Abstract
Purpose: The demand for epilation of large body areas involves using large laser spots. The emitted light causes the desired effect in follicular melanin, but it may also produce collateral effects on pigmented melanocytic nevi. To shield melanocytic lesions, several materials were tested for their capabilities to prevent transmission of alexandrite and diode laser light. Materials and methods: Delivered fluence of the diode laser (808 nm, 30 J/cm2, 12 ms) and the alexandrite laser (755 nm, 30 J/cm2, 40 ms) and transmission rates were measured for glass slides only and additional sunscreen, polyurethane (PU) foam, zinc paste, white kajal, and a wooden spatula. For each method, ten repeated measurements were conducted. An ex vivo human skin explant model was used for histological validation. Results: Using zinc as an absorber reduced transmission to 8.77% (diode) and 7.99% (alexandrite laser). Respectively transmissions were measured as following: PU foam 19.25% versus 20.78%, sunscreen 19.85% versus 16.91%, white kajal 76.43% versus 71.03% and wooden spatula 8.05% versus 3.62%. Histologically, a single application of therapeutic fluences (755 nm) in uncovered congenital nevi leads to immediate formation of atypical nucleoli, a ballooning degeneration of melanocytes, and subepidermal clefting within the treated area. In the sites of the lesions that were covered by zinc paste, PU foam, sunscreen and wooden spatula, no immediate histological changes were visible. Conclusion: Applying a sufficient amount of zinc paste (approximately 1 g/cm2) onto melanocytic lesions allows complete coverage during laser epilation.
Introduction
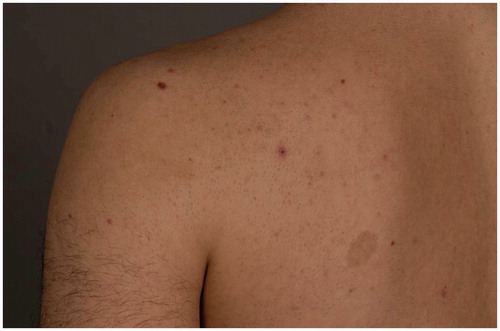
Epilation is a growing trend worldwide [Citation1]. With an increasing demand for the treatment of larger areas, newer devices are built more often with scanner systems and larger treatment tips to allow faster and, to some extent, more effective treatments. Among the most widely used devices are the diode and alexandrite laser systems [Citation2]. Based on the principle of selective photothermolysis, the emitted light causes the desired effect in the follicular melanin, but it may also produce collateral effects on pigmented nevi located within the treatment area. These nevi are frequently found within treatment areas () and therefore targeted accidentally during therapy with scanned or large-tipped laser devices. Histologically, thermal effects of melanocytes with enlarged nuclei and cytoplasm can be observed post laser exposure.
Figure 1. A 23-year-old male requested epilation of the back. He has nevi, acne papules, and café-au-lait spots in the treatment area (hair had been shaved 1 day before the photograph in preparation for laser treatment).
There are various materials already in use for blocking the skin from ultraviolet light [Citation3] and laser light [Citation4]. ‘The European guidelines for care’ from the European Society for Laser Dermatology (ESLD) recommend avoiding laser energy on melanocytic nevi by covering them with white adhesive tape [Citation5]. However, to what extent those materials shield skin from laser light by blocking its transmission and whether they are able to prevent histological changes in melanocytic lesions has never been examined, to the best of our knowledge.
The purpose of this study was to test several materials for their absorption potential of alexandrite (755 nm) and diode (808 nm) laser light. We wanted to examine whether these materials were effective in shielding melanocytic lesions from accidental collateral effects during scanned laser epilation. We also looked ex vivo at histological changes induced by residual laser radiant exposures onto experimentally shielded nevi to estimate biological relevance.
Materials and methods
In vitro absorption assay
Transmission rates in the absence of intervening materials were measured for both laser systems. The two laser systems included a scanned diode laser (Leda, Alma Lasers, formerly Quantel Derma, formerly Wavelight, Buffalo Grove, IL), 808 nm, fluence: 30 J/cm2, pulse duration: 12 ms) and an alexandrite laser (Arion, Alma Lasers), 755 nm, fluence: 30 J/cm2, pulse duration: 40 ms). Fluences and pulse durations were set as used in clinical treatments and in hair removal studies. A blank glass slide (Menzel, Thermo Scientific, Braunschweig, Germany) was brought into each of the laser beams to evaluate the resulting transmission reduction rates, and, subsequently, additional cover materials were applied. Transmission through the glass slide was set as 100%. Tested cover materials included the following: sunscreen (Anthelios XL 50+ creme, La Roche-Posay, Duesseldorf, Germany, physical + organic ultraviolet (UV) filters (photostable filter system containing Mexoryl XL (drometrizole trisiloxane), Mexoryl SX (terephthalylidene dicamphor sulfonic acid), titansorb S (titanium dioxide), Parsol 1789 (butyl methoxydibenzoylmethane), Uvinul N539, Uvinul T150, ethylhexyl salicylate), app. 1 g/cm2, the slides were weighed before and during application to ensure equal amounts), polyurethane (PU) foam (Mepilex®, Molnlycke Health Care, Erkrath, Germany, approximately 1 mm thick), zinc paste (Pasta zinci mollis, Bombastus Werke, Germany, approximately 1 g/cm2, the slides were weighed before and during application to ensure equal amounts), white kajal (BK Cosmetics, no. 4, Hattersheim, Germany; material was applied until the area was fully covered by white color). A wooden spatula (Paul Hartmann, Heidenheim, Germany; approximately 0.5 mm thickness) was brought into the laser beam without the supporting glass slide. The delivered fluence was measured using a laser power meter (Nova, Ophir, Darmstadt, Germany, ). For each cover material, ten measurements were conducted. The mean values and the resulting transmission rates were calculated.
Figure 2. (a) Measuring alexandrite transmission through a glass slide covered with clear glass, (b), measuring diode transmission through a glass slide covered with sun screen, (c) linear scanned hair removal laser emitting at 808 nm in test position, (d) application of a sufficient amount of zinc paste onto a skin explant using a cotton bud.

Ex vivo absorption assay
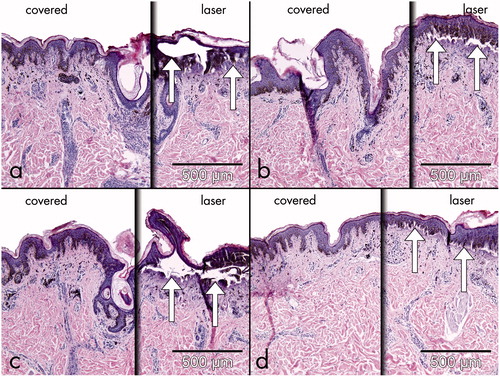
In addition, parts of human skin explants of congenital melanocytic nevi (n = 5) were tested for histological changes. After covering half of the surface of the explants with either zinc paste, PU foam, sunscreen and wooden spatula, a single pass of the alexandrite laser at the above–mentioned settings was applied, and the skin was analysed histologically by two dermatopathologists ().
Results
In vitro absorption assay
The detected fluence of both laser systems without intervening materials and with a blank glass slide was measured. Even without covering material, this led to a decrease of transmission to 90.8% and 91.3% in the diode and alexandrite laser, respectively. As the other materials (with the exception of the wooden spatula) were applied to the glass slide, the transmission rate of the blank slide was defined as 100% (control). After zinc paste application, transmission was reduced to 8.77% and 7.99% in the diode and alexandrite laser, respectively (). PU foam diminished the fluence to 19.25% (diode) and 20.78% (alexandrite) compared to the control. The use of sunscreen led to a reduction of 19.85% (diode) and 16.91% (alexandrite). White kajal reduced transmission to 76.43% (diode) and 71.03% (alexandrite). A wooden spatula slide reduced transmission to 8.05% (diode) and 3.62% (alexandrite). During our research, we found that a wooden spatula starts smoldering after repetitive laser applications.
Table I. Measured transmissions of various materials.
Ex vivo absorption assay
To test the efficacy of the proven systems, parts of beforehand excised melanocytic nevi (n = 5) that were not necessary for pathological investigation were irradiated ex vivo with the informed consent of the patients. Histologically, the single application of the alexandrite laser using therapeutic fluences in an uncovered congenital nevus led to immediate formation of atypical nucleoli, a ballooning degeneration of melanocytes, and subepidermal clefting within the treated area. In the sites of the lesions that were covered by zinc paste, PU foam, sunscreen and wooden spatula, no immediate histological changes could be detected (Figure 3a–d). These observations demonstrate that efficient shielding might help to prevent unwanted irradiation of pigmented nevi.
Discussion
The demand for epilation over a large proportion of the body is a growing trend worldwide. Consequently, scanner systems and larger treatment tips are being used to allow faster and more effective treatments. Based on the principle of selective photothermolysis, the emitted light causes the desired effect in follicular melanin, but may also produce collateral effects on pigmented melanocytic nevi.
UV light, although used intensively to treat inflammatory skin conditions by means of lasers and other light sources [Citation6] is also associated with harmful potential to induce cancer in human skin. In analogy to UV-induced skin cancer, the possibility of induction of malignancy of nevi through laser light should be considered. Few studies have examined this topic. On a molecular base, an up-regulation of p16INK4a protein in p16INK4a positive melanoma cells could be shown through sub-lethal 755 q-switched alexandrite laser light [Citation7]. There are also reports of pseudomelanoma [Citation8] and melanoma formation [Citation9] following laser therapy of pigmented lesions. However, a clear de novo induction of malignant pigmented skin tumours has never been demonstrated. Most likely, suspicious lesions seen on a microscopic level may have appeared due to the healing responses. In general, laser are used safely to remove benign tumors [Citation10]; those with malignant potential are subjected to laser interventions in specific circumstances only. If it is not feasible to spare melanocytic lesions from laser therapy, they should at least be shielded from laser light. There is a recommendation to use white tape as a covering material in the European guidelines [Citation5]. Yet, as no standardization of white tape exists, some products may not be effective enough to avoid collateral melanocytic damage.
To the best of our knowledge, there has been no systematic study of commonly used protective shielding materials [Citation5]. Therefore, the purpose of this study was to evaluate transmission rates of different cover materials during laser treatments with the alexandrite (755 nm) and diode (808 nm) laser light. We also examined histological changes of covered nevi ex vivo after laser radiation.
Here, we show that zinc paste is effective, economic, and easy to apply. The zinc oxide in the applied paste was neither micronized nor included as nano-particles, so it stayed on the epidermal surface and was not absorbed into the skin. Zinc oxide is tested as non-allergenic, non-irritant and non-comedogen; it is approved as a broad spectrum (UVA and UVB) sunscreen by the FDA and is completely photostable [Citation11]. In the current study it reduced transmission of alexandrite laser energy to less than 9% and no histological changes were observed in shielded melanocytic nevi. The shielding capacities of zinc oxide as well as titanium dioxide in opaque sunscreens on epilation laser systems have previously been shown [Citation4]. Those findings are supplemented by the results of our study. However, due to the study design we could not differentiate whether alexandrite laser energy was reflected or absorbed.
Although less effective, the specific sunscreen tested did prevent approximately 80% of the laser irradiation. The effect might be attributed rather to the sunscreen containing low amounts of titanium dioxide, a white pigment with a very high index of refraction, than to the chemical filters it contained that typically protect against UVB and UVA and to a lesser or no extent against visible or near infrared light. For that reason, the results may not be transferred onto other sunscreen products without further testing, as their composition varies.
Transmission data of red light (λ = 625 nm) in various amounts of creams with 25% and 50% zinc oxide and titanium dioxide are in concordance with our results. Moreover, the absorption spectra of zinc oxide and titanium dioxide measured in the same study match the protective effects of both substances against alexandrite and diode laser light [Citation12].
The white kajal pen did also contain titanium dioxide. However, the amount which was applied by colouring the nevus was obviously too low to reduce transmission of the alexandrite and diode laser energy effectively. The PU foam reduced transmission as much as the sunscreen did. However, application was more inconvenient and expensive. Wooden spatulas shielded the nevi sufficiently but cannot be recommended because they also endangered our patients by catching fire with repeated laser application.
Conclusion
Application of a sufficient amount of zinc paste on melanocytic lesions led to significant coverage during laser epilation. This seems to be an economic and effective way to avoid accidental collateral effects when using scanned epilation laser devices. More systematic evaluations within this field are required.
Although zinc paste has no FDA approval for shielding melanocytic nevi from laser light, this seems the best option so far during laser epilation procedures. It has superior physical absorption capacities, widespread availability, and low costs. Long-term follow-up studies will be necessary to confirm the clinical benefit.
Declaration of interest
The authors report no declarations of interest. The laser power meter was loaned from Quantel-Derma, Erlangen, Germany. The authors alone are responsible for the content and writing of the paper.
References
- Moretti M. Weathering the storm - Market forecast predicts continued growth for aesthetics. Aesthetic Guide 2009;January/February:1–8
- Goldberg DJ. Laser- and light-based hair removal: An update. Expert Rev Med Devices 2007;4:253–60
- Forestier S. Rationale for sunscreen development. J Am Acad Dermatol 2008;58:S133–8
- Fisher G. Opaque titanium and zinc sunscreen as an effective and versatile masking agent during laser hair removal: The batik-masking approach. Dermatol Surg 2009;35:985–6
- Drosner M, Adatto M. Photo-epilation: Guidelines for care from the European Society for Laser Dermatology (ESLD). J Cosmet Laser Ther 2005;7:33–8
- Rogalski C, Grunewald S, Schetschorke, M, Bodendorf MO, Kauer F, Simon JC, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia 2012;28:184–90
- Chan H H, Xiang L, Leung JC, Tsang KW, Lai KN. In vitro study examining the effect of sub-lethal QS 755 nm lasers on the expression of p16INK4a on melanoma cell lines. Lasers Surg Med 2003;32:88–93
- Lee HW, Ahn SJ, Lee MW, Choi JH, Moon KC, Koh JK. Pseudomelanoma following laser therapy. J Eur Acad Dermatol Venereol 2006;20:342–4
- Zipser MC, Mangana J, Oberholzer PA, French LE, Dummer R. Melanoma after laser therapy of pigmented lesions – Circumstances and outcome. Eur J Dermatol 2010;20:334–8
- Klein A, Baumler W, Landthaler M, Babilas P. Laser thermal therapy of benign skin tumours: Review and update. Int J Hyperthermia 2011;27:762–70
- Mitchnick MA, Fairhurst D, Pinnell SR. Microfine zinc oxide (Z-cote) as a photostable UVA/UVB sunblock agent. J Am Acad Dermatol 1999;40:85–90
- Schwarz VA, Klein SD, Hornung R, Knochenmuss R, Wyss P, Fink D, et al. Skin protection for photosensitized patients. Lasers Surg Med 2001;29:252–9