Abstract
Tumour-derived chaperone-rich cell lysate (CRCL) when isolated from tumour tissue or when embedded with peptide antigens is a potent anti-cancer vaccine consisting of numerous chaperone/heat shock proteins, including the highly immunogenic Hsp70, Hsp90, glucose regulated protein 94, and calreticulin. We have previously documented that CRCL provides both a source of tumour antigens and danger signals triggering antigen presenting cell activation. In this report we describe the ‘peptidome’ of potential antigens extracted from CRCL prepared from a murine tumour. Using mass spectrometry techniques we identify almost 60 different proteins of origin for the CRCL peptides; we determine that the parental proteins come from essentially all parts of the cell, and are involved in a broad range of functions. Further in silico analysis demonstrates that the parental proteins are components of major signalling networks of vital importance for cancer cell survival, proliferation, and migration. In many instances the peptides identified possess amino acid sequences that would allow their putative binding and display by murine major histocompatibility complex class I and II molecules, and there are also predicted binding motifs for Hsp70-type chaperones. By mixing fractionated pools of peptides with antigen-free (normal liver) CRCL, we were able to reconstitute effective anti-tumour activity of the vaccine, showing that the peptides are indeed the major purveyors of CRCL vaccines’ efficacy.
Introduction
Immunotherapy continues to gain traction as a cancer treatment modality [Citation1, Citation2] and anti-cancer vaccination shows promise in the repertoire of immunotherapeutic options [Citation3]. Autologous single heat shock protein/chaperone protein vaccines have reached stage II and III clinical trials [Citation4–Citation6] and HSPPC-96/Oncophage/vitespen (the endoplasmic reticulum chaperone GRP94) has been approved in Russia for treatment of renal cancer in patients at intermediate disease recurrence risk [Citation7]. Effective vaccines require both adjuvant and antigen for sufficient and specific stimulation of immune responses capable of rejecting residual tumour cells. Chaperone proteins/heat shock proteins (HSPs) are widely regarded as potent ‘danger signals’, activating innate immune cells and antigen presenting cells (APCs) [Citation8–Citation10]. The proposed mechanism by which such vaccines generate tumour-specific immunity is through the delivery of tumour-associated peptide antigens to professional APCs, accompanied by innate stimulation of APCs to yield higher activation states of tumour-specific T cells [Citation11–Citation15]. While the stimulatory capacity of chaperones/HSPs is generally accepted, there has been considerable controversy over the nature of the antigenic peptides presumably associated with the tumour-derived chaperone protein vaccines [Citation16–Citation19], with some thought that there may even be no peptides specifically associated with chaperones/HSPs [Citation20, Citation21]. Obviously, this raises numerous questions about the mechanisms responsible for the efficacy of chaperone protein vaccines. Demonstration of such peptides (or lack thereof) in the autologous, patient tumour-derived GRP94 vaccines even became a significant regulatory agency concern in Europe [Citation22]. Clearly, biochemical evidence of tumour peptides in chaperone protein-based vaccines will be necessary to accompany the immunological responses these vaccines generate.
We have devised a multiple chaperone protein vaccine by employing free solution isoelectric focusing (FS-IEF) to enrich for chaperone-peptide complexes which we have called chaperone-rich cell lysate (CRCL) [Citation23–Citation26]. This vaccine has proven very effective at reducing tumour burden, prolonging survival, and even promoting cures in numerous murine tumour models [Citation24–Citation28] as well as in in vitro immune assays with human cells and clinical tumour samples [Citation29, Citation30]. In a relevant clinical setting we have used autologous CRCL to treat a canine with a grade 3 metastatic lung tumour [31]. In a murine model of BCR-ABL(+) chronic myelogenous leukaemia (CML) we have demonstrated immunologically the presence of BCR-ABL fusion peptides [Citation32], and we have also shown that antigenic peptides may be embedded into the vaccine with relevant immunological activity [Citation30, Citation33]. While these studies bode well for the peptide-carrying capacity of CRCL, we have not previously identified specific peptides that are naturally associated with the chaperone complex as derived from a tumour. Given the aforementioned controversy, as well as the need for antigen identification both as validation of a product’s vaccine potential and as targets for immune monitoring, we have undertaken a ‘peptidomic’ approach using mass spectrometry to identify almost 60 peptides from the murine leukaemia 12B1 CRCL. These peptides originate from proteins that are representative of all the major organelles within a cell, and the proteins of origin encompass a wide range of ascribed functions and roles. The proteins are functionally integrated into important networks related to cancer cell proliferation, early development and survival, and they encompass critical signalling pathways. Many of the peptides are predicted as presentable by (murine) H2Kd and I-Ad major histocompatibility molecules, and some of the peptides possess sequences with putative Hsp70 binding domains. Particular pooled fractions, when combined with liver CRCL (i.e. a tumour antigen-free platform), were able to reconstitute anti-tumour vaccine activity. These data indicate that CRCL contains a wide array of potentially antigenic peptides that are responsible for the vaccine’s specific anti-tumour activity, and that the antigens are importantly linked in networks essential to tumour biology.
Materials and methods
12B1 BCR/ABL leukaemia cell line
12B1 is a murine leukaemia line (BALB/c strain) expressing human BCR-ABL protein, and has been described previously [Citation34]. This is a very aggressive, non-immunogenic leukaemia resembling blast phase of chronic myelogenous leukaemia, despite the presence of the xenogenic p210 BCR-ABL protein. Inoculation with as few as 100 cells intravenously or 1000 cells subcutaneously results in uniform lethality within 25 days due to disseminated disease.
Tumour generation
All tissue/cell culture reagents were purchased from Invitrogen (Gaithersburg, MD, USA). 12B1 cells were cultured at 37°C in 5% CO2 in RPMI medium containing 10% heat-inactivated foetal calf serum and supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin sulphate, 0.025 μg/mL amphotericin B, 0.5 × minimal essential medium non-essential amino acids, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol. Cells were prepared for injection by washing and resuspending in Hanks balanced salt solution. The cells were counted and adjusted to a concentration of 25 × 106 cells/mL. Female BALB/c mice were injected with 0.2 mL (5 × 106) 12B1cells, subcutaneously in both flanks and were monitored for tumour development. Tumours more than 1 cm in diameter were harvested from euthanised mice. In vivo passaging of tumours involved harvesting and mincing the tumour to produce a cell suspension. The cell suspension was filtered through a cell strainer (BD Biosciences Discovery Labware, Bedford, MA, USA) to remove debris and was then centrifuged. The cell pellet was resuspended, washed, counted, and injected into mice.
Preparation of chaperone-rich cell lysate
12B1 tumours were used in the making of tumour-derived CRCL. Normal livers were harvested from BALB/c mice for the preparation of (normal tissue-derived) liver CRCL. FS-IEF enrichment of tumour and normal tissue-derived CRCL was performed as described previously [Citation24]. Briefly, tumour or liver tissues were homogenised in detergent-containing buffers, and high-speed supernatants were obtained. The dialysed supernatants were mixed with detergents and conjugate acid–base pairs (for pH gradient establishment), and the solution was made to 6 M urea. The mixture was subjected to FS-IEF in a Rotofor device (Bio-Rad Laboratories, Hercules, CA, USA) at 15 W constant power, and fractions were harvested and analysed for chaperone content (Hsp70, Hsp90, GRP94, and calreticulin). Overlapping fractions containing each of the above chaperones were pooled and prepared as vaccines by dialysis, detergent removal, and centrifugal concentration. Vaccines were tested for endotoxin with the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA) and were found to contain less than 0.01 EU/µg protein [Citation25]. Liver CRCL was prepared as described by Kislin et al. [Citation33].
Peptide stripping from CRCL and reverse phase high pressure liquid chromatography of peptide fractions
Reverse-phase high pressure liquid chromatography (rpHPLC) fractionation of 12B1 CRCL eluted peptides was performed as described previously [Citation32]. Peptides were stripped from CRCL by procedures similar to those used to elute peptides from major histocompatibility complex (MHC) molecules [Citation35, Citation36]. CRCL (3–10 mg) was acidified by addition of trifluoroacetic acid (TFA) (all HPLC-grade solvents were from Mallinkrodt Baker, Phillipsburg, NJ, HPLC grade) to 0.2% and left on ice for 1 h. Peptides dissociated from CRCL were harvested by centrifugation through a Centricon-3 device (3-kDa cut-off membrane; Millipore, Billerica, MA). The Centricon device was pre-cleared prior to addition of peptides by passage of 0.2% TFA in phosphate-buffered saline (PBS) through the membrane. One mL HPLC-grade water was added to the retentate, and was centrifuged through the membrane. The initial flow-through material was pooled with the water-wash flow-through, and the combined materials were vacuum evaporated (SpeedVac, Thermo Savant, Farmingdale, NY, USA). The peptides were resuspended in 0.1% TFA in HPLC-grade water (TFA/H2O, solvent A) and chromatographed over a YMC ODS-A S5 C18 reverse-phase column, 3.0 × 250 mm (5 μm beads, 120-Å (12 nm) pore size, Waters, Milford, MA). The chromatography was performed using a WatersAlliance 2690 separation module equipped with a PDA 996 photodiode array detector (Waters). The column was run at a flow rate of 0.4 mL/minute with a solvent gradient of 0–30% solvent B (0.1% TFA in HPLC-grade acetonitrile) over 30 min, then to 100% B by 40 min. Fractions (0.4 mL) were collected each minute, and eluent was monitored from 190 nm to 400 nm, with results shown for 214-nm wavelength. Between samples, the column was extensively washed with solvent B before returning to equilibration in solvent A. Fractions were dried (SpeedVac), resuspended in 0.1% TFA in water, and fractions were pooled (1–6, 7–13, 14–20, 21–26, 27–33, 34–40) before mass spectrometry.
Nanoflow high performance liquid chromatography-tandem mass spectrometry
A microbore HPLC system (Surveyor, Thermo Finnigan, San Jose, CA, USA) was modified to operate at capillary flow rates using a simple T-piece flow splitter. Columns (6 cm × 100 mm internal diameter) were prepared by packing 100 Å, 5 μm Zorbax C18 resin at 500 psi pressure into columns with integrated electrospray tips made from fused silica, pulled to a 5 mm tip using a laser puller (Sutter, Novato, CA). Peptides were eluted in a gradient using buffer A (5% v/v ACN, 0.1% formic acid) and buffer B (90% v/v ACN, 0.1% formic acid), at a flow rate of 400 nL/min. Following an initial wash with buffer A for 10 min, peptides were eluted with a linear gradient from 0 to 100% buffer B over a 30 min interval. Samples were introduced onto the analytical column using a Surveyor autosampler. The HPLC column eluent was eluted directly into the ESI source of a Thermo Finnigan LCQ-Deca XP Plus IT mass spectrometer. Spectra were scanned over the range 400–1500 mass units. Automated peak recognition, dynamic exclusion, and daughter ion scanning of the top three most intense ions were performed using the Xcalibur software as previously described [Citation37].
Database searching and data interpretation
Raw MS/MS data were converted to .dta files for each set of spectra acquired using Xcalibur Bioworks Cluster version 3.1 (Thermo Finnigan) with the following parameters: peptide molecular weight range, 500–3500; threshold, 1000; precursor mass tolerance, 1.4; group scan = 1; minimum group count, 1; minimum ion count, 25; fragment ion tolerance = 0; no charge state determination applied. MS/MS spectral data were analysed using SEQUEST (Bioworks Cluster version 3.1, Thermo Finnigan). Since the peptides isolated here came already pre-processed, no enzyme specificity was used during the searching process. Parent and fragment masses were both considered as average values. In this work, the criteria for a preliminary positive peptide identification for a singly charged peptide were a correlation factor (XCorr) greater than 1.8, a δ cross-correlation factor (δCn) greater than 0.08 (indicating a significant difference between the best match reported and the next best match), and a high preliminary scoring. For doubly charged peptides the correlation factor threshold was greater than 2.5, and for triply charged peptides the correlation factor threshold was greater than 3.5. At least one spectrum meeting these criteria from each identified protein was visually examined to confirm the presence of a strong Y- or B-ion series that could be matched to the assigned sequence for at least four amino acids. Peptide (protein) identities were determined by searches against NCBI and UniProt databases.
Peptide/protein subcellular localisations and functions were determined from literature searches and Ingenuity Systems software (Redwood City, CA). Pathway analyses and network constructions were assembled using the Ingenuity software. Peptides were screened for potential binding to MHC-I molecules in the BALB/c strain (H2Kd) using NetMHC (3.2 server) (cbs.dtu.dk/services/NetMHC/), SYFPEITHI, and the Bioinformatics and Molecular Analysis Section (BIMAS) HLA peptide binding predictions site (bimas.cit.nih.gov/molbio/hla_bind/). ‘Model’ comparator peptides SYFPEITHI and GFKQSSKAL (from the fusion junction of the BCR-ABL oncoprotein) were used as predicted binders. For MHC class II binders, we used the RANKPEP program (imed.med.ucm.es/Tools/rankpep.html) to query peptide (15-mer) affinities for I-Ad molecules. We used the LIMBO algorithm analysis (limbo.switchlab.org/limbo-analysis) to predict Hsp70 (DnaK) chaperone binding of the peptides.
Peptide incorporation into chaperone-rich cell lysate by simple mixing
12B1 CRCL peptides that had been stripped from the vaccine were fractionated by rpHPLC as described above. In these experiments, peptide fractions were combined into four pools (i.e. fractions 1–10, 11–20, 21–30, 31–40) as before [Citation32]. The fractions were dried (SpeedVac), resuspended in tissue culture-grade water, and dried again [Citation32]. Fractions were resuspended in 25 µL PBS and were mixed with liver CRCL (25 µL peptide + 75 µL liver CRCL [25 µg CRCL protein]) and incubated at room temperature for 40 min. The peptide/CRCL solution was centrifuged at 9.5 × 103 rpm via a 10 kDa cut-off membrane filter (Vivaspin 500, ISCBioexpress, Kaysville, UT). The retentate was then washed three times with PBS by centrifugation through the filter and then collected for use.
Animal studies
Female BALB/c (H2Kd) mice (National Cancer Institute, Frederick, MD) 6- to 10-weeks old were used for the experiments. The animals were housed in a dedicated pathogen-free facility and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines. Following 12B1 tumour injection on Day 0, mice (four per group) were given subcutaneous injections (groin opposite the tumour site) with ‘empty’ liver CRCL (25 µg/injection in 100 µL of PBS), or with liver CRCL + fractionated peptides (CRCL + fractions 1–10, 11–20, 21–30, or 31–40). One group’s vaccine consisted of all the peptide fractions reconstituted with liver CRCL. Tumour growth was monitored as described [Citation24]. These experiments were performed twice.
Statistics
Tumour growth experiments were analysed by analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison tests (SPSS 20, Chicago, IL), where p < 0.05 was chosen as significant. Error bars depict standard error of the mean. Statistics used for IPA can be found at ingenuity.com.
Results
CRCL vaccines prepared from a murine leukaemia carry a wide array of peptides
We prepared 12B1 CRCL vaccines as previously described [Citation23, Citation24] using FS-IEF with a Rotofor device. We analysed the fractions by gel electrophoresis and western blotting for the chaperones GRP94, Hsp90, Hsp70, and calreticulin (). Fractions of the appropriate pH range (pH 5.1–6.2) and possessing all four of the chaperones were pooled and prepared as vaccine product [Citation23–Citation25, Citation32]. The vaccine was then ‘stripped’ of peptides using acid treatment similar to previous work isolating peptides from MHC complexes [Citation35, Citation36] and as we have done before [Citation32]. Peptides were separated from the bulk of the denatured proteins by centrifugation through a 3-kDa cut-off membrane (), were harvested as described in Methods above, and separated by rpHPLC (). Fractions were pooled (six pools in total) and subjected to mass spectrometry (MALDI-MS/MS) for sequence determination and protein identification. The peptides identified are listed in Supplementary Table I, including which fractions/pools contained the peptides and the actual sequences. Also in Supplementary Table I are listings of the systematic (unique) gene/protein names, subcellular localisations, and consensus functions. Notable among those data are the size distributions of the peptides (ranging from 8- to > 30-mers, the latter being near the size limit of the 3-kDa cut-off membrane), the intracellular distribution of the peptides’ proteins of origin (essentially every cellular compartment, as well as extracellular entities), and the functions of those proteins (broad functional activities). These are recapitulated in and . At least 15 of the proteins have been previously identified as antigens in tumour and autoimmune settings. Those include ARHGDIB [Citation38], CALR [Citation39], CCNB1 [Citation40], GB5A [Citation41], HspA1B [Citation42], HspA4 [Citation43], HspB1 and TPI1 [Citation44], LAMA1 [Citation45], LMNA [Citation46], NKX2-1 [Citation47], RARA [Citation48], STK31 [Citation49], TPD52L2 [Citation50], and VAMP3 [Citation51].
Figure 1. Results of CRCL preparation, scheme for peptide separation, and reverse-phase high pressure liquid chromatography (rpHPLC) of peptides. (A) Coomassie Blue stained gel (top) showing total protein profile of fractions 4–16 of a Rotofor separation of 12B1 tumour proteins. Fraction numbers are listed at the top, and beneath the gel is the pH range of those fractions. Molecular weight markers (in kDa) are indicated on the left. The bottom portion shows western blots for the chaperones GRP94, Hsp90, Hsp70, and calreticulin (CRT). Vertical lines depict the fractions chosen to pool to generate CRCL (i.e. fractions 10–13). (B) shows a schematic of the procedure to strip peptides from CRCL (peptides represented by red dots) and their collection and separation away from the bulk of the proteins by centrifugation thru a 3-kDa cut-off membrane. (C) A chromatogram of peptides fractionated by C18 rpHPLC detected by absorbance readings at 214 nm, following elution with a 0–30% gradient of solvent B (0.1% TFA in ACN) over 30 min with a 30–95% gradient over the last 10 min (red trace). Fractions were collected every minute, and were combined into six pools (fractions 1–6, 7–13, 14–20, 21–26, 27–33, 34–40) with each pool subjected to mass spectrometry for peptide identification.
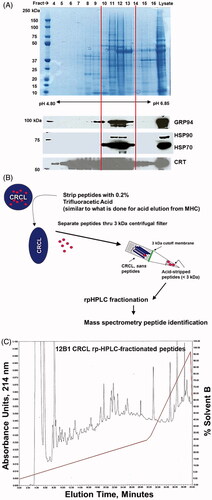
Figure 2. Ingenuity Pathway Analysis (IPA) and gene/protein ontology of CRCL peptides and their parent proteins. Peptides (and thus, proteins) identified by mass spectrometry were grouped by subcellular location (A) and function (B), and as a percentage of the total peptides categorised. (C and D) show the two high-scoring ‘top networks’ categorised by IPA in the form of interactomes; the network in C is Cellular movement, Cellular assembly and organisation, Cellular function and maintenance, with a score of 43, and 20 focus molecules. The network in D is Amino acid metabolism, Drug metabolism, Molecular transport, with a score of 24, and 13 focus molecules. The ‘scores’ (−log (p values)) reflect the probabilities of such associations occurring by chance (significance threshold set at 1.25), and ‘focus molecules’ are nodes for initiating networks. Peptides/proteins identified are shown in large red font with a yellow background. Interactions are depicted by lines (‘edges’), as described in the text.
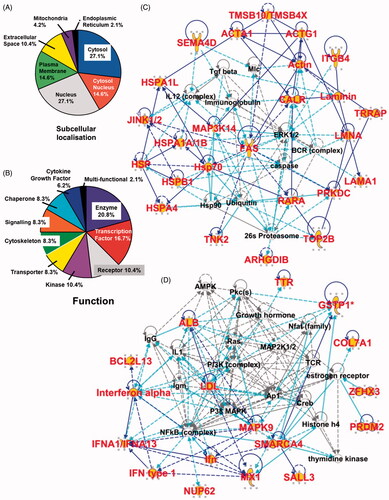
12B1 CRCL peptides come from proteins that span multiple subcellular locations and assorted protein functions
The proteins of origin of the identified peptides can be grouped into expected subcellular localisations based on literature searches and Ingenuity Pathway Analysis algorithms. Shown in , the peptides derive from all the major subcellular compartments with the vast majority coming from the cytosol and nucleus, including proteins that shuttle from one location to the other. These are also cellular regions with active proteasomes capable of generation of peptides that could be bound by chaperones [Citation52]. In addition, the putative functions of the proteins are also listed with percentage distributions (); again, the functions are diverse, indicating that potential CRCL antigens are derived from numerous classes of proteins.
Gene ontogeny/Ingenuity Pathway Analysis of the isolated peptides reveal networks critical to tumour biology
The proteins of origin of the CRCL peptides could be grouped into two high-scoring categories (of five ‘top networks’ discerned); those networks/associated functions are shown with their interactomes in and (); their network descriptions, ‘scores’ and number of ‘focus molecules’ are listed in the figure legend. The scores (−log (p values)) reflect the probabilities of such associations occurring by chance (significance threshold set at 1.25); as evident, the scores are highly significant. Focus molecules are starting points for the generation of biological networks. Parent proteins identified via peptidomics appear with larger font and yellow highlights; direct connections (i.e. known or documented interactions) between identified proteins are indicated by solid blue lines, and indirect connections (either suspected or known via intermediaries) are shown with broken lines. Light blue/turquoise lines show identified molecular interactions with proteins in the network, but we did not specifically identify those other proteins. The lengths of the lines (called ‘edges’) between the protein ‘nodes’ is indicative of amount of literature supporting and documenting those interactions; however, we have shortened many of those edges to fit the interactomes into the figure. As is apparent from the interactomes, many of the proteins are either key points in signalling (e.g. FAS) or they interact with prominent players in cancer signalling pathways (e.g. ERK, PI3K, MAPK, RAS). Of interest, the BCR complex appears in consistent with 12B1 being a BCR-ABL positive leukaemia.
Notable in the IPA ‘Top bio functions’ category were the high-scoring associations with diseases and disorders, with ‘cancer’ receiving the highest score, followed by ‘respiratory disease’, ‘reproductive system disease’, ‘dermatological diseases and conditions’, and ‘inflammatory disease’. Associations with molecular and cellular functions included ‘cell death and survival’, ‘gene expression’, ‘cellular growth and proliferation’, ‘cellular movement’, and ‘cellular assembly and organisation’. Physiological system development and function associations included ‘organismal survival’, ‘haematological system development and function’, ‘organismal development’, ‘tumour morphology’, and ‘tissue morphology’ (data not shown for these). Clearly these networks/associations reflect properties of cancer and haematological development, as might be expected for a leukaemia. The sum of these data suggests that the peptide repertoire obtained from the 12B1 CRCL vaccine canvasses the entire cell, and represents targets of importance in cancer biology.
12B1-CRCL peptides possess putative binding/presentation capacities for MHC class I and MHC class II, as well as Hsp70 binding motifs
Using several publicly available predictive servers we have found that many of the 12B1 CRCL peptide sequences identified have putative high-affinity determinants for BALB/c MHC I (H2Kd), and almost half of the peptides may bind MHC II (IAd) (Supplementary Table II). Obviously, the various programs sometimes yield contradictory results, even for our ‘model’ comparator peptides (SYFPEITHI from the JAK-1 kinase family, and GFKQSSKAL from the fusion junction of the CML BCR-ABL oncoprotein). The SYFPEITHI and BIMAS programs had frequent overlap in their predictions, while NetMHC predicted relatively few binders. We did not show results from RANKPEP for MHC I binding evaluation since only one peptide scored above the predicted threshold. However, RANKPEP did have murine MHC II predictors (based on 15-mers), so we employed it for our peptides with the results shown. These predictive algorithms differ in their methodologies and types of read-outs, which may explain some of the variances [Citation53].
We have previously found that Hsc70 is one of liver CRCL’s primary chaperones active in binding exogenously added peptide [Citation32], and that peptide antigens designed with canonical Hsp70 binding motifs (so-called ‘javelin sequences’, [Citation54, Citation55]) could effectively and efficaciously incorporate into CRCL [Citation30]. A number of the peptides identified here also contain consensus sequences indicating affinity for the peptide-binding pocket of the Hsp70 member DnaK (LIMBO analyses, Supplementary Table II). Combined with the suggestive MHC binding affinities, the probability of Hsp70 interactions implies that CRCL chaperones bona fide antigens in its peptide repertoire.
Immunisation with 12B1 CRCL peptide fractions incorporated into antigen-free CRCL inhibits tumour growth
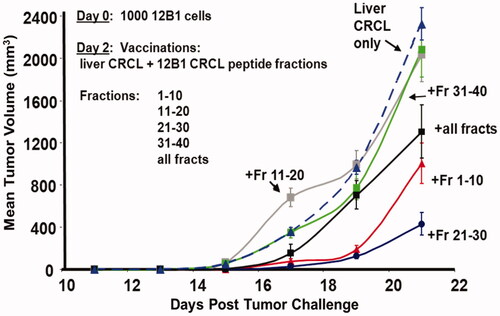
We had previously shown that particular antigenic peptides (such as that encompassing the fusion region of the BCR-ABL protein) could be ‘embedded’ into antigen-free CRCL (i.e. prepared from a normal tissue such as mouse liver) and could lead to significant tumour rejection and prolonged survival in murine vaccination studies [Citation30, Citation32]. Here we performed similar addition of peptides/presumed antigens to murine liver CRCL by mixing pooled fractions of rpHPLC-separated peptides that had been stripped from 12B1 CRCL. The fractions were pooled in groups of 10 (e.g. fractions 1–10, 11–20, etc.) and the pools were mixed with murine liver CRCL prepared from syngeneic (BALB/c) tissue. Those pooled peptide fractions + liver CRCL vaccines were used to treat mice that had been inoculated with 12B1 tumour 2 days prior. In addition, other vaccines included all of the rpHPLC fractions combined. As seen in , mice receiving liver CRCL vaccines reconstituted with (all 40) 12B1-CRCL peptide fractions showed significant reduction in tumour growth compared to groups of mice immunised with liver CRCL as controls. Notably, mice treated with liver CRCL combined with fractions 1–10 or 21–30 displayed even more dramatically reduced tumour growth kinetics. These findings strikingly correlate with ELISPOT data from our previous work [Citation32], where splenocytes from naïve mice vaccinated with 12B1 CRCL-pulsed dendritic cells responded to stimulation from rpHPLC-fractionated peptides derived from 12B1 CRCL. The peptides were chromatographed under the same conditions used here. In those experiments, peak re-stimulation responses to CRLC-derived peptides were driven by fractions 7–10 and 20–23, which were encompassed in the pooled fractions we used in reconstituting the CRCL vaccine. Thus, reconstitution of CRCL with tumour-derived peptides results in a functional vaccine capable of eliciting anti-tumour responses.
Figure 3. Reconstitution of liver CRCL with fractionated 12B1 CRCL peptides yields vaccines with anti-tumour activity. Mice were injected subcutaneously (s.c.) with 1000 in vivo passaged 12B1 tumour cells (LD100) on day 0. On day 2, mice were injected s.c. with ‘empty’ liver CRCL (25 µg/injection in 100 µL of PBS), or with liver CRCL + fractionated peptides (CRCL + pools of fractions 1–10, 11–20, 21–30, or 31–40). One group’s vaccine consisted of all the peptide fractions combined and mixed with liver CRCL as described. Tumour growth was measured every other day with calipers along two axes to determine volume; averages of four mice are shown per curve, with error bars = SEM. CRCL + fractions 1–10 and 21–30 differed significantly from all other groups by day 19; CRCL + all fractions was significantly different from liver CRCL, CRCL + fractions 11–20, and CRCL + fractions 31–40, by day 21 (ANOVA). These data are typical of two experiments.
Discussion
In this report we described the ‘peptidome’ of potential antigens extracted from CRCL prepared from a murine tumour. Using mass spectrometry techniques we identified almost 60 different proteins of origin for the peptides released from CRCL; we determined that the parental proteins come from essentially all parts of the cell, and are involved in a broad range of functions. Further in silico analyses demonstrated that the parental proteins are components of major signalling networks of vital importance for cancer cell survival, proliferation, and migration. In many instances the peptides identified possess amino acid sequences that would allow their putative binding and display by murine MHC class I and II molecules, and there are also predicted binding motifs for Hsp70-type chaperones. By mixing fractionated pools of peptides with antigen-free (normal liver) CRCL, we were able to reconstitute effective anti-tumour activity of the vaccine, showing that the peptides are indeed the major purveyors of CRCL vaccines’ efficacy. However, there appear to be limited numbers of antigenic peptides present in the total peptide pool, since upon reconstitution into CRCL, only certain fractions enabled anti-tumour responses. One issue here might be inadequate antigen quantities, as we undoubtedly experienced losses of material during the procedures. Another concern may be that the simple mixing of peptides into the vaccine may not result in a stable, immunogenic complex for all peptides in a given fraction. Indeed, this was the case for a number of known CD8+ T cell antigens upon addition into ‘naked’ GRP94/gp96 and Hsp70 vaccines [Citation56]. On the other hand, we indeed were able to regenerate immunity by reconstituting certain peptide fractions with CRCL, despite the loss of the original native complexes, and despite the presumed presence of liver peptides in those CRCL preparations.
One of the key features of anti-cancer or anti-pathogen vaccines is the presence of T cell epitopes [Citation57], peptide sequences that originate in an antigenic protein, which are processed by the proteasome and the TAP transporters, and are loaded onto MHC I (and MHC II molecules by different mechanisms) for display by APCs to T cells. T cells with the appropriate receptors capable of recognising the antigenic peptide in the context of MHC molecules will be induced to activate and proliferate. This process is aided by stimulation from the APC, which itself needs stimulation from the environment. In a vaccine setting, this stimulation comes from the adjuvant components of the vaccine, while the peptide epitopes are presumably incorporated within the vaccine as well. In the case of heat shock protein-based vaccines, the adjuvant (HSPs) and the antigens (peptides chaperoned by the HSPs) are all from the same source (i.e. the autologous tumour). The innate immune stimulus provided by individual extracellular/exogenous heat shock/chaperone proteins has been demonstrated [Citation58, Citation59], invoking the term ‘chaperokine’ to describe this [Citation60]. CRCL as a collection of chaperones is also capable of APC and natural killer (NK) cell stimulation [Citation10, Citation61]. Certain chaperone-associated peptides have been identified both immunologically and biochemically upon purification of particular HSPs [Citation62–Citation68] but as yet there are no publications describing relatively large numbers of peptides culled from HSP-based vaccines. In this case, we were able to reconstitute the anti-cancer activity of the vaccine by adding back fractionated peptides to CRCL prepared from normal tissue (from liver, lacking in tumour antigens), with the caveats mentioned above. Further research is needed to discern the identities of the antigenic peptides within those fractions, which would require scale-up to obtain sufficient material for isolation, identification, and vaccine-reconstitution testing of individual peptides.
The use of predictive programs to validate the potential immune utility of the peptides we identified proved somewhat disconcerting. BIMAS [Citation69] and SYFPEITHI [Citation70] were consistent at least in identification of peptides with putative H2Kd binding potential, although their scoring methods and outputs differ. NetMHC [Citation71, Citation72] yielded only five peptide results compared to more than 30 of the identified peptides channelled through SYFPEITHI. The programs all differ substantially in their matrix use (SYFPEITHI and BIMAS) versus artificial neural networks (NetMHC) to predict binding capacity and affinity, which may explain some of the differences; the various programs and algorithms, including RANKPEP, have been reviewed [Citation53]. While RANKPEP [Citation73] also only predicted MHC I binding affinity for one of the peptides (GBP5, 9-mer), the program accommodates murine MHC II (I-Ad in this case), so we used it to predict binding ‘hits’ for 14 of the peptides shown. Ultimately, the value of a given antigen will be assessed by the induced immune responses and also by the necessity of that antigen in the biology of the tumour cell or pathogen.
Kislin et al. [Citation32] found that exogenous peptide (GFKQSSKAL) embedded into CRCL was avidly bound by Hsc70 among the components of CRCL vaccines, and Bleifuss et al. [Citation30] used a modified tyrosinase peptide with the sequence HWDFAWPW fused near the N-terminus to enhance immunogenicity to the peptide (HWDFAWPW is a so-called ‘javelin’ sequence for high-affinity Hsp70 family binding [Citation54]). We queried a publicly available program (LIMBO) [Citation74] which predicts Hsp70/DnaK binding to proteins/peptides, and found that 13 of our identified peptides would have putative Hsp70 binding potential. Thus, we suspect that Hsp70 family members in CRCL are likely important for chaperoning at least some of the peptides within the vaccine. We saw no obvious sequence strings amongst these peptides that would indicate a common affinity signature for Hsp70, but further bioinformatics work may reveal motifs.
Based on our previous reports where we added peptide antigens directly into CRCL [Citation30, Citation32], in this work we added entire fractions of peptides to reconstitute CRCL, using the chaperone complex from an innocuous source (liver), and supplying the vaccine with antigens stripped and separated from an original tumour CRCL (prepared from 12B1 leukaemia). Particular pools of peptides did indeed show effective anti-tumour activity, and curiously those pools corresponded to peptide pools we had previously found stimulatory to 12B1-CRCL primed splenocytes in ELISPOT assays [Citation32]. This indicates the durability and reproducibility of the peptide antigen content of CRCL, as well as the capacity for CRCL to incorporate antigen and effectively stimulate adaptive immune responses. It also strongly suggests that the specific immunity of CRCL is enclosed in its peptide content.
One demonstrated role for HSPs has been their contribution to peptide trafficking during antigen presentation [Citation67] which may help explain HSP involvement in cross-presentation [Citation75]. The endoplasmic reticulum chaperone GRP94 has elutable MHC I binding peptides of β-galactosidase [Citation76], and exogenous, extracellular Hsp90 and Hsp110 can deliver antigenic proteins for processing and presentation by DCs [Citation77, Citation78], indicating the extensive size range of antigen delivery accomplishable by chaperones and HSPs. While we identified peptides with a wide range of sizes (some larger than 30 amino acids, and 8-mers at the smallest), we did use a 3-kDa cut-off membrane in selection and therefore would not have identified whole proteins/polypeptides or fragments much larger than 30 amino acids in the antigen pool. In a complex amalgamation of proteins and peptides such as CRCL, distinctions between carriers and antigens may become blurred, and the use of size as a criterion may be largely semantic. Our procedure for peptide harvest and identification undoubtedly is very conservative, and there are likely far more antigens in CRLC than alluded to in this work, including those that would never appear in the peptide pool due to the size separation. For instance, the large chaperones HSP110 and GRP170 both have isoelectric points in the range that would include them in fractions pooled for CRCL vaccine preparation [Citation79, Citation80]. These chaperones can bind and maintain relatively long interactions with full-length client proteins [Citation81], which is the basis for their use as anti-cancer vaccines [Citation78]. Thus, CRCL may also contain whole protein antigens, or at least polypeptide antigens larger than 3 kDa.
A complete vaccine provides both adjuvant stimulation along with antigens for adaptive responses. As with other chaperones/HSPs, CRCL has adjuvant properties capable of stimulating DCs [Citation10, Citation29, Citation82], but it can also enable APC resistance to immune suppression from regulatory T cells [83]. CRCL can even stimulate other effector cells such as NK cells [Citation61] and B cells [Citation27], the latter leading to bioactive antibody production against a known cancer antigen. Combined with a wide array of antigens, CRCL’s innate stimulation of APCs and non-T cell effectors provides broad immunological coverage in a single package. With high yields from minute tumour quantities (e.g. 12 clinical vaccine doses from 100 mg of tumour (Tehila Sonnenfeld, personal communication)), and a long history of effective animal model treatments, CRCL is currently moving towards clinical trials in the USA and elsewhere.
Conclusion
Chaperone-rich cell lysate (CRCL) vaccines derived from tumours envelop peptide antigens as part of their immune stimulatory repertoire. The peptide content, as demonstrated here, includes peptides whose parental proteins have a wide range of subcellular localisations and functions, including some within critical signalling pathways for tumour cell survival, proliferation, and migration. The peptides have motifs that allow their putative presentation by MHC class I and II molecules, as well as binding potential for Hsp70 family members. Fractionated peptides (via rpHPLC), when added back to antigen-free CRCL, can reconstitute the anti-tumour activity of CRCL, indicating that the peptides are indeed the purveyors of adaptive immunity for CRCL.
Declaration of interest
This work was supported by the US National Institutes of Health grant NIH R01 CA104926-1 (to E.K.), and by the University of Colorado Cancer Center (to M.W.G.). M.W.G. is a consultant for Immunovative Therapies, which has licensed CRCL for clinical use. E.K. was the Chair of the Scientific Advisory Board of Immunovative Therapies from 9/12 to 1/13. The authors alone are responsible for the content and writing of the paper.
Supplementary Material
Download PDF (135.8 KB)Acknowledgements
The authors would like to thank Marilyn Marron and Sylvia Thompson Romm for help with the CRCL preparations and animal work, Tehila Sonnenfeld for sharing unpublished data, and Linda Breci and Paul Haynes for the mass spectrometry work at the University of Arizona.
References
- Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer 2011;11:805–12
- Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy – Revisited. Nat Rev Drug Discov 2011;10:591–600
- Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P. Cancer immunotherapy: Sipuleucel-T and beyond. Pharmacotherapy 2011;31:813–28
- Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96;vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 2008;372:145–54
- Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Lamaj E, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother 2006;55:958–68
- See AP, Pradilla G, Yang I, Han S, Parsa AT, Lim M. Heat shock protein-peptide complex in the treatment of glioblastoma. Expert Rev Vaccines 2011;10:721–31
- Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: An overview. Jpn J Clin Oncol 2009;39:73–80
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int J Hyperthermia 2009;25:610–6
- Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood 2002;100:4108–15
- Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood 2003;101:245–52
- Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: Agents of antigen cross-presentation. Expert Rev Vaccines 2008;7:1019–30
- Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH, et al. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today 2006;11:534–40
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002;20:395–425
- Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother 2006;55:329–38
- Graner MW, Bigner DD. Chaperone proteins and brain tumors: Potential targets and possible therapeutics. Neuro-Oncology 2005;7:260–78
- Demine R, Walden P. Testing the role of gp96 as peptide chaperone in antigen processing. J Biol Chem 2005;280:17573–78
- Fleischer K, Schmidt B, Kastenmuller W, Busch DH, Drexler I, Sutter G, et al. Melanoma-reactive class I-restricted cytotoxic T cell clones are stimulated by dendritic cells loaded with synthetic peptides, but fail to respond to dendritic cells pulsed with melanoma-derived heat shock proteins in vitro. J Immunol 2004;172:162–9
- Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol 2003;3:427–32
- Gullo CA, Teoh G. Heat shock proteins: To present or not, that is the question. Immunol Lett 2004;94:1–10
- Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med 2002;196:1447–59
- Reits E, Griekspoor A, Neijssen J, Groothuis T, Jalink K, van Veelen P, et al. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity 2003;18:97–108
- European Medicines Agency. Withdrawal Assessment Report for Oncophage. London, 2009. Available from: http://www.emea.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/03/WC500075459.pdf
- Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother 2000;49:476–84
- Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003;52:226–34
- Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood 2003;101:4485–91
- Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother 2005
- Li G, Andreansky S, Helguera G, Sepassi M, Janikashvili N, Cantrell J, et al. A chaperone protein-enriched tumor cell lysate vaccine generates protective humoral immunity in a mouse breast cancer model. Mol Cancer Ther 2008;7:721–9
- Graner MW, Cumming RI, Bigner DD. The heat shock response and chaperones/heat shock proteins in brain tumors: Surface expression, release, and possible immune consequences. J Neurosci 2007;27:11214–27
- Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol 2007;148:136–45
- Bleifuss E, Bendz H, Sirch B, Thompson S, Brandl A, Milani V, et al. Differential capacity of chaperone-rich lysates in cross-presenting human endogenous and exogenous melanoma differentiation antigens. Int J Hyperthermia 2008;24:623–37
- Epple LM, Bemis LT, Cavanaugh RP, Skope A, Mayer-Sonnenfeld T, Frank C, et al. Prolonged remission of advanced bronchoalveolar adenocarcinoma in a dog treated with autologous, tumour-derived chaperone-rich cell lysate (CRCL) vaccine. Int J Hyperthermia, 2013;29:390-8
- Zeng Y, Graner MW, Thompson S, Marron M, Katsanis E. Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells. Blood 2005;105:2016–22
- Kislin KL, Marron MT, Li G, Graner MW, Katsanis E. Chaperone-rich cell lysate embedded with BCR-ABL peptide demonstrates enhanced anti-tumor activity against a murine BCR-ABL positive leukemia. Faseb J 2007
- He L, Feng H, Raymond A, et al. Dendritic-cell-peptide immunization provides immunoprotection against BCR-ABL-positive leukemia in mice. Cancer Immunol Immunother 2001;50:31–40
- Rotzschke O, Falk K, Wallny HJ, Faath S, Rammensee HG. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science 1990;249:283–7
- Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991;351:290–6
- Haynes PA, Fripp N, Aebersold R. Identification of gel-separated proteins by liquid chromatography-electrospray tandem mass spectrometry: Comparison of methods and their limitations. Electrophoresis 1998;19:939–45
- Cui JW, Li WH, Wang J, Li AL, Li HY, Wang HX, et al. Proteomics-based identification of human acute leukemia antigens that induce humoral immune response. Mol Cell Proteomics 2005;4:1718–24
- Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: Ins and outs. Trends Immunol 2013;34:13–21
- Junker N, Kvistborg P, Kollgaard T, Straten P, Andersen MH, Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol 2012;273:1–9
- Fellenberg F, Hartmann TB, Dummer R, Usener D, Schadendorf D, Eichmuller S. GBP-5 splicing variants: New guanylate-binding proteins with tumor-associated expression and antigenicity. J Invest Dermatol 2004;122:1510–17
- Chumpitazi BF, Bouillet L, Drouet MT, Kuhn L, Garin J, Zarski JP, et al. Biological autoimmunity screening in hepatitis C patients by anti-HepG2 lysate and anti-heat shock protein 70.1 autoantibodies. Eur J Clin Microbiol Infect Dis 2009;28:137–46
- Zappasodi R, Bongarzone I, Ghedini GC, Castagnoli L, Cabras AD, Messina A, et al. Serological identification of HSP105 as a novel non-Hodgkin lymphoma therapeutic target. Blood 2011;118:4421–30
- Mojtahedi Z, Safaei A, Yousefi Z, Ghaderi A. Immunoproteomics of HER2-positive and HER2-negative breast cancer patients with positive lymph nodes. OMICS 2011;15:409–18
- Paez MC, Matsuura E, Diaz LA, Shoenfeld Y, Serrano NC, Anaya JM. Laminin-1 (LM-111) in preeclampsia and systemic lupus erythematosus. Autoimmunity 2013;46:14–20
- Scanlan MJ, Welt S, Gordon CM, Chen YT, Gure AO, Stockert E, et al. Cancer-related serological recognition of human colon cancer: identification of potential diagnostic and immunotherapeutic targets. Cancer Res 2002;62:4041–7
- Gosney JR, Williams IJ, Dodson AR, Foster CS. Morphology and antigen expression profile of pulmonary neuroendocrine cells in reactive proliferations and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). Histopathology 2011;59:751–62
- Padua RA, Larghero J, Robin M, le Pogam C, Schlageter MH, Muszlak S, et al. PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nat Med 2003;9:1413–7
- Yokoe T, Tanaka F, Mimori K, Inoue H, Ohmachi T, Kusunoki M, et al. Efficient identification of a novel cancer/testis antigen for immunotherapy using three-step microarray analysis. Cancer Res 2008;68:1074–82
- Barbaric D, Byth K, Dalla-Pozza L, Byrne JA. Expression of tumor protein D52-like genes in childhood leukemia at diagnosis: Clinical and sample considerations. Leuk Res 2006;30:1355–63
- Pontes ER, Matos LC, da Silva EA, Xavier LS, Diaz BL, Small IA, et al. Auto-antibodies in prostate cancer: humoral immune response to antigenic determinants coded by the differentially expressed transcripts FLJ23438 and VAMP3. Prostate 2006;66:1463–73
- Wojcik C, DeMartino GN. Intracellular localization of proteasomes. Int J Biochem Cell Biol 2003;35:579–89
- Lin HH, Ray S, Tongchusak S, Reinherz EL, Brusic V. Evaluation of MHC class I peptide binding prediction servers: Applications for vaccine research. BMC Immunol 2008;9:8
- Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA 2000;97:3485–90
- Flechtner JB, Cohane KP, Mehta S, Slusarewicz P, Leonard AK, Barber BH, et al. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J Immunol 2006;177:1017–27
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 1997;186:1315–22
- Chang CX, Dai L, Tan ZW, Choo JA, Bertoletti A, Grotenbreg GM. Sources of diversity in T cell epitope discovery. Front Biosci 2011;16:3014–35
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. Hsp70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J Biol Chem 2002;277:15107–12
- Tamura Y, Torigoe T, Kukita K, Saito K, Okuya K, Kutomi G, et al. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy 2012;4:841–52
- Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones 2000;5:425–31
- Zeng Y, Chen X, Larmonier N, Li G, Sepassi M, Marron M, et al. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer 2006;119:2624–31
- Nieland TJ, Tan MC, Monne-van Muijen M, Koning F, Kruisbeek AM, van Bleek GM. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein gp96/GRP94. Proc Natl Acad Sci USA 1996;93:6135–9
- Lammert E, Arnold D, Nijenhuis M, Momburg F, Hämmerling GJ, Brunner J, et al. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol 1997;27:923–7
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med 1995;182:885–9
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science 1995;269:1585–8
- Meng SD, Gao T, Gao GF, Tien P. HBV-specific peptide associated with heat-shock protein gp96. Lancet 2001;357:528–9
- Ishii T, Udono H, Yamano T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins Hsp70, Hsp90, and gp96. J Immunol 1999;162:1303–9
- Stocki P, Wang XN, Morris NJ, Dickinson AM. Hsp70 natively and specifically associates with an N-terminal dermcidin-derived peptide that contains an HLA-A*03 antigenic epitope. J Biol Chem 2011;286:12803–11
- Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol 1994;152:163–75
- Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999;50:213–9
- Buus S, Lauemoller SL, Worning P, Kesmir C, Frimurer T, Corbet S, et al. Sensitive quantitative predictions of peptide-MHC binding by a ‘Query by Committee’ artificial neural network approach. Tissue Antigens 2003;62:378–84
- Nielsen M, Lundegaard C, Worning P, et al. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci 2003;12:1007–17
- Reche PA, Reinherz EL. Prediction of peptide-MHC binding using profiles. Methods Mol Biol 2007;409:185–200
- Van Durme J, Maurer-Stroh S, Gallardo R, Wilkinson H, Rousseau F, Schymkowitz J. Accurate prediction of DnaK-peptide binding via homology modelling and experimental data. PLoS Comput Biol 2009;5:e1000475
- Udono H, Ichiyanagi T, Mizukami S, Imai T. Heat shock proteins in antigen trafficking – Implications on antigen presentation to T cells. Int J Hyperthermia 2009;25:617–25
- Binder RJ, Kelly JBIII, Vatner RE, Srivastava PK. Specific immunogenicity of heat shock protein gp96 derives from chaperoned antigenic peptides and not from contaminating proteins. J Immunol 2007;179:7254–61
- Oura J, Tamura Y, Kamiguchi K, Kutomi G, Sahara H, Toshihiko T, et al. Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. Int Immunol 2011;23:223–37
- Wang XY, Sun X, Chen X, Facciponte J, Repasky E, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol 2010;184:6309–19
- Subjeck JR, Shyy T, Shen J, Johnson RJ. Association between the mammalian 110,000-Dalton heat-shock protein and nucleoli. J Cell Biol 1983;97:1389–95
- Calvert ME, Digilio LC, Herr JC, Coonrod SA. Oolemmal proteomics – Identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol 2003;1:27
- Easton DP, Kaneko Y, Subjeck JR. The Hsp110 and Grp1 70 stress proteins: Newly recognized relatives of the Hsp70s. Cell Stress Chaperones 2000;5:276–90
- Cantrell J, Larmonier C, Janikashvili N, Bustamante S, Fraszczak J, Herrell A, et al. Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells. Immunobiology 2010;215:535–44
- Larmonier N, Cantrell J, Lacasse C, Li G, Janikashvili N, Situ E, et al. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol 2008;83:1049–59