Abstract
Purpose: This study aimed to investigate two readily available electrophilic reagents, acetyl chloride (AcCl), and acetic anhydride (Ac2O), for their potential in tissue ablation.
Materials and methods: Reagents were diluted in diglyme as solutions up to 8 mol/L and tested in a gel phantom with NaOH solutions and ex vivo in porcine liver. Temperature, pH, and volume measurements were obtained. Infrared and gross pathological images were obtained in bisected specimens immediately after injection.
Results: AcCl was much more reactive than Ac2O and AcCl was therefore used in the tissue studies. Temperature increases of up to 37°C were noted in vitro and 30°C in ex vivo tissues using 4 mol/L AcCl solutions. Experiments at 8 mol/L were abandoned due to the extreme reactivity at this higher concentration. A change in pH of up to 4 log units was noted with 4 mol/L solutions of AcCl with slight recovery over time. Ablated volumes were consistently higher than injected volumes.
Conclusions: Reaction of electrophiles in tissues shows promise as a new thermochemical ablation technique by means of only a single reagent. Further studies in this area are warranted.
Introduction
Hepatocellular carcinoma is a lethal disease with a grim prognosis. There are approximately as many deaths each year as there are new diagnoses (approximately 700 000) and this situation has not changed appreciably in many years Citation[1], Citation[2]. Unfortunately, 75–90% of patients present with too many tumours or too late in the course of their disease for surgery to remove a tumour and there simply are not enough organs available for transplant assuming that the patients meet accepted criteria. Progress has been slow but newer drugs, most notably sorafenib Citation[3] and improved image-guided interventions have resulted in some survival benefits Citation[4].
Currently, most non-resectable cases are treated with one of several minimally invasive methods. These have lower morbidity than surgery as there are no incisions to heal and often general anaesthesia is not necessary. They include transarterial techniques such as embolisation methods or thermal techniques such as radiofrequency (RF) ablation Citation[5]. Use of a particular method is usually guided by algorithms such as the Barcelona Clinic Liver Cancer (BCLC) staging system Citation[6]. These methods represent a shift from the historical use of percutaneous ethanol injection (PEI) or to a lesser extent percutaneous acetic acid injection (PAI) Citation[7], Citation[8].
Cost analyses are complex Citation[9], Citation[10] but must factor in the capital expense for a generator unit and the probes for hyperthermic methods, typically upwards of US$2000 for each disposable probe. Alternatively, transarterial methods typically involve the cost of angiography, embolic agents, drugs, and usually overnight hospital care. Both general categories also depend on the number of procedures. Chemical ablation with ethanol is simple, inexpensive, and can be performed with local anaesthesia on an outpatient basis. It is therefore more available but usually requires several sessions to completely treat a lesion. More recently, a multi-tined needle has been employed with larger doses in a single setting with good results Citation[11], Citation[12]. Local recurrence rates are historically somewhat higher with PEI than with RF ablation but differences in survival are less clear Citation[13]. Given the global disparity in distribution of medical resources for this widespread disease and the low cost of chemical methods, it is of interest to investigate ways to achieve a better result through improving on chemical ablation methods.
Harnessing the energy released from exothermic chemical reactions is an area that has recently been reported with encouraging results both in vitro and in vivo Citation[14–16]. Previous work involved either oxidation–reduction chemistry or acid–base neutralisation chemistry Citation[17–20]. However, an alternative source of energy could be realised through the hydrolysis of electrophiles. This would in principle heat tissue and could at the same time generate acid in the process. Therefore, we investigated and report here our results with two readily available electrophilic reagents, acetyl chloride (AcCl) and acetic anhydride (Ac2O), for their potential in tissue ablation.
Materials and methods
General
Porcine livers were brought to room temperature prior to use. All reagents were obtained from Sigma-Aldrich (Milwaukee, WI, USA) and used without further purification unless otherwise specified. Electrophiles were diluted to the appropriate concentrations in dry diglyme.
In vitro temperature measurements
The electrophiles (1–8 mol/L in diglyme) were reacted with equal volumes of equal concentration solutions of NaOH in a gel phantom as previously reported Citation[21], Citation[22]. Diglyme controls were tested for heats of solution of the reagents without added aqueous base. Triplicate runs of 200 µL NaOH to form a contained, rounded, aqueous reaction chamber in gel were followed by 200 µL injection of electrophile solutions over 3–5 s into the same solutions. The temperature was recorded every 15 s for 5 min by a probe positioned 5 mm from the needle tip. Averages for each experiment were reported.
Ex vivo temperature and pH measurements
The injection of the AcCl solution was done with the needle inserted near the periphery of the livers and approximately 2 cm deep into the liver tissue. Injections were performed via a 20-gauge end-hole needle with a 1 or 3 mL syringe at a rate of 1 mL/min using total volumes of 250–2000 µL with concentrations in increments from 1–8 mol/L. A 3-cm 23-gauge Type T thermocouple temperature probe (Physitemp Instruments, Clifton, NJ, USA) was placed blindly in parallel into the tissue as near as possible to the injection site in the same plane and to the same depth, and temperature was measured using a digital thermocouple thermometer (Digi-Sense® DualLogR™, Cole-Parmer, Vernon Hills, IL, USA). For pH measurements an equilibrated and buffer-calibrated FlexipHet pH electrode (Oakton Instruments, Vernon Hills) was used. Immediately after sectioning the tissue, the centre slice was laid out and the pH probe was lowered onto the tissue in a perpendicular fashion until contact with the treated area was made. The probe was located within 5 mm of the centre of the lesion and was held in place with a clamp to minimise motion. The electrode was used to measure pH of 750 µL injections with measurements taken at 1 min intervals for 15 min using concentrations of reagent ranging from 1–4 mol/L. Injection attempts at higher concentrations were abandoned due to the pronounced reactivity of the reagent.
Infrared imaging
An infrared camera (IRI 4010, Infrared Integrated Systems, Northampton, UK, www.irisys.co.uk) was used to take surface temperature images of selected injections repeated for the purpose of imaging. The IR camera was used to image temperatures of 2D cross sections by bisecting specimens along the needle tract immediately after injection and imaging the sample as soon as feasible thereafter, generally within 10 s or less. A warmed reference object (a quarter dollar coin, 24 mm diameter) was positioned in the image at the same focal distance from the camera to facilitate accurate thermal image size measurements.
Calculation of coagulation volumes
At the conclusion of each experiment, the tissues were cut into 3-mm thick slices and placed in sequence with identifying information in the field for digital photography. Images were processed with ImageJ (freely available from the US National Institutes of Health) and Adobe Photoshop CS2 (Mountain View, CA, USA) software to calculate a surface area map for each slice and summated for the total volume of a given lesion.
Statistics
ImageJ was used to analyse the cross sectional area and width of tissue coagulation, with results imported into Microsoft Excel (Redmond, WA, USA). Using the imported area and width the coagulation volumes were calculated in Excel.
Temperature and pH data were plotted as a function of the gross tissue coagulation volume data in a grid normalising the data with respect to the moles of reagent used, profile over time, and total injection volume.
Results
In vitro
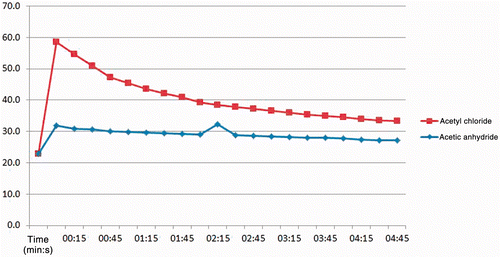
A rapid temperature rise in vitro of 37°C was noted within just a few seconds when a 5 mol/L AcCl solution was reacted with an equal amount and volume of NaOH solution (). When using a higher concentration of base (twice the amount of reagent in the same volume) a temperature rise of 26°C was observed which peaked within 150 s (data not shown). A much smaller temperature change was observed with the same concentration of Ac2O.
Figure 1. Graph of results from gel phantom experiments reacting either AcCl or Ac2O solutions at 5 mol/L with NaOH solutions of equal concentrations. A thermocouple was placed in the reaction chamber to record the degree and duration of the temperature excursions as the reactions took place. Subsequent experiments were performed with AcCl due to the lower reactivity of Ac2O.
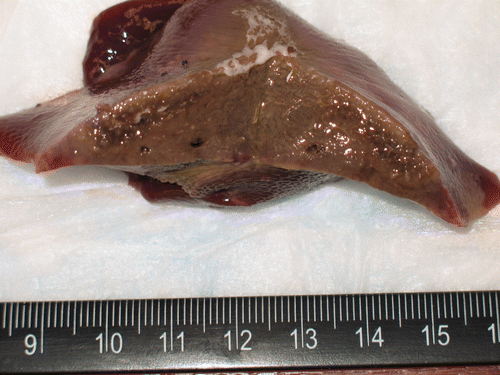
Ex vivo
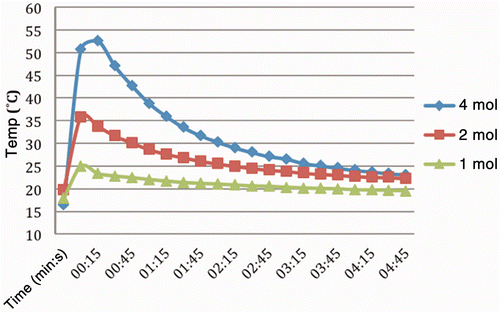
The results of the AcCl injections in tissues, presented according to temperature by molarity are summarised in . Temperature changes of 4.5–54.6°C were obtained. The trials produced average temperature changes of 8.9, 12.7, and 30.3°C increases for 1, 2, and 4 mol/L solutions respectively. Although 8 mol/L solution trials were attempted, data were not collected due to the extremely high reactivity in the liver tissue. Specifically the injections boiled vigorously and erupted through the surface of the liver. These increased temperatures decayed gradually upon completion of the injections, approaching baseline within the 5-min observation period.
Figure 2. Temperature changes recorded over time from reaction of AcCl solutions in liver tissue with thermocouple inserted immediately adjacent and to the same depth. Concentrations ranged from 1–4 mol/L in diglyme as the solvent. Injection rate was 1 mL/min with total volume in each case of 1 mL of solution. Peak temperature using the 4 mol/L solution was 54°C, or an increase of 30°C within seconds after initiating the injection. Experiments at higher concentrations were abandoned due to the pronounced reactivity of the reagent.
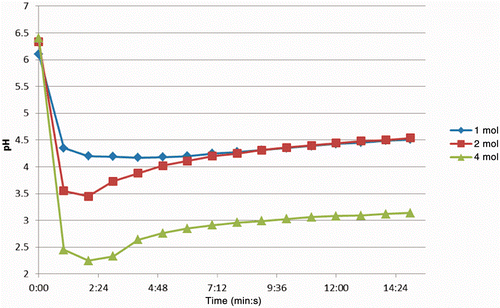
The baseline pH of the liver tissue ranged from 6.1–6.4 and the lowest observed pH of the 1, 2 and 4 mol/L injections was 4.17, 3.45 and 2.25 respectively ().
Figure 3. Change in pH in tissues over time, sectioned after injection of 750 µL solutions of AcCl (concentration range1–4 mol/L). Note in each case the initial drop is followed by a recovery to some degree. The maximum decrease was measured at 2.25, which over time rose to just above 3.
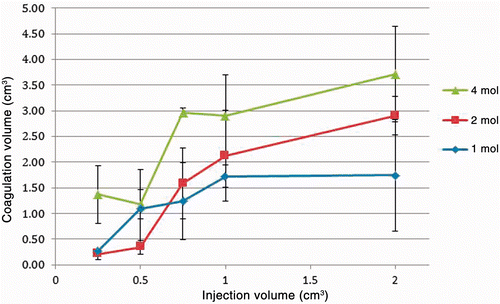
Coagulation volumes from the acetyl chloride injections are summarised in as a function of injection volume. The tissue coagulation volumes exceeded that of the injection volumes in all cases, most notably for a 750 µL injection of 4 mol/L AcCl. In this case an average of 2.96 mL (±0.10 mL) coagulation was achieved.
Figure 4. Relationship of coagulation volume to injection volume and concentration with AcCl solutions. The injected volume ranged from 250–2000 µL at concentrations of 1–4 mol/L. There is some variation but in general the coagulation volume increases as both variables (injection volume and concentration) are increased.
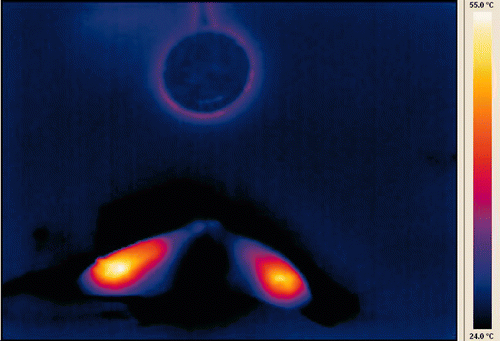
Infrared imaging of a specimen bisected immediately after injection () shows that background parenchyma is at or below the low end of the temperature scale at 24°C. In contrast, the area of the injection is considerably hotter, with the central portion at approximately 55°C. In the corresponding photograph of the gross specimen (), it is apparent that the tan area of coagulated tissue is quite extensive.
Figure 5. Infrared image captured immediately after completion of injection and bisection of specimen. Concentration was 4 mol/L with injection volume of 750 µL. Baseline tissue temperature is below 24°C and central portion of injection is at the peak of the scale, 55°C. A warmed US quarter dollar coin (24 mm diameter) was included in the same focal plane to serve as a size reference.
Discussion
The most common agents in chemical ablation are 50% acetic acid and absolute ethanol Citation[8], Citation[23]. The toxicities in each case limit the amount that can be injected at any one session. In the case of ethanol special precautions may be taken to increase metabolism of ethanol Citation[11]. Acetic acid is reported to dissolve and penetrate through collagen and other components of tumour septa somewhat better than ethanol and therefore less is needed to ablate a specified volume. However, this greater effect is offset by greater toxicity, such that the practical result in most cases is that there is no difference in the treatable volume in one session between the two agents or number of procedures required Citation[24], Citation[25].
Exothermic chemistry applied to tumour ablation is a new field with untapped potential Citation[18], Citation[20], Citation[22]. Use of appropriate chemistry as a heat source rather than deposition of electromagnetic energy into tissues would be attractive because of possible advantages in safety, effectiveness, lesion shape predictability, and especially cost and availability. Further benefit from selected agents, initially chosen for their propensity to release heat, could be derived if the reaction product(s) also had local cytotoxic effects. Such agents could be chosen for favourable profiles that would minimise systemic toxicity. In addition, if a single exothermic agent could be identified, it would simplify matters compared to previously reported acid/base chemistry. This is because a single agent would be simpler to handle than two reagents, and it would also remove the need for a dedicated device to control mixing of two reactants. We thus became interested in reagents that would react in the tissues to release heat energy and at the same time create a denaturing environment. Based on these requirements, we chose to study two electrophiles, Ac2O and acetyl chloride AcCl, as test cases.
It became obvious early on due to the reactive nature of the electrophiles, that an appropriate solvent was needed to dilute the reagents and provide some measure of control. Diglyme, the solvent selected, is a short chain polyether related to polyethylene glycol (PEG). It is one of a class of versatile polyether compounds used as brake fluids, ink bases, dry cleaning solvents, protective coatings, large scale industrial solvents, detergents, cosmetics, and drug adjuvants (PEGylation). It was chosen as the dilution solvent for these experiments primarily because it is chemically inert with respect to the reagents and because of a lack of appreciable toxicity. Other desirable characteristics were the combination of miscibility with water, low viscosity, low vapour pressure and high boiling point.
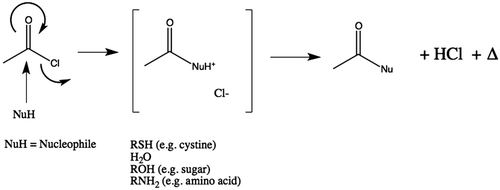
Initial work confirmed that Ac2O was not as reactive or energetic and that AcCl was both more predictable in its reactivity and released more heat energy for ablation. This difference in reagents is not altogether unexpected as it is known that chloride is a better leaving group than acetate. This led us to focus on AcCl for subsequent evaluation. Further, the secondary hydrolysis by-product from AcCl with water is HCl () rather than an additional equivalent of acetic acid as would be seen with Ac2O. The stronger acid HCl would be expected to have more of an ablative effect than acetic acid in ablation due to the lower pH (i.e. more available hydrogen ions) of HCl.
Figure 7. Generalised reaction scheme illustrating release of heat during hydrolysis of AcCl and generation of acetic acid. Where the nucleophile (Nu) is water, two equivalents of acid are produced (one each of acetic acid and hydrochloric acid). In those cases where reagent could react with tissue instead of water, only one equivalent of acetic acid would be produced. However, in those cases this implies that covalent modification of the substrate has occurred.
In tissues, full strength acetyl chloride was noted to cause cavitation when used beyond anything other than very small volumes. After the initial tests with pure AcCl proved too reactive, further experiments were conducted with fresh dilute solutions. Even experiments at 8 mol/L were too reactive to provide meaningful results and were not pursued further.
In reacting with the local tissue, AcCl causes tissue coagulation upon injection into liver by both thermal and chemical effects. First the heat of reaction of AcCl with a nucleophile has been shown to be strongly exothermic. This results in a transient temperature change comparable to that of radio frequency ablation and other thermal ablative methods. As this is happening, AcCl may also cause chemical modification by acetylation of any available nearby nucleophilic molecules in the tissues. Although water is the most abundant it is not the most reactive nucleophilic species present. Nevertheless, when water does react with AcCl, both acetic acid and hydrochloric acid (HCl) are produced from one equivalent of AcCl. These are known to cause denaturation of the tissue even in the absence of any added heat energy. The effects of heat and pH change would be expected to reinforce one another, as is the case when proteins are studied under similar conditions. However, the relative contributions of each aspect in the coagulation are unknown, and it must be noted that the temperature excursion is relatively short in comparison to the pH change. Although the duration of the temperature rise is fairly brief, it does persist longer than the injection. Once the reagent is consumed and no further heat energy is released, the temperature declines as expected. Questions about the interaction of these two factors will be the subject of future studies.
Measuring the temperature and pH near the injection point provided insight into the distribution, temperature change, and localised acidification due to the reaction of AcCl with tissues. Unlike acid–base neutralisation chemistry, however, the thermodynamics of these electrophile hydrolysis experiments in tissue are difficult if not impossible to calculate. Theoretical models of even the simplest cases in the gas phase provide meaningless results regarding the enthalpy of the reaction. Experimental data suggest that the heat of hydrolysis is about −95 kJ/Mole Citation[26]. To put this in some context, it is approaching twice the exotherm from a strong acid reacting with a strong base, and approximately three times the exotherm from the reaction of a weak acid and weak base or from the hydrolysis of ATP. Adding to the complexity is the unknown number of reactants in tissue (e.g. proteins, carbohydrates), their ratios, and exact type of bonds formed (e.g. esters, amides, thioamides). Any calculations of the energetics are thus far from satisfactory. Regardless, the data demonstrate that the exotherm derived from the hydrolysis was quite dramatic.
The intrinsic reserve capacity of the tissue to resist a change in acidity can be appreciated by observing the change of pH over time for the three injection concentrations. After an initial drop in pH following the injection of AcCl, a gradual, slight increase in pH over the next 15 min was noted that began to plateau. The extent of the pH excursion was dependent on the amount of acid formed in the reaction, and this in turn depended on the concentration and volume of the injected reagent. We believe that the slight rise in pH over time reflects the gradual consumption of some portion of the acid. This observation, first reported by Rundback et al., was attributed to the buffering capacity in the tissues as the material diffused through the parenchyma Citation[27].
There were several limitations in this report that reflect the early stage and nature of the work. The use of ex vivo tissue as a model, as is commonly used with RF ablation, meant that perfusion effects were absent and tissue viability studies were not applicable. Live tissue studies may show differences in response due to heat-sink effects, reactive vasoconstriction, and any capillary flow that may occur prior to tissue coagulation, for example. Marginal zones with some viable cells could recover to an unknown extent if stress response mechanisms (e.g. efflux pumps, heat shock proteins) are not overwhelmed. Correlating the coagulation volumes with the volume and concentration data from each experiment allows for some means to assess the effectiveness of the injections. However, it does not necessarily follow that the most concentrated solution will coagulate the greatest amount of tissue. Indeed, pure AcCl was so reactive as to quickly destroy tissue and erupt from the surface. Also, normal tissues were used rather than tumour in a cirrhotic background, as seen in HCC. Finally, although simple and available, an end-hole needle is not optimal for this type of therapy. The use of a multiple tine/multiple side-hole device would be expected to improve the results Citation[28], Citation[29] provided it was stable to the conditions used.
In conclusion, reaction of electrophiles in tissues shows promise as a new thermochemical ablation technique by means of only a single reagent. The temperature changes, pH changes and coagulation volumes observed in ex vivo liver show potential in particular for the use of AcCl in thermochemical ablation. This is notable considering both the small volume and relatively low concentration of acetyl chloride used relative to the corresponding coagulation volumes and temperature changes observed. Further studies are warranted.
Acknowledgements
We are most grateful to Paul Iaizzo for the use of his infrared camera and for ex vivo porcine liver specimens. Theresa Brix Edelman provided assistance in procuring reagents and tissue samples and Kyle Pfeifer and Kevin Turner for assistance in data collection.
Declaration of interest: D.A.J. would like to thank the SIR Foundation for a summer research award and for a Cope Scholar award. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–236
- Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): A global perspective. J Gastrointestin Liver Dis 2010; 19: 311–317
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. The Lancet 2002; 359: 1734–1739
- Lencioni R. Loco-regional treatment of hepatocellular carcinoma in the era of molecular targeted therapies. Oncology 2010; 78: S107–112
- Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: A multicentre, cohort study. Lancet Oncol 2011; 12: 654–662
- Livraghi T, Benedini V, Lazzaroni S, Meloni F, Torzilli G, Vettori C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer 1998; 83: 48–57
- Clark TW, Soulen MC. Chemical ablation of hepatocellular carcinoma. J Vasc Interv Radiol 2002; 13: S245–S252
- Ray CE, Jr, Battaglia C, Libby AM, Prochazka A, Xu S, Funaki B. Interventional radiologic treatment of hepatocellular carcinoma – A cost analysis from the payer perspective. J Vasc Interv Radiol 2012; 23: 306–314
- Ansari D, Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J Gastroenterol 2012; 18: 1003–1008
- Ho CS, Kachura JR, Gallinger S, Grant D, Greig P, McGilvray I, et al. Percutaneous ethanol injection of unresectable medium-to-large-sized hepatomas using a multipronged needle: Efficacy and safety. Cardiovasc Intervent Radiol 2007; 30: 241–247
- Lencioni R, Crocetti L, Cioni D, Pina CD, Oliveri F, De Simone P, et al. Single-session percutaneous ethanol ablation of early-stage hepatocellular carcinoma with a multipronged injection needle: Results of a pilot clinical study. J Vasc Interv Radiol 2010; 21: 1533–1538
- Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol 2008; 43: 727–735
- Cressman EN, Tseng HJ, Talaie R, Henderson BM. A new heat source for thermochemical ablation based on redox chemistry: Initial studies using permanganate. Int J Hyperthermia 2010; 26: 327–337
- Cressman ENK, Geeslin MG, Shenoi MM, Hennings LJ, Zhang Y, Iaizzo PA, et al. Concentration and volume effects in thermochemical ablation in vivo: Results in a porcine model. Int J Hyperthermia 2012; 28: 113–121
- Cressman ENK, Shenoi MM, Edelman TL, Geeslin MG, Hennings LJ, Zhang Y, et al. In vivo comparison of simultaneous versus sequential injection technique for thermochemical ablation in a porcine model. Int J Hyperthermia 2012; 28: 105–112
- Rao W, Liu J. Injectable liquid alkali alloy-based tumor thermal ablation therapy. Minim Invasive Ther Allied Technol 2009; 18: 30–35
- Rao W, Liu J. Tumor thermal ablation therapy using alkali metals as powerful self-heating seeds. Minim Invasive Ther Allied Technol 2008; 17: 43–49
- Rao W, Liu J, Zhou YX, Yang Y, Zhang H. Anti-tumor effect of sodium-induced thermochemical ablation therapy. Int J Hyperthermia 2008; 24: 675–681
- Deng ZS, Liu J. Minimally invasive thermotherapy method for tumor treatment based on an exothermic chemical reaction. Minim Invasive Ther Allied Technol 2007; 16: 341–346
- Freeman LA, Anwer B, Brady RP, Smith BC, Edelman TL, Misselt AJ, et al. In vitro thermal profile suitability assessment of acids and bases for thermochemical ablation: Underlying principles. J Vasc Interv Radiol 2010; 21: 381–385
- Misselt AJ, Edelman TL, Choi JH, Bischof JC, Cressman EN. A hydrophobic gel phantom for study of thermochemical ablation: Initial results using a weak acid and weak base. J Vasc Interv Radiol 2009; 20: 1352–1358
- Clark TW. Chemical ablation of liver cancer. Tech Vasc Interv Radiol 2007; 10: 58–63
- Kulik LM, Mulcahy MF, Omary RA, Salem R. Emerging approaches in hepatocellular carcinoma. J Clin Gastroenterol 2007; 41: 839–854
- de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol 2012; 56: S75–87
- Devore JA, O’Neal HE. Heats of formation of the acetyl halides and of the acetyl radical. J Phys Chem 1969; 73: 2644–2648
- Rundback JH, Hwang D, Susman J, Haskal ZJ, Weintraub JL. pH diffusion of acetic acid in an ex-vivo calf liver model. Paper presented at the Annual Scientific Meeting, Society of Interventional Radiology. Phoenix, AZ, USA 2004
- Sudheendra D, Léger R, Groppo ER, Sun D, Durrani AK, Neeman Z, et al. Comparison of three different needles for percutaneous injections. Cardiovasc Intervent Radiol 2007; 30: 151–152
- Hines-Peralta A, Liu Z, Horkan C, Solazzo S, Goldberg SN. Chemical tumor ablation with use of a novel multiple-tine infusion system in a canine sarcoma model. J Vasc Intervent Radiol 2006; 17: 351–358