Abstract
Background: Hyperthermic intraperitoneal chemotherapy (HIPEC) is more and more used in the treatment of patients with peritoneal carcinomatosis (PC) of different primary tumours. However, survival of patients with PC is still poor. The aim of this study was to evaluate the effects of preoperatively administered bevacizumab and temperature of the perfusion in an experimental model of HIPEC.
Materials and methods: A model for peritoneal carcinomatosis of colorectal origin was created by implantation of tumour fragments (HT29) in the abdomen of athymic nude rats. All animals were treated with oxaliplatin-based HIPEC. Animals were randomised into different treatment groups: hyperthermic treatment was compared to normothermic treatment and in both groups there were animals with and without preoperative administration of bevacizumab. Interstitial fluid pressure (IFP), bio-availability (concentrations of the drug in blood and tumour) and tumour growth delay (TGD) were evaluated.
Results: In this study, preoperative bevacizumab lowered IFP but there was no effect on the bio-availability. The effect on the size of the tumour was ambivalent with no statistically significant effect on TGD. The area under the curve (AUC) of platinum was higher in animals treated with hyperthermic perfusion as compared to normothermic perfusion. In the TGD-study a post-operative mortality of 100% was observed in the hyperthermic group, compared to 1 out of 16 rats in the normothermic group.
Conclusions: The results of this study suggest that bevacizumab is not promising for clinical implementation in the setting of HIPEC: the effects on tumour size were ambivalent and there was no statistically significant benefit in TGD. The effects of hyperthermic perfusion in this study were negative: a higher blood concentration of oxaliplatin as compared to normothermic perfusion, without a higher intra-tumoural concentration. There was a higher mortality rate after hyperthermic perfusion as compared to normothermic perfusion (p < 0.001).
Introduction
Hyperthermic intraperitoneal chemotherapy (HIPEC) is used in the treatment of peritoneal carcinomatosis of different origins. Peritoneal carcinomatosis (PC) occurs in 5% of patients with colorectal carcinoma and in patients with FIGO stage III and IV ovarian cancer. Both synchronous and metachronous PC can occur. Survival rates in these patients are rather low. The median survival time with standard chemotherapy is 50 and 23 months in PC of ovarian and colorectal origin respectively Citation[1], Citation[2]. When treated with HIPEC, patients with PC of ovarian and colorectal origin have a median survival of 66 and 30 months respectively Citation[1], Citation[3].
Oxaliplatin is rapidly absorbed intracellularly, as a result of its lipophilic structure Citation[4]. It is recognised as a standard adjuvant treatment in colorectal cancer Citation[5]. Promising results were also demonstrated in ovarian cancer, gastric cancer and malignant mesothelioma Citation[6–8]. The required perfusion time is shorter for oxaliplatin compared to cisplatin and mitomycin, making it more practical for clinical purposes.
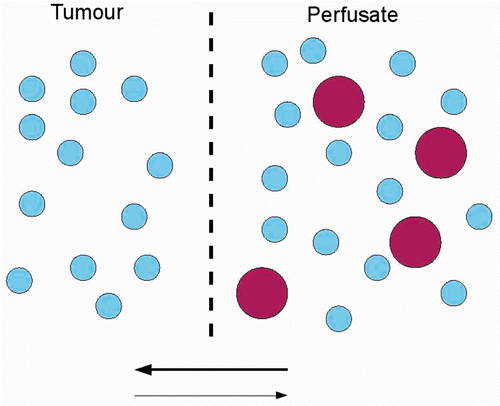
The benefit of intraperitoneal chemotherapy arises from the existence of a peritoneal–plasma barrier. This barrier allows the local administration of higher doses of chemotherapy while minimising systemic side effects. However, cytotoxic drugs penetrate only a few millimetres into the tumour tissue Citation[9]. In local administration, the penetration of drugs can be described as a process of permeation. This process depends on the chemical potential gradient between the solutions on both sides of the membrane Citation[10]. The driving forces for the chemical potential are concentration gradients, temperature, electromotive force and pressure gradient () Citation[10]. Penetration of the drug into the tumour increases with increasing pressure gradient. It is known that the interstitial fluid pressure (IFP) in tumours is increased as compared to normal tissue. Therefore, penetration from the perfusate into tumour tissue is more difficult as compared to normal tissue. The causal mechanism of the increased IFP in tumour tissue is not completely understood, but important factors are leaky blood and lymph vessels Citation[11]. Bevacizumab is an inhibitor of angiogenesis, inducing a decrease in microvascular density and IFP Citation[12]. Our hypothesis was that a lower IFP in the tumour resulting from the preoperative administration of bevacizumab would increase the penetration of oxaliplatin. Two experiments were conducted in order to assess this hypothesis. The first experiment aimed to verify the lowering of the IFP in a rodent model of peritoneal carcinomatosis. The second experiment aimed to analyse the effects of the administration on bioavailability of oxaliplatin.
Figure 1. Schematic presentation of permeation process in IPEC. The drug (big circles) will move from the perfusate to the tumour, passing cell membranes (dotted line), due to the concentration difference (bold arrow). High pressure in the tumour decreases the rate of penetration, due to the lower pressure gradient between perfusate and tumour, as compared to normal tissue.
Hyperthermia induces apoptosis in tumour cells Citation[13–15]. Recently it was demonstrated that hyperthermia increased the peritoneal oxaliplatin concentration while reducing systemic absorption Citation[16]. Therefore intraperitoneal chemotherapy (IPEC) is administered at temperatures above body temperature. However, hyperthermia also induces apoptosis in normal cells Citation[15] and affects the healing of anastomosis Citation[17–19]. Furthermore, it was demonstrated in a rat model of peritoneal carcinomatosis of colorectal origin that hyperthermia did not increase survival as compared to normothermic intraperitoneal treatment Citation[20]. To our knowledge, a clinical benefit from hyperthermia combined with chemotherapy compared to chemotherapy alone was never demonstrated. The high morbidity rates (up to 41% Citation[21]) described in patients after HIPEC, possibly result from the hyperthermia component of the therapy.
The aim of this study was to analyse the effect of preoperative bevacizumab and hyperthermic administration of oxaliplatin on IFP, bioavailability and tumour growth delay (TGD) in a rodent model of peritoneal carcinomatosis and HIPEC. The IFP, TGD and bioavailability between animals treated with hyperthermic and normothermic intraperitoneal oxaliplatin were compared. The same comparison was done for animals treated with different doses of preoperative bevacizumab.
Methods
Animals and tumour model
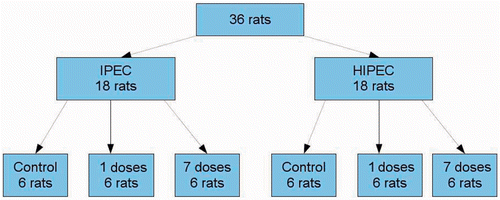
The protocol of this study was approved by the local committee on animal welfare of Ghent University. Male athymic nude rats (Harlan, Horst, the Netherlands), aged 8–10 weeks, were housed in groups of two animals in a 12-h day/night cycle and a room temperature of 24°C. The animals had free access to tap water and standard pellet food. A HT29 (humane colorectal cancer) cancer cell line was cultured and 8 million cells were suspended in saline and injected in the proximal hind leg of donor animals. After two weeks, fragments of the tumours were transplanted into the abdomen of acceptor animals. Acceptor rats were anaesthetised with inhalation anaesthesia (isoflurane). A median laparotomy was performed. Tumour fragments (3–5 mm) were stitched to the peritoneum with Vicryl 3-0 (Ethicon, Johnson & Johnson, New Brunswick, NJ, USA). The abdomen was closed in layers with Vicryl 3-0. In the IFP-study the rats were randomly divided into six treatment groups: normothermic chemoperfusion without preoperative bevacuzimab, hyperthermic chemoperfusion without preoperative bevacuzimab, normothermic chemoperfusion with preoperative bevacizumab, and hyperthermic chemoperfusion with preoperative bevacizumab. Six animals per group were included (). Bevacizumab (5 mg/kg) was administered by intraperitoneal injection, either once 7 days before IPEC or daily, starting 7 days before IPEC. In the bioavailability study and the TGD study there were only four different treatment groups: IPEC and HIPEC without bevacizumab, IPEC and HIPEC with daily bevacizumab, starting 7 days before IPEC. It was planned to treat eight animals per group. In this experiment, there were no groups receiving only one dose of bevacizumab.
Intraperitoneal chemoperfusion
Two weeks after tumour implantation, intraperitoneal chemoperfusion (IPEC) was performed. The animals were anaesthetised with inhalation anaesthesia (isoflurane). A median laparotomy was performed. The abdominal wall was fixed to a metal ring placed over the abdomen. Two tubes were placed in the abdomen. These tubes were connected to a heating element and a roller pump. The temperature of the perfusate was monitored by thermosensors placed in the abdominal cavity. During normothermic perfusion the temperature of the perfusate was kept between 37°C and 38°C; during hyperthermic perfusion the temperature was kept between 41.5°C and 42.5°C. The perfusate consisting of 100 mL glucose 5%, was heated and circulated into the abdomen for 60 min. The dose of oxaliplatin was 460 mg/m2 body surface. The body surface of a rat (in cm2) equals k × (body weight)2/3 (in g), with k = 10.4 cm2/g Citation[22]. The perfusion was given for 60 min in the IFP experiment and the bioavailability experiment and for 45 min in the TGD study. (Perfusion time was reduced for the TGD experiment after a few deaths were observed in the previous experiments.) Afterwards, the perfusate was evacuated. In the bioavailability experiment the tumour was excised and fixed in formol. Blood samples were collected by cardial punction at 5, 15, 30, 45, 60, and 75 min after the start of IPEC. In the IFP experiment and the bioavailability experiment animals were sacrificed after the IPEC procedure by intrapulmonary injection of 0.5 mL T61.
Platinum determination
Platinum concentrations in the blood and perfusate were determined with inductively coupled plasma mass spectrometry (ICP-MS) after acid digestion. Areas under the curve (AUC) in blood and perfusate were calculated for the different groups. From these data the area under the curve ratio (AUC in blood divided by AUC in perfusate) was calculated. The distribution of platinum in the tumours was visualised and semi-quantitatively assessed with laser ablation ICP-MS. Details of these methods are described elsewhere Citation[23]. Maximal tumour diameter was measured on the fixed tumours. Maximal penetration depth (MPD) and percentage of tumour surface covered with platinum were calculated based on images produced by LA-ICP-MS 3 in Photoshop QR (Adobe QR, San Jose, CA, USA). The pictures represented platinum distribution on a cross-cut through the tumours. The coloured pixels (corresponding with a platinum concentration above 0.1 µg/g) on these images were counted and related to the number of pixels in similar pictures of the whole tumour. The result was expressed as a percentage, describing the percentage of surface covered with platinum.
Interstitial fluid pressure measurement
All IFP measurements were performed by means of a fibre optic probe on the Fabry-Perot interferometer (Samba Preclin, Samba Sensors, Västra Frölunda, Sweden). The technique used is described elsewhere Citation[24]. Briefly, half of a polyurethane intravenous catheter (23 gauge) was perforated using an insulin needle (29 gauge). This catheter was placed into the tumour using a syringe technique, fixed with one stitch to the abdominal wall and filled with sterile lubricant eye drops (Allergan Pharmaceuticals, Westport, County Mayo, Ireland). Next the probe was carefully inserted into the catheter and fixed. The IFP was measured continuously and registered at 0, 5, 15, 30, 45 and 60 min. The values are relative to one bar, i.e. 750 mmHg.
Tumour growth delay – MRI imaging and volumetry
Tumour growth delay (TGD) was determined on three sequential T1 MRI scans, without contrast (day 1, 6 and 13) using a 3 Tesla MRI scan (Siemens, Munich, Germany). On day 0, an IPEC-procedure was performed, either hyperthermic or normothermic. Before the IPEC procedure, the volume was calculated in order to compare the volume on the MRI images to in vivo measurements. The animals were sacrificed on day 14 (0.5 mL T61, intrapulmonal). On day 14, the diameter of the tumour was measured in vivo, in order to make sure that the MRI images corresponded to the actual size of the tumour. The images were analysed in Pmod (PMOD Technologies, Zurich, Switzerland), using the volume of interest function. Absolute volumes and relative volume changes were calculated for the different groups.
Statistical analysis
Differences in continuous variables with a normal distribution were analysed with unpaired student's t-test. Non- parametric repeated measurements were assessed with independent samples Kruskal-Wallis test. Independent samples non-parametric variables were assessed with Mann-Whitney U-test. Statistical significance was assumed when p ≤ 0.05. Data analysis was performed by means of SPSS 19.
Results
Interstitial fluid pressure
Thirty-six rats were included in this study. One rat was excluded because of instability of the IFP-measurement. The IFP (±SD) at the start of IPEC was 22.8 ± 19 mmHg, −10.5 ± 20 mmHg, and −2.4 ± 3.3 mmHg at 37°C in the control, bevacizumab ×1, and bevacizumab ×7 groups respectively. Similar results were found in animals which underwent hyperthermic IPEC. Kruskal- Wallis test showed a significant difference in IFP at the start of IPEC (). Pairwise comparison revealed a difference between bevacizumab treated hyperthermic and normothermic groups versus control hyperthermic and normothermic groups. Analysis of the results combined according to bevacizumab treatment reveals that the IFP at the start of IPEC diverged in groups treated with one dose (p < 0.001) or seven doses (p = 0.021) of bevacizumab compared to controls. The difference in IFP between rats treated with one or with seven doses of bevacizumab was not statistically significant (p = 0.646). The difference between IFP at the start and at the end of IPEC was similar in all groups (). Perfusion temperature had no effect on this difference: 6.3 mmHg (95%CI −0.7 to 13.2) in normothermic and 3.4 mmHg (95%CI −5.7 to 12.6) in hyperthermic groups.
Table I. Results of IFP measurements. IFP at start: IFP at the moment the perfusate was administered in the abdominal cave (time = 0 min). IFP difference: IFP at time = 60 min minus IFP at time = 0 min. (1) Normothermic, without bevacizumab; (2) Hyperthermic, without bevacizumab; (3) Normothermic, 1 dose of bevacizumab; (4) Hyperthermic, 1 dose of bevacizumab; (5) Normothermic, 7 doses of bevacizumab; (6) Hyperthermic, 7 doses of bevacizumab.
Bioavailability
Thirty-four rats were included in this study. Two rats died of intrapulmonary bleeding after intracardial punction early during the IPEC-procedure and were excluded from the study. In the normothermic groups, two out of 16 rats died before the end of the IPEC-procedure and 6 out of 16 rats died in the hyperthermic groups. This difference was not statistically significant (p = 0.225).
Some of these results were described in the methodological paper by Gholap et al. Citation[23]. The AUC for platinum in blood was significantly higher during hyperthermic IPEC as compared to normothermic IPEC (p ≤ 0.001) (). AUC Pt was similar in groups with or without preoperative bevacizumab (p = 0.855)(). AUC ratio (AUC in the blood divided by AUC in the perfusate) was similar in groups treated with or without bevacizumab (p = 0.418). The platinum-covered surface was similar in all groups (p = 0.752) (). Mean MPD (±SD) was 2.9 ± 0.9 and 2.8 ± 0.9 mm in the hyperthermic and normothermic groups respectively (p = 0.75) and 2.7 ± 0.7 vs. 3.0 ± 1.0 mm with and without bevacizumab respectively (p = 0.43). Rats treated with preoperative bevacizumab had smaller tumours (maximal diameter of 6.3 ± 1.2 vs. 7.1 ± 0.9, p = 0.045).
Figure 3. Results of LA-ICP-MS. Upper left: normothermic without bevacizumab (63.75%); upper right: hyperthermic without bevacizumab (93.53%); under left: normothermic with bevacizumab (43.18%); under right: hyperthermic with bevacizumab (45.18%). Blue line: tumour border. Coloured parts: platinum concentration from 0.1 µg/g (purple) upwards.
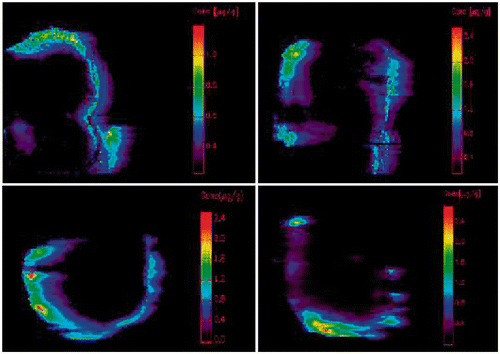
Table II. Effects of temperature on bio-availability. AUC ratio = AUC blood/AUC perfusate.
Table III. Effects of bevacizumab.
Tumour growth delay
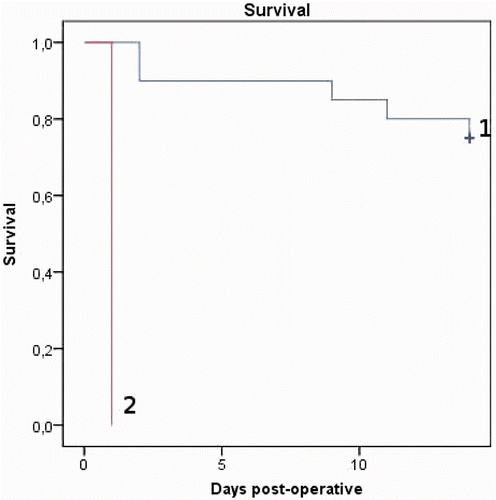
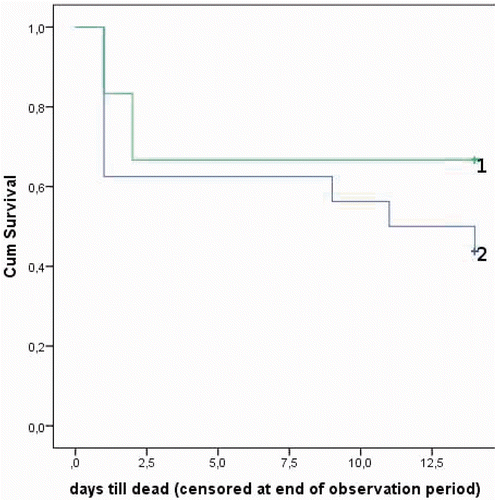
In the hyperthermic groups, a mortality rate of 100% within 24 h after the end of IPEC was observed in the first eight rats. In the normothermic groups only one rat had to be sacrificed early in the post-operative phase, due to severe wound complications. illustrates the difference between the groups receiving normothermic and hyperthermic treatment. The difference between both groups was statistically significant (p < 0.001, log-rank test). It was decided to end the hyperthermic study prematurely. Due to the unexpected deaths in the groups receiving hyperthermic treatment, changes in tumour volumes were only calculated for the groups having received normothermic treatment. All animals were included in the analysis of preoperative tumour volume. Analysis of the first scans showed that tumours were larger in rats treated with bevacizumab (p = 0.012). Volume changes measured on the MRI scans did not differ significantly between both groups on day 6 (p = 0.127) and day 13 (p = 0.662) (). The correlation between measurement on the MRI scans and in vivo volume measurements was good (0.6 Pearson correlation coefficient) with slightly lower volumes measured in vivo. However, in rats treated with bevacizumab a decrease in tumour volume was shown at day 6, whereas in rats not receiving bevacizumab there was an increase. In terms of survival, there was no significant difference in survival between rats treated with bevacizumab and control rats (p = 0.259, ).
Table IV. Tumour volumes and changes.
Discussion
In the above-described experiments the effects of preoperative treatment with bevacizumab and intra-operative temperature of perfusate in a rat model of peritoneal carcinomatosis were analysed.
The study regarding the effects of preoperative administration of bevacizumab, was a negative one. We hypothesised that the inhibition of angiogenesis by the administration of bevacizumab would lower the IFP in tumour tissue in PC. The first experiment did demonstrate a lower IFP in rats treated with preoperative bevacizumab. Interestingly, the IFP values in rats treated with seven doses of bevacizumab and those treated with one dose of bevacizumab were similar. However, the lower IFP did not result in an increased penetration of oxaliplatin in the tumour. The effect of preoperative bevacizumab on the size of the tumour was ambivalent. In the bioavailability study, tumours were smaller in the rats treated with bevacizumab, while in the TGD study tumours turned out to be larger after bevacizumab treatment. No statistically significant difference in tumour growth delay could be demonstrated between groups treated with and without bevacizumab. It is possible that the effects of bevacizumab on IFP and TGD would be different after intravenous administration as compared to the intraperitoneal administration we used in our studies. However, biodistribution of bevacizumab is similar after intraperitoneal and intravenous administration Citation[25].
Regarding the effects of hyperthermic treatment, the results were interesting. First, hyperthermia did not result in a decrease in IFP during the perfusion. Second, the AUC for Pt in blood was significantly higher during hyperthermic perfusion as compared to normothermic perfusion. However, this did not result in a higher penetration of Pt in the tumour tissue. Third, in our TGD study we observed a mortality of 100% within 24 h after hyperthermic IPEC, as compared to one dead within 24 h out of 20 rats in normothermic IPEC.
The rationale for IPEC is to maximise the local concentration of oxaliplatin, and thus the penetration in the tumour tissue, while minimising the systemic uptake. Previously, it was stated that hyperthermia could improve penetration in cancer tissue and enhance cytotoxicity towards cancer cells Citation[26]. Therefore, in clinical settings IPEC is routinely administered at temperatures ranging from 41–44°C Citation[27]. Thus it was expected to find better penetration of oxaliplatin during hyperthermic perfusion. In our study we could not demonstrate better penetration into cancer tissue, while we found two adverse effects associated with hyperthermia: higher systemic concentrations of oxaliplatin and higher post-operative mortality rates. In order to measure the penetration of platinum, the tumour had to be cut out. As a result, there is only one time point where the penetration was measured: at the end of the perfusion. This is the most important time point, as the drug in the tumour at this time can still be active after the operation. However, the lack of dynamic data on the penetration of oxaliplatin is a limitation of our study.
The cause of this mortality in the TGD study could not be determined. Autopsy did not reveal any clear indications for the high death rates. In addition to the described experiments, a few rats were treated with an intraperitoneal perfusion of different hyperthermic solutions. One rat treated with hyperthermic glucose 5% solution without oxaliplatin (temperature between 41.5° and 42.5°C during the perfusion) died three hours after IPEC. Another rat, treated with hyperthermic (41.5° and 42.5°C) saline survived until it was sacrificed after three weeks. Rats treated with normothermic glucose 5% solution (temperature between 37° and 38°C during the perfusion) did survive for longer periods. As a result, our hypothesis on the cause of death is that the combination of glucose 5% solution and hyperthermia has an important causal role regarding the observed mortality. This is an important observation because glucose 5% is the standard carrier solution for intraperitoneal chemoperfusion with oxaliplatin Citation[6], Citation[28]. To our knowledge, no clinical studies comparing different carrier solutions are published. Therefore, it is not known to what extent the side effects of HIPEC are due to the carrier solution. It is also not known whether the effects of intraperitoneal perfusion with hyperthermic glucose 5% solution in our experimental setting are comparable to clinical settings. In clinical studies on HIPEC with oxaliplatin as a cytotoxic drug, the post-operative mortality rate is far from 100% Citation[6], Citation[28]. In clinical settings, patients are monitored closely during the operation, and it is possible to compensate for the effects of the treatment in case homeostasis is affected. In the experimental setting it was not possible to monitor the rats as closely as patients are. As a result, homeostasis in the rats could not be maintained because intra-operative and post-operative changes could not be detected. The administration of hyperthermia may have influenced the metabolism in the rats. This could be an explanation of the high mortality in our experiments.
Further research is required to determine the cause of the mortality in rats treated with hyperthermic IPEC. This research should focus on the effects of different carrier solutions for chemotherapy in IPEC and on the effects of temperature of the perfusate. When mortality in rats can be reduced by changing either the carrier solution or the temperature, this can be of interest for clinical practice. In clinical settings intraperitoneal chemotherapy is routinely administered at temperatures above body temperature. Our results suggest the hypothesis that hyperthermia has possibly some adverse effects in clinical settings as well. To our knowledge, there are no clinical studies comparing hyperthermic IPEC to normothermic IPEC. Several studies described survival benefits of hyperthermic IPEC as compared to standard chemotherapy in selected patients with peritoneal carcinomatosis Citation[1], Citation[3]. However, from our results it seems that hyperthermic IPEC perhaps is less suitable as compared to normothermic IPEC as it resulted in higher systemic concentration without increasing the penetration in tumour tissue and in higher death rates.
Conclusion
We conclude that combined therapy with bevacizumab and oxaliplatin does not appear to be promising for clinical implementation in the setting of IPEC. Our experiments suggest that in rats hyperthermic IPEC possibly has more adverse effects as compared to normothermic IPEC, including higher mortality rates. Several studies described longer survival after HIPEC as compared to standard chemotherapy in selected patients Citation[1], Citation[3]. As a result, HIPEC is becoming a standard treatment. To our knowledge, there are no clinical studies comparing hyperthermic IPEC to normothermic IPEC. Clinical studies where hyperthermic IPEC is not only compared to standard chemotherapy but to normothermic IPEC as well, are needed to find out whether the adverse effects of hyperthermia observed in animals in our study are also present in clinical settings.
Declaration of interest: The author thanks Ghent University for providing a special research grant. The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Armstrong DK, Wenzel L, Huang HQ, Baergen R, Lele S, Walker JL, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43
- Elias D, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009; 27: 681–685
- Glehen O, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, et al. Toward curative treatment of peritoneal carcinomatosis from non-ovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Cancer 2010; 116: 5608–5618
- Levi F, Metzger G, Massari C, Milano G. Oxaliplatin: Pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet 2000; 38: 1–21
- Chua I. Adjuvant therapy in colorectal cancer – What, when and how?. Ann Oncol 2006; 17: 1347–1359
- Frenel JS, Pouplin L, Ferron G, Rigaud DB, Bourbouloux E, Dravet F, et al. Oxaliplatin-based hyperthermic intraperitoneal chemotherapy in primary or recurrent epithelial ovarian cancer: A pilot study of 31 patients. J Surg Oncol 2011; 103: 10–16
- Cunningham D, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36–46
- Fennell DA, Shamash J, Sheaff MT, Evans MT, Goonewardene TI, Nystrom ML, et al. Phase II trial of vinorelbine and oxaliplatin as first-line therapy in malignant pleural meothelioma. Lung Cancer 2005; 47: 277–281
- Tannock IF, Tunggal JK, Cowan DSM, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res 2002; 8: 878–884
- Wijmans JG, Baker RW. The solution-diffusion model: A review. J Membr Science 1995; 107: 1–21
- Heldin C-H, Rubin K, Pietras K, Östman A. High interstitial fluid pressure – An obstacle in cancer therapy. Nat Rev Cancer 2004; 4: 806–813
- Willett CG, Boucher Y, di Tomaso E, Dudal DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–147
- Borkamo E, Fluge O, Mella O, Akslen L, Bruland O, Dahl O. Hyperthermia improves the antitumor effect of metronomic cyclophosphamide in a rat transplantable brain tumor. Radiother Oncol 2008; 86: 435–442
- Lui P, Fan Y, Xu G, Ngai C, Fung K, Tse G, et al. Apoptotic and necrotic effects of tumour necrosis factor-alpha potentiated with hyperthermia on L929 and tumour necrosis factor-alpha-resistant L929. Int J Hyperthermia 2010; 26: 556–564
- Roth M, Zhong J, Tamm M, Szilard J. Mesothelioma cells escape heat stress by upregulating Hsp40/Hsp70 expression via mitogen-activated protein kinases. J Biomed Biotech 2009; 451084
- Pih N, Sidris L, Pichette V, Drolet P, Fortier LP, Mitchell A, et al. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis. Ann Surg 2011; 254: 138–144
- Pelz J, Doerfer J, Decker M, Dimmler A, Hohenberger W, Meyer T. Hyperthermic intraperitoneal chemoperfusion (HIPEC) decreases wound strength of colonic anastomosis in a rat model. Int J Colorectal Dis 2007; 22: 941–947
- Aarts F, Bleichrodt R, Man BD, Lomme R, Boerman O, Hendriks T. The effects of adjuvant experimental radioimmunotherapy and hyperthermic intraperitoneal chemotherapy on intestinal and abdominal healing after cytoreductive surgery for peritoneal carcinomatosis in the rat. Ann Surg Oncol 2008; 15: 3299–3307
- Shimizu T, Maeta M, Koga S. Influence of local hyperthermia on the healing of small intestinal anastomoses in the rat. Br J Surg 1991; 78: 57–59
- Klaver YLB, Lomme RMLM, Rutten HJT, Bleichrodt RP, de Hingh IHJT. Hyperthermia and intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis. Ann Surg 2011; 254: 125–130
- Saxena A, Yan T, Chua T, Morris D. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2010; 17: 1291–1301
- Spector WS. Handbook of Biological Data. Saunders, Philadelphia and London 1956
- Gholap D, Verhulst J, Ceelen WP, Vanhaecke F. A combination of pneumatic nebulization and laser ablation inductively coupled plasma – Mass spectrometry (LA-ICP-MS) for studying the efficacy of intraperitoneal use of a PT-containing chemotherapeutic drug. Anal Bioanal Chem 2012; 402: 2121–2129
- Ozerdem U. Measuring interstitial fluid pressure with fiberoptic pressure transducers. Microvasc Res 2009; 77: 226–229
- Agrawal S, Tessamani J, Tang JY, Yagi Y, Fushida S, Harada S, et al. Biodistribution of humanized anti-VEGF monoclonal antibody/bevacizumab on peritoneal metastatic models with subcutaneous xenograft of gastric cancer in mice. Cancer Chemother Pharmacol 2010;66:745–753
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 2007; 23: 431–442
- Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009; 16: 2152–2165
- Quenet F, Goere D, Mehta SS, Roca L, Dumont F, Hessissen M, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 2011; 254: 294–301