Abstract
Purpose: Magnetic iron oxide nanoparticles (MNPs) are used as contrast agents for magnetic resonance imaging (MRI) and hyperthermia for cancer treatment. The relationship between MRI signal intensity and cellular iron concentration for many new formulations, particularly MNPs having magnetic properties designed for heating in hyperthermia, is lacking. In this study, we examine the correlation between MRI T2 relaxation time and iron content in cancer cells loaded with various MNP formulations. Materials and methods: Human prostate carcinoma DU-145 cells were loaded with starch-coated bionised nanoferrite (BNF), iron oxide (Nanomag® D-SPIO), Feridex™, and dextran-coated Johns Hopkins University (JHU) particles at a target concentration of 50 pg Fe/cell using poly-D-lysine transfection reagent. T2-weighted MRI of serial dilutions of these labelled cells was performed at 9.4 T and iron content quantification was performed using inductively coupled plasma mass spectrometry (ICP-MS). Clonogenic assay was used to characterise cytotoxicity. Results: No cytotoxicity was observed at twice the target intracellular iron concentration (∼100 pg Fe/cell). ICP-MS revealed highest iron uptake efficiency with BNF and JHU particles, followed by Feridex and Nanomag-D-SPIO, respectively. Imaging data showed a linear correlation between increased intracellular iron concentration and decreased T2 times, with no apparent correlation among MNP magnetic properties. Conclusions: This study demonstrates that for the range of nanoparticle concentrations internalised by cancer cells the signal intensity of T2-weighted MRI correlates closely with absolute iron concentration associated with the cells. This correlation may benefit applications for cell-based cancer imaging and therapy including nanoparticle-mediated drug delivery and hyperthermia.
Introduction
Magnetic iron oxide-based nanoparticles (MNPs) continue to generate significant interest for their utility in numerous biomedical applications [Citation1–5]. Central to the appeal of iron oxide MNPs is the unique combination of inherent biocompatibility, and responsiveness to magnetic fields that give rise to multiple applications [Citation1]. When suitably formulated, the magnetic properties of MNPs can be adjusted for use with static magnetic fields to provide contrast for magnetic resonance imaging (MRI) [Citation1,Citation3]. Use of MNPs with gradient magnetic fields extends to high-throughput cell sorting applications, and magnetic vectorisation for targeted drug delivery [Citation4,Citation5]. When exposed to an alternating magnetic field (AMF), MNPs having appropriate magnetic anisotropy can generate heat [Citation1,Citation2,Citation6,Citation7]. The amount of heat generated by MNPs under AMF can be modulated by adjusting the magneto-structural properties of MNPs [Citation7]. Magnetic nanoparticle-mediated heat therapy or hyperthermia is of particular interest for targeted and image-guided therapy of various diseases including cancer.
Magnetic nanoparticle (or magnetic fluid) hyperthermia was first proposed by Gilchrist in 1957 to treat metastatic lymph nodes in recurrent breast cancer by heating the ferrofluid infused into the draining lymph nodes with AMF [Citation8]. Since this first example, efforts have been devoted to developing clinically relevant AMF devices and nanoparticle constructs to enable clinical applications for cancer hyperthermia [Citation1,Citation2,Citation9–12]. In addition, molecular targeting approaches have been explored to enable remote and non-invasive therapy for cancer following systemic (intravenous) delivery [Citation13]. Despite this considerable effort, only one application has been successfully translated into clinical trials for cancer hyperthermia of prostate and brain (glioblastoma multiforme) [Citation14–16].
The paucity of this type of clinically viable technology may, in part be due to the historical lack of nanoparticle formulations that provide sufficient heat at clinically relevant nanoparticle concentrations and AMF frequency and strength [Citation1,Citation6,Citation7]. It has been established that the interactions of AMF with conductive tissues induce eddy currents, which deposit power causing Joule heating of tissues non-selectively in the absence of MNPs [Citation17–21]. The extent of power deposition in tissue, or specific absorption rate (SAR, measured as W/kg tissue) scales with the cross-section of exposed tissue (eddy current path), and AMF frequency and amplitude [Citation17,Citation22]. Thus, for a given volume of a patient exposed to the AMF, both frequency and amplitude are constrained to ensure patient safety. This requirement places a significant burden on MNP performance, requiring that the specific loss power (SLP) normalised to the mass has to be high at low frequency (∼100–200 kHz) and for amplitudes <20 kA/m [Citation6,Citation22].
Early work with magnetic iron oxide nanoparticles was dominated largely by a desire to produce superparamagnetic nanoparticles (SPIOs) as MRI contrast agents, because it was believed that superparamagnetism ensured colloid stability [Citation2]. Stabilised superparamagnetic constructs were desired for MRI contrast, largely because of their inherently high T2/T2* relaxivity and straightforward surface modification chemistry that would enable functionalised MNPs with targeting molecules and/or use them as carrier platforms [Citation23–26]. For hyperthermia, however, superparamagnetism is undesirable because superparamagnetic materials exhibit no hysteresis (this is by definition, hence the terminology ‘paramagnetism’) with alternating magnetic fields yielding no work and thus no heating [Citation7]. Recent efforts have been directed to synthesise magnetic nanoparticle formulations containing iron oxide nanocrystalline compositions that exhibit enhanced magnetic anisotropy through modified crystalline properties, controlled aggregation of crystals to form a collective magnetic state, or some combination [Citation27–32].
Grüttner et al. [Citation28] described synthesis of nanoparticles in which nanocrystalline shape was controlled during crystal growth using high-pressure homogenisation to form anisotropic structures. These crystals were aggregated under high-energy conditions leading to controlled aggregates forming nanoparticle cores comprising 5–9 parallelepipeds that exhibit a collective magnetic state leading to particles exhibiting significant uniaxial anisotropy and thus enhanced heating with AMFs [Citation29–31]. Interestingly, once coated with polysaccharides (i.e. dextran or starch) or surfactant (i.e. citrate) the MNPs form a stable suspension in water or phosphate-buffered saline (PBS) through either steric or ionic mechanisms. This demonstrates that superparamagnetism is not a pre-requisite requirement to ensure colloid stability [Citation28–31].
More recently, Hedayati et al. [Citation29] have described methods and particle synthesis based on high-gravity controlled precipitation and subsequent hydrothermal processing to produce MNP constructs having reduced uniaxial anisotropy while still retaining significant magnetic properties at saturation to enable heating at clinically relevant magnetic field conditions [Citation7,Citation29]. MNP constructs designed for heating, as described above are generally not characterised for their MR imaging contrast, and conspicuously absent are comparisons with formulations designed for MRI contrast.
Non-invasive imaging techniques such as MRI are commonly utilised clinical tools for the diagnosis of many types of cancer. Image contrast is most commonly improved using contrast agents such as gadolinium-based complexes. It is, however, important to recognise that MNPs are inherently MR contrast agents that are uniquely suited to be theranostic agents, i.e. therapeutic and diagnostic, hence ‘theranostic’, because of their multifunctional capability when coupled with magnetic fields. This versatility results from their efficient contrast mechanisms (both negative and positive) that can be exploited, and the influence a local particle distribution can have within a voxel on MRI contrast [Citation33–35]. When MNPs are internalised in cells, the T1 effect is strongly reduced due indirectly to the switch from a dispersed state (freely suspended colloid) to an aggregated state (sequestered in endosomes or other organelles within the cell) [Citation36]. MNP aggregation thus modifies susceptibility contrast and water access to the iron core, thereby reducing the T1 effect. Additionally, T2 and relaxivity effects are modulated by a diffusion-mediated contrast mechanism that depends upon the size of the MNPs or MNP clusters [Citation37,Citation38].
Several MNP constructs and formulations have been studied in clinical trials for multiple indications [Citation39], and some have gained regulatory approval such as Feridex I.V.® (Advanced Magnetics, Cambridge, MA) [Citation39,Citation40] and Resovist® (Bayer Schering Pharma Pharmaceutical, Pittsburgh, PA) [Citation41] for detection and characterisation of liver lesions. MNPs have also been used to visualise liver, spleen, bone marrow, lymph nodes, and renal flow due to non-specific uptake of the reticuloendothelial system (RES) in these organs [Citation4]. Indeed, current and past clinical applications of imaging contrast are based and rely upon the non-specific uptake or phagocytic processes by macrophages and other components of the innate immune system. Future applications may be based upon molecular targeting of the nanoparticles for direct cancer cell detection. Regardless of mechanism or route by which the nanoparticles become associated with the target cell – SPIOs make possible MR imaging of cells when sufficient nanoparticle concentration is associated with the cell. Stated another way, it is established that MRI contrast depends principally upon cell-associated Fe concentration when SPIOs are used as the contrast agent [Citation35–39]. Consequently, the efficacy of particles designed for imaging (i.e. SPIOs) is largely determined by physicochemical properties (e.g. size, surface coating, surface hydrophobicity, surface charge) that in turn affect their physiochemical properties (e.g. toxicity, stability and aggregation, protein adsorption) [Citation1,Citation4,Citation39,Citation42]. Thus considerable attention has been directed to surface modifications using various coatings, targeting ligands, and other biologically active molecules to assist with selective tissue accumulation [Citation43,Citation44]. Most iron oxide nanoparticle formulations developed as contrast agents comprise some biopolymer (polysaccharide such as dextran or carboxydextran) coating of microparticles or nanoparticles of magnetite/maghemite (Fe3O4/Fe2O3). The magnetic properties of these compounds have been thoroughly documented [Citation26,Citation39,Citation41,Citation43,Citation44].
Thus we may ask the question whether MNP formulations designed for hyperthermia, i.e. enhanced anisotropy, will exhibit different or enhanced contrast properties than the SPIOs designed for use as contrast agents only. Recent reports of characterisation studies suggest that the magnetic properties of MNPs can influence their MRI contrast properties [Citation32,Citation45]. In this work we aim to characterise and compare the MRI contrast with heating properties of several MNP formulations designed for hyperthermia or T2-weighted contrast. The particles studied comprise Feridex I.V., an approved (SPIO) contrast agent that does not heat at clinically relevant AMF conditions [Citation6,Citation40]; BNF (Micromod Partikeltechnologie, Rostock, Germany) that heats very well at high amplitude AMF [Citation6,Citation46]; Nanomag-D-SPIO (Micromod), a predominantly superparamagnetic formulation that produces modest heat [Citation6,Citation47,Citation48]; and, JHU MNPs [Citation29]. The latter are citrate-stabilised MNPs produced by high-gravity controlled precipitation with hydrothermal ageing as previously described. All particles studied produced measurable amplitude-dependent heat when exposed to alternating magnetic fields having frequency 140–160 kHz. Feridex produced measurable but negligible amplitude-dependent heating that has no clinical relevance at field conditions tested [Citation6]. Other magnetic nanoparticle constructs are available, and it may be desirable to perform a comprehensive future study. The set chosen for the current study, however, presents a focused and representative sampling of MNPs for which structural and heating properties have been previously measured, and which have demonstrated significant variations of amplitude-dependent SLP at our chosen AMF conditions [Citation6,Citation29]. The MNPs were loaded into human prostate cancer cells (DU-145) which were then suspended in agarose gel for T2-weighted contrast measurements. All data were normalised for total sample iron content measured by inductively coupled plasma mass spectrometry (ICP-MS). T2-weighted contrast correlated with measured total iron (MNP) concentration among the samples and was essentially independent of magnetic properties among MNPs as determined by their heating properties.
Materials and methods
For clarity, a simplified schematic of the experimental design is illustrated in . The specific steps are described in detail following this summary. Human prostate carcinoma DU-145 cells were cultured and exposed to four iron oxide nanoparticle formulations, each having distinct magnetic properties. Standard cell transfection reagent, a synthetic amino acid poly-D-lysine, was used to modify the surface charge of the particles to aid cellular internalisation, following previously described methods [Citation49]. After exposure for 24 h, cells were washed and pelleted to remove any excess particles that had not been internalised. Iron concentration of supernatant was not measured. After re-suspending the cells in PBS, serially diluted aliquots of cells were immobilised in agarose gel for T2/ MR relaxivity measurement and one aliquot from each sample was used for iron quantification to co-register the result with the obtained relaxation times. A sample of nanoparticle-loaded cells was also seeded for clonogenic survival assay and stained for ferric iron using Perls’ (Prussian blue) reaction.
Magnetic iron oxide nanoparticles (MNPs)
Four aqueous iron oxide magnetic nanoparticle formulations – starch-coated BNF MNPs, Nanomag-D-SPIO (both from Micromod), Feridex I.V., and dextran-coated JHU (NanoMaterials Technology, Singapore). MNPs were obtained and characterised (). The hydrodynamic particle diameter of each particle type was determined using dynamic light scattering (DLS) with a Zetasizer Nano ZS-90 (Malvern Instruments, Malvern, UK). Physical and magnetic properties of the particles have been previously reported in several publications [Citation6,Citation28–31,Citation40,Citation41,Citation46–49]. SLP of each formulation over a range of field amplitudes is illustrated in , providing a surrogate comparison of differing total anisotropy energy relevant for hyperthermia at 155 kHz used in this study.
Figure 2. Comparison of amplitude-dependent SLP across peak-to-peak field amplitude spectrum among tested nanoparticle formulations. SLP values for Feridex were obtained from Bordelon et al. [Citation6], and are provided for comparison. Error bars represent standard deviation of the mean calculated from three measurements. AMF frequency was 155 ± 10 kHz.
![Figure 2. Comparison of amplitude-dependent SLP across peak-to-peak field amplitude spectrum among tested nanoparticle formulations. SLP values for Feridex were obtained from Bordelon et al. [Citation6], and are provided for comparison. Error bars represent standard deviation of the mean calculated from three measurements. AMF frequency was 155 ± 10 kHz.](/cms/asset/1a6686c1-a127-4916-9398-727ae3cdbd54/ihyt_a_913321_f0002_b.jpg)
Table I. Physical parameters of MNPs used in the current study.
Nanoparticle loading
Human prostate carcinoma DU-145 cells (ATCC HTB-81, American Type Culture Collection, Manassas, VA, USA) were cultured in flasks with GIBCO® Roswell Park Memorial Institute (RPMI)-1640 medium containing 2 mM L-glutamine and 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Aliquots of 75 µg Fe/mL of each particle type and 1 µg/mL poly-D-lysine (PDL, Sigma-Aldrich, St. Louis, MO) were added to the cell culture media exposing cells for 24 h at 37°C and 5% CO2 [Citation49,Citation50]. Then each flask was washed three times with Dulbecco’s PBS to remove any remaining free particles. Trypsin EDTA (0.25%) was added to detach the cells which were then re-suspended in RPMI for counting (with a Nexcelom Bioscience Cellometer Auto T4, Lawrence, MA).
Clonogenic survival assay
Cells were loaded at twice the target iron concentration, 150 µg/mL and incubated for 24 h. Samples were diluted and plated in triplicate in 100-mm culture dishes. After 14 days, the plates were stained with a crystal violet solution in 50% methanol. Colonies were counted and the survival fraction was determined relative to control unlabelled cell samples.
Intracellular iron quantification
An aliquot of each sample collected was set aside for iron quantification, using methods previously described [Citation49]. Briefly, aliquots reserved for iron quantification were centrifuged, supernatant removed, and the cell pellets were suspended in nitric acid (HNO3) for digestion in a MARS5 Xpress microwave (CEM, Matthews, NC). Cells were digested using a two-stage ramp to temperature method and the cellular digests were diluted for analysis in an Agilent 7500ce inductively coupled plasma mass spectrometer (Agilent Technologies, Santa Clara, CA). Total iron content of each cell sample was calculated based on an eight point calibration curve, blank correction, and the recovery of a standard reference material, SRM 2709a San Joaquin Soil (National Institute of Standards and Technology, Gaithersburg, MD). Total iron content per cell was calculated based on known cell number. Unlabelled controls were included to account for the endogenous iron present in cancer cells.
MR imaging
An aliquot of each sample was collected and set aside for MR imaging. Each aliquot was centrifuged and the cells were suspended in PBS and embedded in 3% agarose gel (1:10 dilution) in 200 µm micro-centrifuge tube. Up to 10 tubes were positioned in a plastic holder in a 40-mm diameter volume RF coil and imaged on a 9.4 T horizontal bore AvanceBiospec system (Bruker, Billerica, MA) at room temperature using a standard multi-slice spin echo imaging pulse sequence. Acquisition parameters used in the experiments were: repetition time TR = 4,000 ms, four spin echo times of TE = 7, 14, 21, and 28 ms, coronal slices with a slice thickness of 0.5 mm. Quantitative T2 maps were reconstructed from the imaging data using a fitting routine developed with IDL programming environment (Exelis, McLean, VA). The final analysis consisted of selecting an appropriate region of interest (ROI) and determining the mean T2 and standard deviation for the ROI using ImageJ software (http://rsbweb.nih.gov/ij). weighted gradient echo MR images were also obtained but were not used for quantitative analysis due to the very rapid signal decay.
Perls’ (Prussian blue) reaction
Perls’ reagent was used to detect ferric iron and ferritin. DU-145 cells were seeded on glass coverslips in flasks and incubated with identical particle concentrations as detailed above. The coverslips were removed, washed with PBS and fixed with 3% formaldehyde at room temperature. Fresh Prussian blue solution prepared from a 1:1 mixture of 4% potassium hexacyano ferrate (II) w/v in PBS and 4% HCl was added. Coverslips were then washed with PBS and incubated with nuclear fast red dye (diluted 1:5 in PBS), washed again and mounted.
Results
Experimental design and nanoparticle properties
In this study, our aim was to compare the T2/ relaxivity and MRI contrast properties of several MNP formulations developed for different applications: Feridex as an MRI contrast agent, Nanomag D-SPIO for biomedical applications such as cell sorting, and magnetic nanoparticle BNF and JHU for hyperthermia. The physicochemical and magnetic properties of these four representative iron oxide MNPs have been well described and differ significantly as measured by characterisation of their heating rates and SLP values measured at a frequency of 155 ± 10 kHz ( and ). Feridex SLP values, which are measurable but negligible at this frequency, have been previously reported and those values are shown in for comparison [Citation6]. Nanomag-D-SPIO MNPs have intermediate SLP, which is at least 100-fold above those of Feridex. Both JHU and BNF MNPs have the highest SLP of the panel of the nanoparticles used in the study, exceeding Feridex by a factor of 400 to 500. Although maximum SLP measured from JHU particles is similar to that observed with BNF particles, the field amplitude (H) dependence appears exponential and qualitatively similar to that of the Nanomag-D-SPIOs. Uniquely, the BNF particles display a strong ‘sigmoidal’ SLP with field at this frequency, consistent with previously reported results [Citation6]. The MNPs used in this study have comparable chemical composition (magnetite/maghemite) and similar hydrodynamic diameter (∼100 nm), but varying iron oxide crystal size and structure, surface coating and surface charge [Citation6,Citation28,Citation29–31,Citation39,Citation40,Citation46–48]. Thus, Feridex and Nanomag D-SPIO are composed of iron oxide nanocrystals embedded into a dextran matrix, whereas BNF and JHU nanoparticles have a single multicrystalline iron oxide core coated with a layer of biocompatible polymer or surfactant. Thus, these MNPs provide a convenient sample set to determine whether the cell-internalised MNPs having different magnetic properties has potential to affect their MRI contrast properties.
Treatment with a cell transfection reagent, poly-D-lysine, provides an established method to modify the surface physicochemical properties of the nanoparticles enabling maximal uptake by cells [Citation49,Citation50]. Even with this modification, differences occur among particles, necessitating ICP-MS quantification of total Fe content to appropriately normalise data for comparison. Further, serial dilutions of each prepared MNP cell stock were measured to provide a robust data set for comparison.
Nanoparticle loading
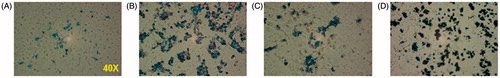
Representative images of DU-145 cells incubated with nanoparticle formulations and stained with Prussian blue are shown in . Nanoparticles appear to be internalised by the cells, with little to no membrane-bound or free MNPs. BNF and JHU MNP formulations appear to have dense intracellular particle distribution, while the Feridex and D-SPIO MNP formulations appear to have much less dense distribution for similar iron concentration.
Figure 3. Prussian blue stained slides of nanoparticle loaded DU-145 cells. Images were obtained after 24 h incubation with (A) D-SPIO, (B) JHU, (C) Feridex, and (D) BNF.
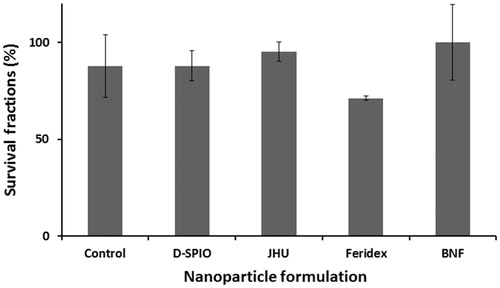
Clonogenic survival assays were conducted to determine the toxicity of internalised MNPs. To determine the level of toxicity to cells, survival fractions of DU-145 cells loaded at twice the target iron concentration (∼100 pg/cell) were performed. No significant concentration-dependent toxicity relative to control samples was observed ().
Figure 4. Survival fraction of DU-145 cells at 100 pg Fe/cell loading. Cells were incubated 24 h with various nanoparticle formulations.
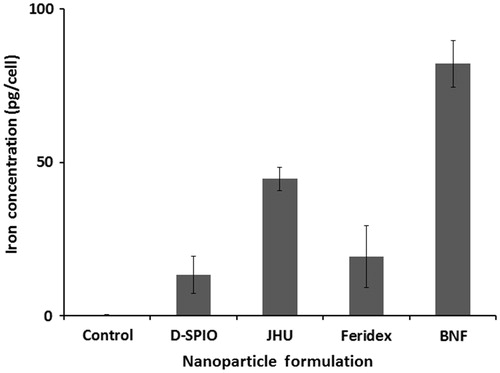
Samples loaded with nanoparticle formulations and assessed for iron content with ICP-MS showed a maximum iron concentration in BNF particle loaded cells, indicating this formulation had the highest iron loading efficiency. Decreased loading efficiency was noted with the JHU, Feridex, and D-SPIO formulations, respectively, in part due to the lower iron oxide content per nanoparticle (see ). Cells in each group were incubated with the same loading concentration of iron, and control cells were incubated in the medium without MNPs ().
Figure 5. Analysis of DU-145 cell-NP loaded iron concentration using ICP-MS. Aliquots ranged from 5 × 105 to 1.1 × 106 cells. Absolute iron concentration was calculated based on known cell number (pg Fe/cell).
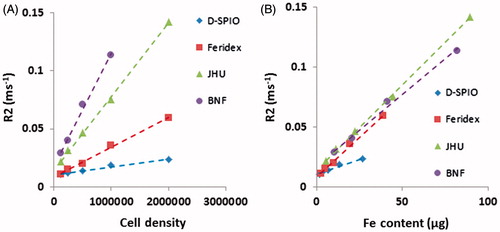
Transverse relaxation rates (R2 = 1/T2) of MNP loaded DU-145 cells demonstrated a linear increase with increased cell numbers (). BNF loaded samples, having the highest loading efficiency, corresponded to the shortest T2 times and thus increased transverse relaxation rates; whereas Nanomag-D-SPIO loaded samples with the lowest loading efficiency, had the longest T2 for all cell numbers. When normalised against total Fe concentration (per sample), MR imaging data demonstrated nearly linear increasing R2 with increasing Fe concentration (). Interestingly, comparable R2 values were detected for a given Fe concentration for all MNP preparations studied. Nanomag-D-SPIO MNPs generated slightly reduced R2 values, possibly due to systematic errors in the determination of low iron concentrations in these samples. relaxation rate was very short in all measured samples and signal intensities detected in the labelled cell pellets were too low for quantitative analysis at TE ≥1 ms. Therefore, no quantitative
data are presented.
Discussion
MR imaging is uniquely suited for in vivo visualisation of iron oxide magnetic nanoparticles in target organs because the iron oxide crystal generates strong local inhomogeneities of magnetic field that can be easily detected as hypo-intense areas in T2 or weighted images [Citation23]. SPIOs have also been extensively used in cell tracking MR experiments in which iron-oxide labelled cells are implanted and imaged longitudinally in vivo using high resolution MRI [Citation45]. First-in-human studies using endogenous human cells labelled in vitro with SPIOs have been recently reported for peripheral blood mononuclear cells [Citation51] and autologous mesenchymal stem cells [Citation52]. This method provides high sensitivity of detection, and a single cell labelled with a single micron-sized magnetic microsphere can be detected [Citation53,Citation54].
Given the considerable potential for biomedical applications in cell sorting and tracking, imaging and hyperthermia, various MNP constructs and formulations have been developed with increasing frequency [Citation54]. Single-purpose MNPs, i.e. superparamagnetic constructs developed only for MRI contrast present a well-studied but limited (and limiting) subset of these materials. For SPIOs, it has been well established that their utility for MRI contrast depends primarily upon cell- or tissue-associated concentration thereby placing a significant burden on their physicochemical properties, which to a large extent, controls the interaction of these particles with biological systems [Citation54].
The more recent recognition that SPIO constructs may fail to realise the full potential of MNPs (i.e. theranostic applications) has motivated development of synthesis techniques to produce MNPs having enhanced magnetic and crystalline anisotropies. These enhanced anisotropies increase the responsiveness of MNPs to magnetic fields, particularly linearly time-varying (or alternating) magnetic fields (AMFs). The focus on these constructs has been directed toward their heating properties (via magnetic hysteresis losses), and less toward their MRI contrast capability. Recent reports suggesting that some of these novel MNP compounds may provide enhanced MRI contrast thus raise the question that established models describing MNP contrast capabilities may necessitate a closer examination [Citation32].
To test this notion we have selected a representative set of MNPs that span both SPIO and high anisotropy samples to determine whether magnetic properties affect the MRI signal, i.e. T2 relaxivity. Of the chosen samples, three are commercially available and have been extensively characterised. All MNPs chosen for this study have been characterised for their heating properties, a surrogate measure of their anisotropy, at our chosen AMF frequency and amplitude conditions. Other nanoparticle constructs are available, yet this group was selected because extensive structural data and heating data provided a convenient sample set for initial comparative study [Citation6,Citation29,Citation39,Citation40]. Heat generated by MNPs when exposed to AMFs is the result of loss mechanisms that depend upon the frequency and amplitude of the AMF, and upon the total anisotropy energy of the MNPs [Citation6,Citation7]. Measurements of both heating rate and (mass normalised) amplitude-dependent SLP show that each of the particles has a unique combination of both total heating capability and loss power as a function of AMF amplitude. Thus, the set of MNPs selected for this study is appropriate to test the notion whether magnetic properties are potentially capable to modulate or enhance MRI contrast.
Given the dependence of hysteresis on total anisotropy energy [Citation7], measurements of SLP extending through magnetisation saturation (fixed frequency) of particles can provide a convenient relative measure of this energy. Magnetisation saturation of nanoparticles can differ significantly from that of the corresponding bulk material because of the heightened influence of anisotropies and surface effects, which make up a much larger proportion of the sample [Citation6,Citation7]. Typical values of saturation magnetisation for iron oxide nanoparticles (∼100 nm) can vary between 0.1 T to 0.2 T [Citation1,Citation6,Citation7,Citation9–11]. SLP measurements potentially provide a qualitative indication of the nature or extent of inter- or intra-particle dipole–dipole interactions [Citation6,Citation7]. In particular, it is useful to note the dependence of SLP on field amplitude, i.e. SLP(H) as the shape or nature of this curve can distinguish between ‘hard’ or ‘soft’ ferrite behaviour, suggesting extent of dipole–dipole interactions that are influenced by interactions with the external field (H) as recently described [Citation55]. One possible explanation is that a sigmoidal SLP(H) suggests a ‘hard’ magnetic (ferrite) structure, likely due to strong dipole–dipole coupling between particles. Conversely, weaker dipole–dipole interactions give rise to a ‘soft’ response to the external field which is manifest by an exponential SLP(H). These are speculative considerations with regard to the current sample set of particles; however, preliminary results suggest different internal (collective) magnetic structures among the particles [Citation7,Citation30,Citation55], and further study is needed.
Loading MNPs can be problematic, particularly for those constructs (e.g. coating, surface charge, hydrophilicity) that are designed to minimise non-specific interactions with cells. This is a design of MNPs used in many biomedical applications, and is especially important for MNPs used in clinical applications. Commonly used transfecting agents, e.g. poly-D-lysine, are established agents to coat materials and enhance their interactions with cells in order to maximise internalisation. Thus, we have used this material to increase the total amount of MNP uptake by cells, even though such a modification has no direct clinical application. In short, a sufficient intra-cellular (and relatively consistent) MNP concentration is needed to perform comparisons.
Interestingly, from our results it is apparent that the major determinant of MR efficiency of MNPs, as a T2 contrast agent, is the absolute iron content per cell. Complimentary results demonstrating linear dependence of T2-weighted MRI signal from the number of cells loaded with equal amounts of MNP were reported recently for different cell types [Citation54]. The specific magnetic parameters of the iron core such as magnetic anisotropy, residual ferro- or ferrimagnetism and the capacity to generate heat are secondary, and seemingly do not contribute significantly to the resulting T2 relaxivity. Indeed, it has been shown that the T2 relaxivity of water protons (R2 = 1/T2) generated by MNPs in the so-called motional average regime (MAR) is described as:
where ν is the volume fraction occupied by MNPs, the diffusion time, τD, for MNP size d and water diffusion coefficient D is defined as τD = d2/4D. Beq is the equatorial magnetic field generated by the MNP located in the external magnetic field B0, and γ is the gyromagnetic ratio of protons (≈42.6 MHz/T).
where μ0 is the magnetic permeability of vacuum (4π × 10−7 V s/(A m), and Msat is the saturation magnetisation of MNP [Citation56–58]. Generally, the magnetisation of iron oxide is saturated if the external field B0 is above ∼1.5 T. MAR conditions are fulfilled when τD < 1/γBeq. R2 reaches its maximum in the static dephasing regime (SDR) when τD ≈ 1/γBeq ∼ 150 ns and can be written as
As long as the radii of different MNPs are within an optimal narrow range, 20–30 nm, the R2 relaxivity and hence the T2 contrast of labelled cells in T2-weighted MRI is just a function of the total iron content of the samples as has been demonstrated in our experiments. While all our measurements have been performed using a preclinical high-field MR system operating at the B0 field of 9.4 T, these results are independent of the B0 strength and should have similar implication for MRI performed at typical clinical fields of 1.5 to 3.0 T.
Conclusions
This study demonstrates that for a range of iron oxide MNP magneto-structures and concentrations internalised by cancer cells, the signal intensity of T2-weighted MRI correlates closely with absolute iron concentration associated with the cells. This finding supports models that describe T2-shortening by clusters of magnetised spheres as dominated by unrestricted diffusion by water in large spheres when in the dephasing regime. Simultaneously, this study challenges recent claims that some nanoparticle formulations having enhanced magnetic anisotropy for hyperthermia may also display enhanced MRI contrast.
Declaration of interest
Robert Ivkov is an inventor on several issued and pending patents that disclose nanoparticle formulations for imaging and hyperthermia. All patents on which Robert Ivkov is an inventor are assigned to either Johns Hopkins University or Aduro Biotech, LLC. All other authors report no conflicts of interest. This work was funded by an award from the Safeway Foundation and the Prostate Cancer Foundation and by MD TEDCO Maryland Stem Cell Research Fund 2010-MSCRFE-0096. ICP-MS work was supported in part by the Maryland Cigarette Restitution Fund Program at Johns Hopkins Bloomberg School of Public Health and the National Institute of Environmental Health Sciences Center P30 ES00319.
References
- Pankhurst Q, Tranh N, Jones S, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys 2009;42:22401
- Jordan A, Wust P, Scholz R, Faehling H, Krause J, Felix R. Magnetic fluid hyperthermia (MFH). In: Hafeli U, Zborowski M, Schutt W, Teller J, editors. Scientific and Clinical Applications of Magnetic Carriers. New York: Plenum Press; 1997. pp 569–95
- Corot C, Robert P, Idée JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Delivery Rev 2006;58:1471–504
- Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 2005;293:483–96
- Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Del Rev 2008;60:1252–65
- Bordelon D, Cornejo C, Grüttner C, Westphal F, DeWeese TL, Ivkov R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl Phys 2011;109:124904
- Dennis CL, Ivkov R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2013;29:715–29
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg 1957;146:596–606
- Hergt R, Dutz S. Magnetic particle hyperthermia – Biophysical limitations of a visionary tumor therapy. J Magn Magn Mater 2007;311:187–92
- Carrey J, Mehdaoui B, Respaud M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J Appl Phys 2011;109:083921
- Dutz S, Kettering M, Hilger I, Müller R, Zeisberger M. Magnetic multicore nanoparticles for hyperthermia – Influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011;22:265102
- Stauffer PR, Sneed PK, Hashemi H, and Phillips TL. Practical induction heating coil designs for clinical hyperthermia with ferromagnetic implants. IEEE Trans Biomed Eng 1994;41:17–28
- DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, et al. Thermal dosimetry predictive of efficacy of 111In-ChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. J Nuc Med 2007;48:437–44
- Wust P, Gneveckow U, Johannsen M, Böhmer D, Henkel T, Kahmann F, et al. Magnetic nanoparticles for interstitial thermotherapy – Feasibility, tolerance and achieved temperatures. Int J Hyperthermia 2006;22:673–85
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 2005;21:637–47
- Jordan A, Maier-Hauff K. Magnetic nanoparticles for intracranial thermotherapy. J Nanosci Nanotechnol 2007;7:4604–6
- Lin JC, Bernardi P. Computational methods for predicting field intensity and temperature change. In: Barnes FS, Greenebaum B, editors. Bioengineering and Biophysical Aspects of Electromagnetic Fields, 3rd ed. Boca Raton: CRC Press; 2007. pp 293–380
- Liu F, Zhao H, Crozier S. On the induced electric field gradients in the human body for magnetic stimulation by gradient coils in MRI. IEEE Trans Biomed Eng 2003;50:804–15
- Adair ER, Black DR. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics 2003;6:S17–38
- Trakic A, Liu F, Crozier S. Transient temperature rise in a mouse due to low-frequency regional hyperthermia. Phys Med Biol 2006;51:1673–91
- Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, et al. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res 2005;11:S7093–103
- Atkinson WJ, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng 1984;31:70–5
- Artemov D. Molecular magnetic resonance imaging with targeted contrast agents. J Cell Biochem 2003;90:518–24
- Artemov D. MRI for molecular imaging applications: Overview, perspectives, and challenges. In: Willard HF, Ginsburg GS, editors. Genomic and Personalized Medicine, vol. 1. San Diego, CA: Elsevier Academic Press; 2009. pp 512–23
- Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol 2009;70:258–64
- Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 2008;108:2064–110
- Ivkov R. Magnetic nanoscale particle compositions, and therapeutic methods related thereto. US Patent 7,731,648
- Grüttner C, Müller K, Teller J, Westphal F, Foreman A., Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J Magn Magn Mater 2007;311:181–6
- Hedayati M, Attaluri A, Bordelon D, Goh R, Armour M, Zhou H, et al. New iron-oxide particles for magnetic nanoparticle hyperthermia: An in-vitro and in-vivo pilot study. Proc SPIE 2013, 8584, 858404-1–10
- Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Lau JW, et al. The influence of collective behavior on the magnetic and heating properties of iron oxide nanoparticles. J Appl Phys 2008;103:07A319
- Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology 2009;20:395103
- Lartigue L, Hugounenq P, Alloyeau D, Clarke SP, Levy M, Bacri JC, et al. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012;6:10935–49
- Girard OM, Du J, Agemy L, Sugahara KN, Kotamraju VR, Ruoslahti E, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: Comparison of T1, , and synergistic T1– contrast mechanisms. Magn Reson Med 2011;65:1649–60
- Liu W, Dahnke H, Jordan EK, Schaeffter T, Frank JA. In vivo MRI using positive-contrast techniques in detection of cells labeled with superparamagnetic iron oxide nanoparticles. NMR Biomed 2008;21:242–50
- Girard OM, Ramirez R, McCarty S, Mattrey RF. Toward absolute quantification of iron oxide nanoparticles as well as cell internalized fraction using multiparametric MRI. Contrast Media Mol Imaging 2012;7:411–17
- Billotey C, Wilhelm C, Devaud M, Bacri JC, Bittoun J, Gazeau F. Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging. Magn Reson Med 2003;49:646–54
- Bowen CV, Zhange XW, Saab G, Gareau PJ, Rutt BK. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magn Reson Med 2002;48:52–61
- Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime. Magn Reson Med 1994;32:749–63
- Wang Y-XJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur Radiol 2001;11:2319–31
- Feridex® prescribing information. Bayer Healthcare Pharmaceuticals, 2007. Available at: http://www.rxlist.com/feridex-iv-drug/overdosage-contraindications.htm
- Reimer P, Balzer T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur Radiol 2003;13:1266–76
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995–4021
- Schlorf T, Meincke M, Kossel E, Glüer CC, Jansen O, Mentlein R. Biological properties of iron oxide nanoparticles for cellular and molecular magnetic resonance imaging. Int J Mol Sci 2011;12:12–23
- Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Coro C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol 2004;39:56–63
- Lee N, Choi Y, Lee Y, Park M, Moon WK, Choi SH, et al. Water-dispersible ferromagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett 2012;12:3127–31
- Grüttner C, Müller K, Teller J, Westphal F, Foreman A., Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J Magn Magn Mater 2007;311:181–6
- Grüttner C, Teller J, Schütt W, Westphal F, Schümichen C, Paulke BR. Preparation and characterization of magnetic nanospheres for in vivo application. In: Hafeli U, Zborowski M, Schutt W, Teller J, editors. Scientific and Clinical Applications of Magnetic Carriers. New York: Plenum Press; 1997. pp 53–68
- Rudershausen S, Grüttner C, Frank M, Teller J, Westphal F. Multifunctional superparamagnetic nanoparticles for life science applications. Eur Cells Mater 2002;3:81–3
- Hedayati M, Thomas O, Abubaker-Sharif B, Zhou H, Cornejo C, Zhang Y, et al. The effect of cell cluster size on intracellular nanoparticle-mediated hyperthermia: Is it possible to treat microscopic tumors? Nanomedicine (Lond) 2013;8:29–41
- Frank JA, Zywicke H, Jordan EK, Mithcel J, Lewis BK, Miller B, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol 2002;9:S484–7
- Richards JMJ, Shaw CA, Lang NN, Williams MC, Semple SIK, MacGillivray TJ, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: Feasibility and safety in humans. Circ Cardiovasc Imaging 2012;5:509–17
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010;67:1187–94
- Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci 2004;101:10901–6
- Lee H, Yoon T-J, Figueiredo J-L, Swirski FK, Weissleder R. Rapid detection and profiling of cancer cells in fine-needle aspirates. PNAS 2009;106:12459–64
- Branquinho LC, Carriao MS, Costa AS, Zufelato N, Sousa MH, Miotto R, et al. Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia. Sci Rep 2013;3:2887
- Gossuin Y, Gillis P, Bue FL. Susceptibility-induced T2-shortening and unrestricted diffusion. Magn Res Med 2002;47:194–5
- Gillis P, Moiny F, Brooks RA. On T2-shortening by strongly magnetized spheres: A partial refocusing model. Magn Res Med 2002;47:257–63
- Pöselt E, Kloust H, Tromsdorf U, Janschel M, Han C, Maβlo C, et al. Relaxivity optimization of a PEGylated iron-oxide-based negative magnetic resonance agent for T2-weighted spin-echo imaging. ACS Nano 2012;2:1619–24