneous tumours were found to require more thermal energy than heterogeneous tumours to increase their temperature, i.e. a higher mHC. These findings are also supported by mathematical modelling of the bioheat equation which confirmed that the greater the tumour vascularity, the greater the thermal sink and inability to undergo heating by RFA Citation[19].
Heat transfer from tissue to adjacent vessels occurs predominantly by conduction, governed by Fourier's Law Citation[25] and is directly related to the area of contact and the temperature gradient between the two Citation[26]. Logically, as heat is transferred to blood within a vessel, the temperature gradient between tissue and vessel declines and so does heat transfer. The influx of unheated blood or volumetric blood flow should therefore directly affect the temperature gradient. The higher the volumetric blood flow within a vessel, all else constant, the better the temperature gradient is maintained and the ability to transfer heat by conduction (act as a heat sink). Because volumetric blood flow in a vessel is determined by both cross‐sectional area and blood velocity, for a similar sized hepatic vein, portal vein and hepatic artery, the volumetric flow can vary widely due to differences in blood velocity. This argues that these vessels should be evaluated separately with respect to their HSE rather than pooled as done previously, especially for HCC in a cirrhotic liver. The vascular parameters we assessed were more robust than in previous studies, besides making the distinction between vessel type, we looked at all vessels greater than 1 mm in diameter, abutting and within 3 mm of tumour edge. Interpretation included measurements on extent of tumour contact, extent of contact relative to tumour size, vessel volume and vessel distance from tumour edge as a function of vessel type. Although actual area of vessel contact with tumour is an important part of heat transfer, this obviously was not possible to assess with any accuracy on our CT images.
The modified heat capacity showed no relationship to the length of abutting hepatic and portal veins or to the volume of abutting portal veins and arteries. It did however, correlate with the volume of hepatic veins in contact with tumour. Because volume was determined by multiplying vein length of contact with tumour by vessel cross‐sectional area and no relationship between mHC and contact length was found, it follows that cross‐sectional area, and thus vein diameter directly impacted on mHC. This is in comparison to hepatic arteries where the modified heat capacity was found to correlate with HATC and HATCn but not hepatic artery volume. Both of these results may be explained by again considering the impact of blood volumetric flow on heat transfer. For the size of hepatic veins dealt with in our study, volumetric blood flow probably relied more on vessel size rather than blood velocity, while in the hepatic arteries velocity was the dominant factor.
The lack of correlation of mHC with any portal vein parameter is likely due to our patient population which consisted of individuals with chronic liver disease (25/29 with cirrhosis). These patients have relatively decreased portal flow and compensatory increased arterial flow Citation[27]. Because of poor portal flow, these vessels probably act as poor heat sinks in the true clinical setting and it logically follows that they should have little to no effect on mHC of HCC in a cirrhotic liver. Additionally we found no correlation of mHC with hepatic arteries within 3 mm of but not in contact with HCC. One would think hepatic arteries close by but non‐abutting tumour could have an effect on mHC given the strong correlation found with abutting hepatic arteries. Perhaps, because we had only eight patients with this scenario and with a mean hepatic artery size of only 1.2 mm, this relationship was not adequately tested. Alternatively, the insulatory effect proposed for cirrhotic tissue Citation[20], Citation[28] which was interposed between vessel and tumour may explain this finding. Clearly a larger number of patients with a wider range of non‐abutting arterial diameters are needed to fully assess this relationship.
There are study limitations that need to be identified. First, we assumed that vessels abutting or within 3 mm of tumour would have an effect on temperature measured by the RFA electrode that was positioned for tumour ablation rather than at multiple positions along the tumour's edge. This assumption seems valid for our study population, given that the modified heat capacity had a strong correlation with abutting arteries and hepatic vein volume and was unrelated to tumour volume. Thus, statistically these relationships cannot be random or due to chance. Additionally, this assumption was made in all cases so the relative differences among mHC values and what they reflect should be relevant. Other published studies concerning overall thermal clearance within tumours have also relied on only temperatures obtained from the tumour's epicentre Citation[11]. Finally, in clinical practice electrode temperature is considered the standard surrogate marker for overall tumour temperature and the tumouricidal effect of RFA on the entire lesion and not just tumour adjacent to the electrode. We acknowledge that all our tumours were small (mean diameter of 18.6 mm) and perhaps in larger tumours, electrode position relative to tumour's edge could influence the results, i.e. in larger lesions determining the mHC per gram of tissue or true heat capacity may be necessary to assess the HSE.
We also assumed that tissue heating for 1 min at constant wattage had no effect on tumour tissue, intratumoural vessels and adjacent vascularity. This may not be accurate given that final temperatures 1 min after heating did reach above 50°C in all but five patients. Above 50°C, tissue necrosis and vessel thrombosis can occur Citation[29] which would inhibit heat conductivity within tumour and result in lower values of heat capacity. However, we argue that achieving a temperature above 50°C after 1 min of heating indicates an HCC's intrinsic inability to tolerate thermal energy and thus its intrinsic low heat capacity to start with. In all five patients in whom the temperature did not exceed 50°C, their HCCs had abutting arteries which we believe inhibited their temperature rise. Finally, we also want to again stress that our results are applicable to HCCs below 3 cm and predominantly in a cirrhotic liver. They should not be generalised to larger lesions or other types of hepatic primary or metastatic malignancies in otherwise normal liver parenchyma.
In conclusion, the modified heat capacity of small hepatocellular carcinomas, the ability of tumour adjacent to the electrode to undergo heating, was assessed numerically, in vivo, using the thermocouple within an RFA electrode. The mHC values in our study population were found to correlate with abutting arteries and the volume of abutting hepatic veins, and therefore may be a quantitative indicator of the heat sink effect unique to each small HCC. Initial observations indicate that the mHC also seems to substantiate a relationship between the heat sink effect and potential local recurrence of small HCCs after RFA.
Declaration of interest: No conflict of interest exists among any of the authors. The authors alone are responsible for the content and writing of the paper.
References
- Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: Increased lesion diameter with a perfusion electrode. Acad Radiol 1996; 3: 636–644
- Organ LM. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol 1976; 39: 69–76
- Lu DSK, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003; 14: 1267–1274
- Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol 2007; 188: 480–488
- Goldberg SN, Charboneau JW, Dodd GD III, Dupuy D, Gervais D, Gillams A, et al. Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria. Radiology 2003; 228: 335–345
- Patterson EJ, Scudamore CH, Owen DA, Nagy A, Buczkowski A. Radiofrequency ablation of porcine liver in vivo: Effects of blood flow and treatment time on lesion size. Ann Surg 1998; 227: 559–565
- Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the ‘heat sink’ effect. Am J Roentgenol 2002; 178: 47–51
- Plengvanit U, Suwanik R, Chearanai O, Intrasupt S, Sutayavanich S, Kalayasir C, et al. Regional hepatic blood flow studied by intrahepatic injection of 133Xenon in normals and in patients with primary carcinoma of the liver, with particular reference to the effect of hepatic artery ligation. Aust N Z J Med 1972; 2: 44–48
- Kim Y-S, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: Analysis of the pattern and risk factors. Eur J Radiol 2006; 59: 432–441
- Lu DKS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: Treatment success as defined by histologic examination of the explanted liver. Radiology 2005; 234: 954–960
- Masunaga S, Ono K, Mitsumori M, Nishimura Y, Hiraoka M, Akuta K, et al. Clinical usefulness of determining the rate of thermal clearance within heated tumors. Jpn J Oncol 1996; 26: 428–437
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis 1999; 19: 329–338
- Goldberg SN, Stein M, Gazelle S, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: Optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc Interv Radiol 1999; 10: 907–916
- Asayama Y, Yoshimitsu K, Nishihara Y, Irie H, Aishima S, Taketomi A, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: Radiologic-pathologic correlation. Am J Roentgenol 2008; 190: W28–W34
- Wang B, Gao ZQ, Yan X. Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol 2005; 46: 353–358
- Feldman JP, Goldwasser R, Mark S, Schwartz J, Orion I. A mathematical model for tumor volume evaluation using two dimensions. J Appl Quant Methods 2009; 4: 455–462
- Wright AS, Sampson LA, Warner TF, Mahvi D, Lee FT. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005; 236: 132–139
- Steinke K, Haghighi KS, Wulf S, Morris DL. Effect of vessel diameter on the creation of ovine lung radiofrequency lesions in vivo: Preliminary results. J Surg Res 2005; 124: 85–91
- Liu Z, Lobo SM, Humphries S, Horkan C, Solazzo S, Hines-Peralta A, et al. Radiofrequency tumor ablation: Insight into improved efficacy using computer modeling. Am J Roentgenol 2005; 184: 1347–1352
- Ahmed M, Karim A, Weeks D, Lobo SM, Kruskal J, Lenkinski R, et al. Radiofrequency ablation: Effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 2004; 230: 761–767
- Kim Y-S, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progession: 3D quantitative assessment using CT image fusion. Am J Roentgenol 2010; 195: 758–765
- Zytoon AA, Ishii H, Murakami K, Ramden El-Kholy M, Furuse J, El-Dorry A, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol 2007; 37: 658–672
- Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg 2006; 93: 440–447
- Kolios MC, Sherar MD, Hunt JW. Large blood vessel cooling in heated tissues: A numerical study. Phys Med Biol 1995; 40: 477–494
- Bird B, Stewart S, Lightfoot E. Transport Phenomena. Wiley, New York 1960; 243–247
- dos Santos I, Haemmerich D, da Silva Pinheiro C, Ferreira da Rocha A. Effect of variable heat transfer coefficient on tissue temperature next to a large vessel during radiofrequency tumor ablation. Biomed Eng Online 2008; 7: 21
- Richter S, Mücke I, Menger MD, Vollmar B. Impact of intrinsic blood flow regulation in cirrhosis: Maintenance of hepatic arterial buffer response. Am J Physiol Gastrointest Liver Physiol 2000; 279: G454–G462
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: Treatment with radiofrequency ablation versus ethanol injection. Radiology 1999; 210: 655–661
- Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: Effect of electrode size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995; 2: 399–404