Abstract
Objective: The aim of this study was to assess whether high-intensity focused ultrasound (HIFU), a new and promising method for the treatment of benign hot and cold thyroid nodules using thermal ablation, has an impact on thyroid function, and to evaluate its feasibility in outpatient settings. Additionally, a possible difference in the treatment of solid and complex thyroid nodules was evaluated. Method: Ten patients with one thyroid nodule each (six cold and four hot nodules) underwent HIFU in January 2014. Four nodules were solid and six nodules were complex. Serum levels of triiodothyronine (T3), thyroxine (T4), thyrotropin (TSH), thyroglobulin (hTg) and additionally antibodies against hTg (TAK), TSH receptors (TRAK) and thyroid peroxidase (TPO) were measured at enrolment and 24 h after the HIFU treatment. The pre- and post-thyroglobulin reduction was measured to evaluate the scale of ablation. In addition, patients’ pain was recorded on a numeric rating scale from 0 to 10. Results: The HIFU treatment did not affect thyroid function, since hormone levels stayed stable (p < 0.05). No serious immune reaction was induced. Thyroglobulin serum levels increased significantly (p < 0.05) and were correlated to the total energy emitted by HIFU (p < 0.1). The results of complex thyroid nodules did not differ from solid thyroid nodules. Similarly, the results of hot thyroid nodules did not differ from cold thyroid nodules. All patients tolerated the whole treatment and no severe complications were observed. Conclusion: HIFU is a safe and effective method to treat benign, solid, complex, hot and cold thyroid nodules preserving thyroid function. Further developments of the system are needed to gain suitability for daily use.
Introduction
Thyroid nodules are common in clinical practice and can be found in about 20–50% of the patients examined, depending on the technique of detection [Citation1–6]. While most thyroid nodules are benign and do not require treatment, some benign nodules require treatment for associated symptoms such as compression, discomfort and cosmetic concerns [Citation7,Citation8,Citation9]. Surgical intervention and radioiodine therapy (RIT) still constitute the standard therapy options [Citation10,Citation11], but more minimal invasive alternatives, such as laser ablation [Citation12], radiofrequency ablation [Citation8], ethanol sclerotherapy [Citation13], microwave ablation [Citation7,Citation9,Citation14,Citation15] and highly intense focused ultrasound (HIFU), developed to treat thyroid nodules under local anaesthesia on an outpatient basis using ultrasound guidance, have been researched in the last decade. Nowadays some of these alternative techniques are already established as useful alternatives. Yet the majority of those standard therapies and alternatives still contain several drawbacks such as inducing serious immune reactions, for example Graves’ disease, scar formation, hypothyroidism and hyperthyroidism, thyrotoxicosis or possible inflammation [Citation10–19].
Among these alternative therapies, HIFU, using focused high-energy ultrasound beams seems promising. HIFU is already in use for breast lesions [Citation20] and prostate cancer [Citation21]. Furthermore, it is in development for the treatment of various tumours [Citation22–24]. Yet so far there have been only two studies investigating ablation of human thyroid tissue with HIFU [Citation25,Citation26] and two preceding animal studies [Citation27,Citation28].
This technique delivers several advantages over other ablative procedures: (1) being non-invasive, (2) great accuracy of planning and treatment itself due to the use of a robot arm, and (3) the simplicity of its practice. Furthermore, to our knowledge no serious immune reaction has yet been reported after any HIFU treatment of thyroid nodules.
The aim of this study was to observe the effects of HIFU on the thyroid gland function and evaluate feasibility under an outpatient setting. Pre- and post-ablative thyroid hormone statuses were compared and analysed.
Material and methods
Patients
Ten patients (two men, eight women, average age 56.7 years, range 36–80 years) were treated as outpatients. One nodule each (median volume 3.2 mL, range 0.8–7.67 mL, six cold and four hot nodules) was treated in one HIFU session. Four were solid and six were complex nodules. Inclusion criteria were (1) symptomatic thyroid nodules, (2) cosmetic concerns, and (3) refusal of or contraindications for surgery. Exclusion criteria were (1) asymptomatic nodules, (2) nodule volume exceeding 10 mL, (3) histological evidence for malignancy, (4) conspicuous Tc-99m methoxy-isobutyl-isonitrile (MIBI) uptake in cold nodules, and (5) conspicuous calcitonin measurement.
Baseline assessment
All patients underwent a pre-ablation assessment, including laboratory blood tests, ultrasound imaging, scintigraphic imaging and fine needle aspiration biopsy of the targeted nodule.
B-mode-ultrasound (Sonix Touch Ultrasound system, Ultrasonix Medical, Richmond, Canada) was used to evaluate volume, size, number and composition of the nodules.
Serum levels were determined with commercially available immuno-radiometric assay and radioimmunoassay (RIA) kits. Laboratory blood tests included a complete thyroid hormone status with triiodothyronine (T3) (normal range 1.0–3.3 nmol/L) determined by RIA (T3(125I) RIA kit, Izotop, Budapest, Hungary), thyroxine (T4) (normal range 55–170 nmol/L) determined by RIA (T4(125I) RIA kit), thyrotropin (TSH) (normal range 0.3–4.0 mU/L) determined by image retrieval in medical applications (IRMA) (SELco® TSH rapid, Medipan, Dahlewitz, Germany) and thyroglobulin (Tg) (normal range 2–70 ng/mL) determined by IRMA (Riason® Tg c.t., Iason, Graz-Seiersberg, Austria).
Moreover, calcitonin measurement, blood count and coagulation diagnostic were measured.
Additionally, the presence of antibodies was checked, namely against thyroid peroxidase (TPO) (positive > 50 U/mL) determined by RIA (anti-TPO magnum, Medipan), thyreoglobulin (TAK) (positive > 50 U/mL) determined by RIA (anti-Tg magnum, Medipan) and thyrotropin receptor (TRAK, positive > 1.5 IU/L) determined by RIA (TRAK Human RIA, Brahms, Henningsdorf, Germany).
All patients underwent Tc-99m pertechnetate imaging; images were taken 20 min after administration of 75 MBq (2 mCi) Tc-99m pertechnetate with a scintillator-camera (Mediso Nucline® TH/22, Mediso GmbH Münster, Germany). Patients with ‘cold’ nodules were re-evaluated with a Tc-99m MIBI scan to exclude malignancy. Images were taken 10 and 60 min after injection of 500 MBq (13.5 mCi) Tc-99m MIBI.
To exclude malignancy, fine needle aspiration biopsy, calcitonin measurement and Tc-99m MIBI-imaging was performed. No evidence for malignant transformation has been found.
HIFU treatment procedure and equipment
In this study the system that was used (Echopulse® THC900888-H, Theraclion, Malakoff, France) required assembly of its cooling system and a general validation of function at the start of each treatment. Depending on the patient’s individual pain tolerance and safety margins, the treatment was supported by local anaesthesia or without anaesthesia at all. The HIFU treatment was performed on an outpatient basis and all patients were able to leave immediately after the therapy.
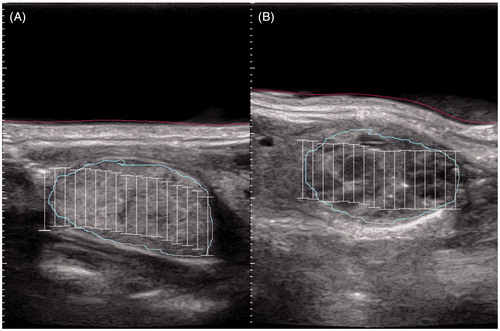
Eight out of 10 patients received local anaesthesia (Mecain 1%), followed by the positioning of the ultrasound-probe on the hyperextended neck of the patient, guided by sonographic imaging to position precisely on the intended nodule. If the nodule was located close to sensitive structures such as the trachea or carotid artery, local anaesthesia was applied in the tissue separating those structures from the nodule under sonographic imaging to increase the distance. The skin, targeted nodule, trachea and carotid artery were marked on the monitor on a starting layer in transverse and sagittal view (), sequentially followed in all transverse layers to precisely define the nodule and the sensitive structures to the system. After creating a one-layer voxel map filled with the planned ellipsoid focal points, each with a diameter of 2 mm and a height of 9 mm, a first test beam with medium energy was delivered to the centre of the nodule to test pain acceptance and effectiveness. Energy level was corrected and the system executed the ablation automatically following the computed voxel map in a screw pattern. A beam lasted 4 s followed by various cooling pauses, depending on the distance to the skin and the energy level. During the whole time the voxel map, sonographic imaging, and the planned sonographic image for comparison were visible to control the location of the ultrasound probe.
Figure 1. Exemplary ultrasound imaging of (A) a solid nodule and (B) a complex nodule during HIFU planning. The investigating physician drew the light blue line constituting the nodule and the red line constituting the skin. The white bars symbolise the planned treatment sites.
Manual pausing, repositioning, selecting or removing particular voxels and termination of the treatment were possible at all times. If the patient swallowed or moved during a beam, a laser-controlled movement detector directed at the laryngeal prominence would stop the treatment automatically and revert to manual repositioning or recalibration of the measured laser distance.
The imaging system worked with frequencies of 7.5–12 MHz and the therapy system with frequencies of 3 MHz, reaching temperatures approximately 80–90 °C. Heat is formed by absorbing acoustic energy and turning it into thermal energy.
Only one probe with a maximal penetration depth of 1.5 cm and an exchangeable cooling kit was used. The system automatically selected the following safety margins: (1) to the skin 0.5 cm, (2) to the trachea at least 0.3 cm, and (3) to the carotid artery 0.2 cm. The mean output per treated voxel varied between 87.6 and 192.8 W.
Efficacy evaluation and follow-up
All patients had a second scinitgraphic imaging and laboratory blood tests 24 h after the ablation treatment as well as a sonographic control immediately after the therapy.
Statistical analysis
Statistical analyses were done with the free R statistical software (developed by the R Development Core Team). As normal distribution could not be assumed, the tests for significant changes of any laboratory parameters were performed with non-parametric methods (Wilcoxon signed rank test). Due to small sample size and unknown underlying distributions, correlations are reported using Kendall’s tau and tested against the null hypothesis of tau = 0. Results were considered to be significant at p < 0.1.
Ethical standards
The study was approved by the local ethics committee and all patients signed a written informed consent prior to participation.
Results
The 10 HIFU treatment sessions were completed in a mean time of 68 ± 21 min (range 42–96 min). The treatment was well tolerated by all patients and an interruption was not necessary. The results for complex thyroid nodules in this study did not differ from the results for solid thyroid nodules (p > 0.1).
Laboratory tests
With the exception of hTg, no laboratory parameter, antibody measurement nor blood count changed significantly at the follow-up 24 h after ablation (p > 0.1). All patients were euthyroid after ablation.
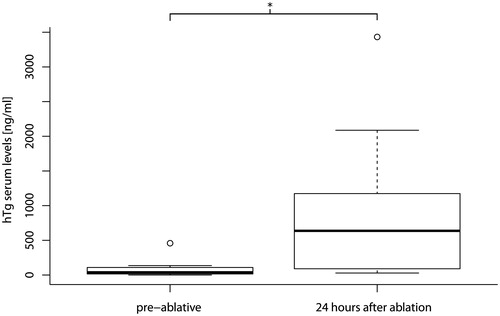
Thyroglobulin levels increased significantly (p < 0.05, ) with a mean pre-ablative hTg serum level of 91 ng/mL (range 0.1–459 ng/mL) and a mean post-ablative hTg serum level of 964 ng/mL (range 28.9–3431 ng/mL). A significant statistical correlation between the total energy emitted by the system and the increase of the thyroglobulin level was found (p < 0.1, tau = 0.5).
Treatment tolerance and efficacy
All patients reported a slight pain in the throat during the treatment. Some patients reported a spreading backwards to the neck, trapezius muscle, scapula and arm over time. The pain was captured on a numeric rating scale ranging from 0 to 10. The median pain during the treatment was 5.5 (range 3–7), and 2 (range 0–4) right after the treatment.
By 24 h after the treatment most patients developed flushing and slight swelling due to the pressure and cooling of the ultrasound probe over the long treatment time, which did not need any therapy. Furthermore one patient developed a mild haematoma at the injection site of the local anaesthesia. There were no serious adverse events such as infection, nodule rupture, secondary haemorrhage, hypoparathyroidism, voice change or injuries of the vagal or recurrent laryngeal nerve.
In the post therapeutic observation period no other complications were observed.
Pain during the treatment was not correlated to the energy per treated site (p > 0.1), to the time of the treatment (p > 0.1) nor to the treated volume (p > 0.1).
Discussion
Nowadays thyroidectomy and RIT are the standard therapy for benign thyroid nodules [Citation29]. Nevertheless those therapies, especially surgery, involve a high risk of major side effects such as a loss of function in the thyroid gland, nerve lesions, immune reactions such as Graves’ disease and scar formation. RIT is reported to have fewer side effects but at the same time it is only possible for patients with a high uptake of iodine in the region of interest.
Due to these drawbacks, there is a high demand to find new alternatives to surgery and RIT in the treatment of thyroid nodules. Thermal ablative procedures seemed to play a key role in this indication and produced convincing results [Citation30,Citation31]. HIFU represents the next logical step to a non-invasive, well-tolerated and efficient method with the lowest side effects possible and without any risk of infection. Based on the results of this study there is no evidence that HIFU either interferes with the function of the thyroid gland or induces thyrotoxicosis or serious immune reactions such as Graves’ disease. Thus the treatment of cold nodules, hot nodules or goitres with HIFU has no effect on the hormone status of the patients, whereas other thermal ablative treatments of hot thyroid nodules and goitres hold the risk of inducing transient thyrotoxicosis [Citation16]. According to Bhuyan [Citation32] and Vogl [Citation33] temperatures higher than 60 °C apply irreversible damage to tissue and thus destroy thyroid hormones, whereas unnatural temperatures under 60 °C apply reversible damage, in this case with a risk of leaking thyroid hormones. It is possible that HIFU, due to its small focal points, short ablation time of 4 s, constant cooling system, great accuracy of positioning, and delivering very little energy to the surrounding tissue, limits reversible heat damage that could induce thyrotoxicosis. However, other thermal ablative treatments using large ablation areas with rather large ablation time deliver much more energy to the surrounding tissue with a risk of inducing thyrotoxicosis. Furthermore, the automatic safety margins and the accuracy in planning the treatment reduce the risk of damage to nerves, vessels or trachea.
Increasing hTg levels 24 h after an ablative treatment of thyroid tissue can be attributed to the mass of the successfully damaged tissue. In this study hTg levels significantly increased in solid thyroid nodules as well as in complex thyroid nodules 24 h after ablation. It has already been proven that HIFU is a feasible and effective tool for treatment of solid nodules [Citation26]. It was also found in this study that both solid thyroid nodules and complex thyroid nodules are responsive to HIFU, due to the increase of hTg serum levels.
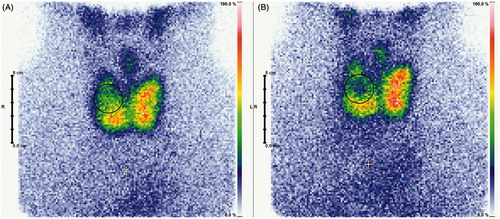
Furthermore, a correlation between the elevation of hTg serum level and the total energy emitted by the HIFU system was seen. Thus the aim of inducing coagulation necrosis by thermal ablation was successful, and it might be possible to value the effect of each single therapy by evaluating the emitted energy. Yet the global Tc-99m pertechnetate uptake should be considered for evaluation of the success of the therapy (). In comparison to benign hot thyroid nodules, the global Tc-99m pertechnetate uptake of a benign cold thyroid nodule should stay constant after treatment, whereas decrease is expected for treatment of benign hot thyroid nodules and goitres.
Figure 3. Exemplary scintigraphic imaging of a goitre pre-ablative (A), and 1 day post-ablation (B). Nodular Tc-99m pertechnetate uptake reduction is clearly visible.
Thus, in the future HIFU constitutes an interesting alternative to surgery. Yet HIFU’s current state is nowhere near technical perfection and still holds some disadvantages. The most impactful drawback was the treatment duration, a mean time of 68 min, which is mostly related to long cooling intervals and readjustments after frequently occurring movements. It was very hard for patients to lie still with a hyperextended neck and the pressure of the ultrasound probe for that long without swallowing or hawking. This being said, it is also possible that some voxels were partially treated multiple times or not at all. Furthermore, the long time for one session may not cover therapy for the whole volume of the nodule (). The automatically defined safety margins may reduce the treated volume as well as the geometric qualities of the nodule. One session of treatment was limited to one layer of ellipsoid voxels with a height of 9 mm. Hence every nodule extending over the height of 9 mm would only be treated partially in one session of HIFU treatment and would need a further session of treatment.
Table 1. Mean and range of volume calculated by ultrasound, calculated doses to fulfil the planned volume, calculated planned volume.
It was shown in this study that patients’ pain levels did not correlate to any limiting factors such as time, energy or treated volume. Thus in theory there could be multiple HIFU sessions or even multiple layer sessions to complete the treatment of the whole nodule volume. In addition, it was recognised that an announcement briefly prior to each beam could ease the patients’ pain noticeably. Naturally the patients’ pain tolerance could be still limited for extended therapy time or skin damage.
Conclusion
HIFU is safe, effective and easy to perform for benign hot thyroid nodules, benign cold thyroid nodules and goitres without any risk of inflammation. HIFU is an effective treatment for benign solid thyroid nodules and benign complex thyroid nodules. The function of the thyroid gland is preserved and no hints of serious immune reactions or thyrotoxicosis were found. Feasibility is not limited to any limited factors such as patients’ pain, energy level or treated volume. Yet technical effectiveness of HIFU ablation needs further development to fulfil its promising expectations. Thus far the long duration of a single HIFU session constitutes the major disadvantage of HIFU compared to other thermal ablative techniques. But at the same time HIFU contains the major advantage of being non-invasive and thus erases any risk of inflammation. Summarising, it may be possible to classify HIFU in its current state as effective for the treatment of nodules in the range of 1–4 mL, and provides appropriate treatment duration and treatment coverage.
Due to the small sample size, findings of this study need to be verified by larger studies. This applies particularly for the laboratory parameters that did not change significantly (thyroid hormones, antibodies). Also long-term outcomes as well as the feasibility of multiple HIFU treatments on benign thyroid nodules should be investigated further.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Etzel M, Happel C, von Müller F, Ackermann H, Bojunga J, Grünwald F. Palpation and elastography of thyroid nodules in comparison. Nuklearmedizin 2013;52:97–100
- Reiners C, Schumm-Draeger PM, Geling M, Mastbaum C, Schönberger J, Laue-Savic A. Schilddrüsenultraschallscreening [Thyroid ultrasound screening]. Internist 2003;44:412–19
- Schicha H, Hellmich M, Lehrmacher W, Eschner W, Schmidt M, Kobe C, et al. Should all patients with thyroid nodules ≥1 cm undergo fine-needle aspiration biopsy? Nuklearmedizin 2009;48:79–83
- Schmid KW, Sheu S-Y, Görges R, Ensinger C, Tötsch M. Tumoren der Schilddrüse [Tumours of the thyroid]. Pathologe 2003;24:357–72
- Völzke H, Lüdemann J, Robinson DM, Spieker KW, Schwahn C, Kramer A, et al. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid 2003;13:803–10
- Happel C, Truong PN, Bockisch B, Zaplatnikov K, Kranert WT, Korkusuz H, et al. Colour-coded duplex-sonography versus scintigraphy. Can scintigraphy be replaced by sonography for diagnosis of functional thyroid autonomy? Nuklearmedizin 2013;52:157–203
- Korkusuz H, Happel C, Heck K, Ackermann H, Grünwald F. Percutaneous thermal microwave ablation of thyroid nodules. Nuklearmedizin 2014;53:123–30
- Jeong WK, Baek JH, Rhim H, Kim YS, Kwak M, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: Safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244–50
- Yue W, Wang S, Wang B, Xu Q, Yu S, Yonglin Z. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: Safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11–16
- Dietlein M, Dressler J, Grünwald F, Leisner B, Moser E, Reiners C, et al. Leitlinie zur Radioiodtherapie (RIT) bei benignen Schilddrüsenerkrankungen [Guideline for radioiodine therapy (RIT) for benign thyroid diseases] (Version 4). Nuklearmedizin 2007;46:220–23
- Lin CM, Doyle P, Tsan YT, Lee CH, Wang JD, Chen PC, et al. 131I treatment for thyroid cancer and risk of developing primary hyperparathyroidism: A cohort study. Eur J Nucl Med Mol Imaging 2014;41:253–59
- Papini E, Rago T, Gambelunghe G, Valcavi R, Bizzarri G, Vitti P, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 2014;jc20141826 [e-pub ahead of print]
- Martino E, Murtas ML, Loviselli A, Piga M, Petrini L, Miccoli P, et al. Percutaneous intranodular ethanol injection for treatment of autonomously functioning thyroid nodules. Surgery 1992;112:1161–5
- Korkusuz H, Happel C, Grünwald F. Ultrasound guided percutaneous microwave ablation of hypofunctional thyroid nodules: Evaluation by scintigraphic 99mTc-MIBI imaging. Nuklearmedizin 2013;52:205–57
- Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: Initial experience. Int J Hyperthermia 2014;30:150–57
- Christou N, Mathonnet M. Complications after total thyroidectomy. J Visc Surg 2013;150:249–56
- Han EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes 2011;18:310–14
- Tarantino L, Francica G, Sordelli I, Sperlongano P, Parmeggiani D, Ripa C, et al. Percutaneous ethanol injection of hyperfunctioning thyroid nodules: Long-term follow-up in 125 patients. Am J Roentgenol 2008;190:800–8
- Feng B, Liang P, Cheng Z, Yu X, Yu J, Han Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: Experimental and clinical studies. Eur J Endocrinol 2012;166:1031–7
- Kaiser WA, Pfleiderer SO, Baltzer PA. MRI-guided interventions of the breast. J Magn Reson Imaging 2008;27:347–55
- Rewcastle JC. High intensity focused ultrasound for prostate cancer: A review of the scientific foundation, technology and clinical outcomes. Technol Cancer Res Treat 2006;5(6):619–25
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumors in a western population. Br J Cancer 2005;93:890–95
- Wang Y, Wang W, Tang J. Primary malignant tumours of the bony pelvis: US-guided high intensity focused ultrasound ablation. Int J Hyperthermia 2013;29:683–7
- Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: Preliminary results. Int J Hyperthermia 2011;27:648–53
- Esnault O, Rouxel A, Le Nestour E, Gheron G, Lennhardt L. Minimally invasive ablation of a toxic thyroid nodule by high-intensity focused ultrasound. Am J Neuroradiol 2010;31:1967–8
- Esnault O, Franc B, Ménégaux F, Rouxel A, De Kerviler E, Bourrier P, et al. High-intensity focused ultrasound ablation of thyroid nodules: First human feasibility study. Thyroid 2011;21:965–73
- Esnault O, Franc, B, Chapelon JY. Localized ablation of thyroid tissue by high-intensity focused ultrasound: Improvement of noninvasive tissue necrosis methods. Thyroid 2009;19:1085–91
- Esnault O, Franc B, Monteil JP, Chapelon JY. High-intensity focused ultrasound for localized thyroid-tissue ablation: preliminary experimental animal study. Thyroid 2004;14:1072–6
- Pacini F, Burroni L, Ciuoli C, Di Cairano G, Guarino E. Management of thyroid nodules: A clinicopathological, evidence-based approach. Eur J Nucl Med Mol Imaging 2004;31:1443–9
- Gharib H, Hegeduüs L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 2013;98:3949–57
- Papini E, Pacella CM, Laszlo H. Thyroid ultrasound and ultrasound assisted procedures. From the shadows into an array of applications. Eur J Endocrinol 2014. doi:10.1530/EJE-13-0917
- Bhuyan BK. Kinetics of cell kill by hyperthermia. Cancer Res 1979;39:2277–84
- Vogl TJ, Zegelman A, Bechstein WO, Zeuzem S, Zangos S. Treatment of liver metastases of colorectal carcinoma: Overview of hyperthermal ablation methods. Dtsch Med Wochenschr 2013;138:792–8