Abstract
Purpose: The aim of this study was to demonstrate that multi-wall carbon nanotubes can be functionalised with antibodies to group A streptoccocus (GAS) for targeted photothermal ablation of planktonic and biofilm residing bacteria.
Materials and methods: Antibodies for GAS were covalently attached to carboxylated multi-wall carbon nanotubes and incubated with either planktonic or biofilm GAS. Bacterium was then exposed to 1.3 W/cm2 of 800 nm light for 10–120 s, and then serially diluted onto agar plates from which the number of colony forming units was determined. Photothermal ablation of GAS on the surface of full thickness ex vivo porcine skin and histological sectioning were done to examine damage in adjacent tissue.
Results: Approximately 14% of the GAS antibody-functionalised nanotubes attached to the bacterium, and this amount was found to be capable of inducing photothermal ablation of GAS upon exposure to 1.3 W/cm2 of 800 nm light. Cell viability was not decreased upon exposure to nanotubes or infrared light alone. Compared to carboxylated multi-wall carbon nanotubes, antibody-labelled nanotubes enhanced killing in both planktonic and biofilm GAS in conjunction with infrared light. Analysis of GAS photothermally ablated in direct contact with ex vivo porcine skin shows that heat sufficient for killing GAS remains localised and does not cause collateral damage in tissue adjacent to the treated area.
Conclusions: The results of this study support the premise that carbon nanotubes may be effectively utilised as highly localised photothermal agents with the potential for translation into the clinical treatment of bacterial infections of soft tissue.
Introduction
Virulent bacterial infections are a major therapeutic challenge in all areas of clinical practice. Within the USA there are approximately 60–90,000 deaths from hospital-acquired infections alone, resulting in treatment costs ranging from $17 to $29 billion [Citation1]. One of the major pathogens encountered is Streptococcus pyogenes, alternatively termed group A streptococcus (GAS). GAS is a Gram-positive human pathogen that routinely causes a variety of infections, including pharyngitis, upper and lower respiratory tract infections, and dermal infections such as impetigo and cellulitis. An invasive form of GAS disease is necrotising fasciitis, an infection that occurs in deep tissue and spreads along the fascial planes, destroying fat and muscle. During 2012, the US Centers for Disease Control and Prevention surveyed a population of over 32,000,000 people and documented 10,700 cases of invasive GAS, resulting in 1,115 deaths [Citation2]. Unlike less severe infections which can be treated with antibiotics, necrotising fasciitis requires aggressive surgical debridement of necrotic tissue and sacrifice of adjacent compromised tissue. Invasive infections involving GAS can progress quickly and serial aggressive surgeries are usually necessary to completely clear diseased tissue. A major challenge for treating GAS infections with surgery and antibiotics is that the bacteria thrive in biofilms. Biofilms consist of colonies of bacteria embedded within a three-dimensional extracellular matrix composed of polysaccharides, proteins and DNA. Biofilms inhibit penetration of antibiotics and hinder the immune response, thus promoting survival of the bacteria.
Within the past decade, nano-structured materials such as specific coatings on orthopaedic implants have been mechanistically evaluated as an option to disrupt biofilm formation and growth [Citation3]. Similarly, carbon nanotubes have been explored as coatings on filters to decrease biofilm growth and to trap pathogens [Citation4–6]. Individual nanoparticles such as gold nanorods, graphene and carbon nanotubes have been employed for their ability to generate sufficient heat to kill bacteria upon exposure to infrared light [Citation7–14].
The spectral region where water and haemoglobin have optical absorption minima is between 700–900 nm, therefore photothermal therapies using near infrared light (NIR) are ideal for penetrating soft tissue [Citation15]. Over the past decade the majority of nanoparticle-mediated photothermal therapies for ablation of tissue (PTA) have been developed for targeted treatment of cancer. Much higher temperatures are needed to effectively eliminate bacteria than to kill cancer cells. Most pathogenic bacteria require temperatures in excess of 70 °C, whereas eukaryotic cells begin to suffer irreparable damage at temperatures as low as 45 °C [Citation16–18]. The length of time at which bacteria are subjected to elevated hyperthermia and the degree of hyperthermia have a major influence on complete eradication. Van Asselt et al. [Citation19], calculated that a log reduction of pathogenic bacteria per minute of exposure to elevated temperature is linearly related to increasing temperature. For S. pyogenes there was a 1-log/min reduction minute for every 10 °C increase. The calculated predictions from van Asselt et al. also showed that the rate of thermal inactivation was similar for Gram-positive bacteria such as S. aureus and S. pyogenes. Hossain et al. [Citation20] experimentally demonstrated that, using a steam autoclave, both S. aureus and S. pyogenes had a 5-log reduction when subjected to 131 °C for 5 min, and complete killing of the bacterium when they were treated at 131 °C for 15 min. Hossain et al. also showed that higher temperatures and longer times are needed to thermally inactivate Gram-positive bacteria compared to Gram-negative bacteria. Recent studies have shown that very rapid temperature spikes from nanoparticles can cause cancer cell death [Citation21]. This suggests that rapidly induced high temperatures at the bacterial cell surface may be bactericidal.
Carbon nanotubes have excellent photo-absorption in the NIR, and generate heat by absorbing incident light which induces phonon resonances along the tube length leading to very specific peaks of thermal intensity that correspond to the length of the nanotubes [Citation22]. In one of the first uses of carbon nanotubes for generating hyperthermia, Kam et al. [Citation17] showed that single-wall carbon nanotubes (SWNT) could generate temperatures in small volumes of aqueous media of up to 75 °C with 2 min of exposure to 808 nm light. Boldor et al. [Citation23] determined that multi-wall carbon nanotubes (MWNT) are also capable of generating elevated temperatures (ΔT∼70 °C) using 1064 nm light. Previously, both single-wall and multi-wall carbon nanotubes have been used for targeting individual cancer cells for photothermal ablation. Some of the agents that have been attached to the nanotube side walls for targeting include DNA, polysaccharides, folic acid, growth factors, and antibodies [Citation17,Citation24–29]. In a similar fashion, we predict that MWNTs targeted to the bacterial surface should induce sufficient heat to irreversibly damage the bacterium upon exposure to infrared light. Due to their small size and high aspect ratios (one to tens of nanometers in diameter, and micron lengths), carbon nanotubes may be able to effectively penetrate biofilms and attach to the individual bacterial cell wall. Herein we describe an alternative approach for eradicating GAS through the use of MWNT for photothermal ablation of GAS biofilms, specifically by targeted killing of individual bacterium.
Experimental section
Materials
MWNTs (>99% purity; outer diameter 13–18 nm, length 3–30 µm) were purchased from Cheap Tubes (Cheap Tubes, Inc., Cambridgeport, VT) and used as received. Concentrated nitric acid (70%) and concentrated sulphuric acid (98%) were purchased from Fisher Scientific (Waltham, MA). 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and 2-(N-morpholino)ethanesulphonic acid (MES) were purchased from Aldrich (St. Louis, MO). Todd Hewitt broth was purchased from Becton-Dickinson (Franklin Lakes, NJ). Yeast extract was purchased from Fisher Scientific. Polyclonal antibodies for GAS were purchased from US Biological (Salem, MA). All reagents were used as received without further purification.
Synthesis of oxidised MWNTs (MWNT-COOH)
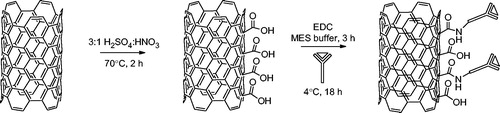
As-grown multi-walled carbon nanotubes (100 mg) were oxidised through addition of a 3:1 mixture of concentrated sulphuric acid to concentrated nitric acid (40 mL total) and the mixture was heated to 70 °C for 2 h. The suspension was filtered through a 0.2 µm filter and washed with copious amounts of water until the pH of the filtrate solution was neutral. The tubes were then washed with ethanol and acetone and allowed to dry at room temperature in air overnight to yield oxidised MWNTs (MWNT-COOH).
Synthesis of functionalised MWNTs (GAS-Ab-MWNTs)
Oxidised MWNTs (3 mg) were dispersed in MES buffer (pH 6, 1 mg/mL) through horn sonication. 1EDC was added and the solution was stirred at room temperature for 4 h. Then 75 μL of GAS antibody (1 mg/mL in phosphate buffered saline (PBS)) was added and the solution was stirred for a further 18 h at 4 °C. The tubes were centrifuged at 14,000 rpm and washed twice with MES buffer and finally re-suspended in MES buffer and stored at 4 °C until use. The GAS-Ab-MWNTs were washed repeatedly in sterile PBS, followed by centrifugation and re-suspension in sterile PBS solution. The Thermo Scientific™ Pierce™ bicinchoninic acid (BCA) assay was used according to the manufacturer’s instructions to quantify the amount of GAS antibody bound to MWNT-COOH after functionalisation.
Spectral characterisation
Fourier transform infrared spectra (FT-IR) were recorded on either a Mattson (Fremont, CA) Genesis II FT-IR spectrometer or on a Perkin–Elmer (Akron, OH) Spectrum 10 spectrometer with an ATR sampling accessory equipped with a diamond anvil. Raman spectra were recorded on a DeltaNu (Laramie, WY) Advantage 532 Raman spectrometer at 532 nm. The absorbance of MWNT solutions at 800 nm for MWNTs was measured using a Beckman Coulter (Brea, CA) DU730 Life Science UV/Vis spectrophotometer and the concentration determined using a standardised concentration curve of MWNT-COOH.
Hyperthermic potential of MWNT
Aliquots of 200 μL aqueous solutions containing MWNT-COOH at concentrations of 0–100 μg/mL were placed in wells of a covered 48-well plate. The plate was incubated at 37 °C for 20 min to reach a baseline temperature of 37 °C. During laser exposure the ambient temperature of the plate was maintained by placing the plate on a hot water bottle that was 37 °C. Each well was exposed to 800 nm light (1.3 W/cm2 for 30 s) using the Cube™ continuous wave diode laser from K-Laser, Nasheville, TN. The initial and final temperatures were measured using a Fluke (Everett, WA) 714 thermocouple calibrator and type k 80Pk-1 bead probe wire thermocouple immediately before and after laser exposure of each nanotube concentration. The experiment was repeated using GAS-Ab-MWNT.
Bacterial strains and growth conditions
This study utilised Streptococcus pyogenes MGAS5005, a clinical M1T1 serotype strain isolated from a case of invasive GAS disease [Citation30,Citation31]. Planktonic and biofilm cultures were grown in Todd-Hewitt broth supplemented with 2% yeast extract (THY) at 37 °C, 5% CO2.
GAS planktonic cultures exposed to laser treatment
GAS cultures were grown overnight in THY, 37 °C, 5% CO2. The following day a 1:10 subculture was grown in fresh THY, 37 °C, 5% CO2 to logarithmic phase (OD600∼0.6). Cultures of 3 mL in THY, THY supplemented with 0.1 mg/mL MWNT-COOH, or THY supplemented with 0.1 mg/mL GAS-Ab-MWNT were added to 15-mL conical tubes and incubated shaking (on low) for 1 h at 37 °C. Cultures were spun down at 2000 rpm for 2 min at 4 °C. Supernatants were removed and pellets vortexed and resuspended in 3 mL of fresh THY. Cultures of 200 µL per well were aliquoted into 48-well polystyrene plates and incubated for 20 min at 37 °C to acclimate to a baseline temperature of 37 °C. Cultures were then exposed to an 800-nm continuous wave laser (1.3 W/cm2) for 0, 10, 20, or 30 s. After laser exposure, samples were serially diluted and plated on THY agar plates. The number of colony-forming units (CFUs) was visually counted 24 h after incubation at 37 °C.
GAS biofilms exposed to laser treatment
GAS cultures were grown overnight in THY, 37 °C, 5% CO2. The following day a 1:10 subculture was grown in fresh THY in 37 °C, 5% CO2 to logarithmic phase (OD600∼0.6). Culture volumes of 200 µL per well were aliquoted into 48-well polystyrene plates. After 24 h incubation the media was removed from the biofilms and replaced with 200 µL of fresh THY, THY supplemented with 0.1 mg/mL MWNT-COOH, or THY supplemented with 0.1 mg/mL GAS-Ab-MWNT and incubated 1 h at 37 °C, 5% CO2. Supernatants were removed and replaced with 200 µL of fresh THY. Biofilms were incubated in 37 °C for 20 min to acclimate to a baseline temperature of 37 °C. Biofilms were then exposed to an 800 nm continuous wave laser (1.3 W/cm2) for 0, 30, 60 or 120 s. After laser exposure, supernatants were removed and biofilms were scraped and re-suspended in 200 µL of fresh THY. These samples were serially diluted and plated on THY agar plates. After 24 h incubation at 37 °C, the number of CFUs was visually counted.
Microscopy of biofilms exposed to laser treatment
GAS cultures were grown overnight in THY at 37 °C, 5% CO2. The following day a 1:10 subculture was grown in fresh THY at 37 °C, 5% CO2 to logarithmic phase (OD600∼0.6). GAS cultures of 357.9 µL per well were aliquoted into a Lab-Tek®II Chambered 1.5 German Coverglass System (Fisher Scientific) (culture volume calculated to be comparable to the area covered by 200 µL GAS culture in a multi-well 48-well plate). After 24 h of incubation, biofilms media was removed and replaced with 357.9 µL of fresh THY, THY supplemented with 0.1 mg/mL MWNT-COOH, or THY supplemented with 0.1 mg/mL GAS-Ab-MWNT and incubated 1 h at 37 °C, 5% CO2. Supernatants were removed and replaced with 357.9 µL of fresh THY. Biofilms were incubated in 37 °C for 20 min to acclimate to a baseline temperature of 37 °C. Biofilms were then exposed to an 800 nm laser (1.3 W/cm2) for 0 or 2 min. After laser exposure, biofilms were analysed for viability by LIVE/DEAD® BacLight™ bacterial viability kit (Molecular Probes, Eugene, OR) staining. Supernatants from samples were removed and replaced with 357.9 µL of 1 × PBS with equal amounts of SYTO 9 and propidium idodide and incubated for 15 min. Nikon C1Si confocal laser-scanning microscopy (CLSM) and EZ-C1 software were used to visualise and capture images. The analytical software, ImageJ (http://imagej.nih.gov/ij/), was used to quantify the percentage of live and dead bacterial populations in the representative images.
Histological evaluation of photothermal damage in ex vivo porcine skin
Full thickness sections of skin, including the dermis, epidermis, subcutaneous fat and muscle were obtained from freshly euthanised swine. The skin was shaved, cleaned with ethanol and a cylindrical chamber approximately 7 mm in diameter secured to the skin using polyacrylamide glue. GAS incubated with GAS-Ab-MWNT were added to the chamber and exposed to infrared light similar to the procedures described above. The size of the chamber was specifically chosen to allow overlap of the incident laser beam (beam diameter of 1 cm) onto the skin adjacent to the chamber. It was already known from previous in vitro results that the planktonic bacteria would be killed, thus the goal was to observe whether thermal damage occurs in skin adjacent to the ablative treatment area. Following laser irradiation, the chamber was removed and the number of CFUs in the suspension enumerated to confirm GAS killing. The skin was fixed in 4% paraformaldehyde, embedded in paraffin, sectioned for light microscopy and stained with Mason’s trichrome and haematoxylin and eosin stain to visualise cells as well as collagen. Microscopy images were taken at a magnification of 5× using a Zeiss (Jena, Germany) Axioplan 2.
Results
Characterisation of functionalised MWNT
GAS-Ab-MWNTs were synthesised according to a similar literature procedure and can be seen in [Citation28]. Pristine MWNTs were heated to 70 °C in a mixture of 3:1 concentrated H2SO4:HNO3 to give MWNT-COOH. Covalent attachment of the GAS antibody was carried out using EDC in MES buffer. EDC activates the carboxylic acid groups on the oxidised MWNTs and facilitates the coupling of primary amines on lysine and arginine residues of the antibody to the MWNT through amide bonds [Citation32].
As shown in ), the FT-IR of oxidised MWNT (MWNT-COOH) shows O-H stretching from carboxyl groups near 3430 cm−1 [Citation33]. The peaks near 1700 cm−1 are indicative of oxidative groups on the MWNT [Citation34]. The antibody has a broad peak near 1650 cm−1, which is indicative of N-H bending vibrations [Citation35]. The weak stretches of the GAS antibody appearing near 1050 cm−1 are indicative of C-N stretching [Citation35]. These peaks are significantly dampened upon conjugation to MWNT-COOH. This dampening effect, as well as the shifting to higher wavenumbers (3325 cm−1 to 3430 cm−1) supports the conclusion that GAS-Ab is attached to MWNT.
Figure 1. (A) FT-IR spectra of pristine MWNT-COOH, GAS antibody, and the GAS-Ab-MWNTs. (B) Raman spectra of MWNT-COOH and GAS-Ab-MWNTs.
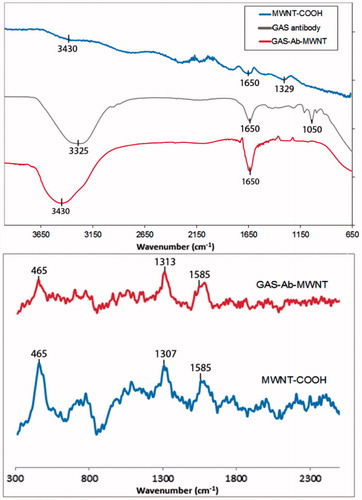
Raman spectroscopy was also used to determine whether antibodies were covalently attached to the surface of the carbon nanotubes. The characteristic D- and G-bands for the MWNT-COOH and GAS-Ab-MWNTs are shown at near 1300 and 1585 cm−1, respectively [Citation36]. The peak near 465 cm−1 stems from the radial breathing mode (RBM) of the innermost tube within MWNT-COOH [Citation37]. Quenching of the RBM upon attachment of the GAS antibody supports the premise that the antibody has attached to the exterior sidewall of the MWNT.
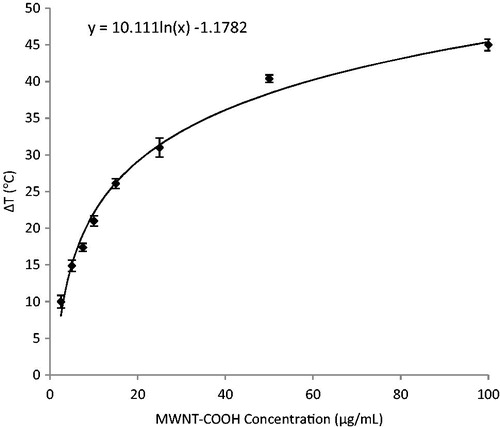
Variable concentrations of MWNT-COOH or AS-Ab-MWNT exposed to 1.3 W/cm2 of 800 nm light were used to evaluate temperature increases in a 200 µL volume of water. As shown in , there is a logarithmic increase in temperature change with increasing nanotubes concentration. A temperature increase of 40 °C for the concentration of 100 µg/mL is sufficient for inducing irreversible damage in GAS, according to Kennedy et al. [Citation16], since 40 °C over the baseline of 37 °C results in 77 °C. Therefore, this concentration was used for photothermal ablation experiments. BCA assay was used to quantify the amount of GAS antibody attached to MWNT-COOH. This assay revealed that 10 µg of GAS antibody bound per 1 mg of MWNT-COOH, or alternatively 66.7 pmol antibody per mg nanotubes. The increase in temperature from GAS-Ab-MWNT fitted the same curve as for MWNT-COOH, as shown in (data not shown).
Quantifying MWNT bound to GAS
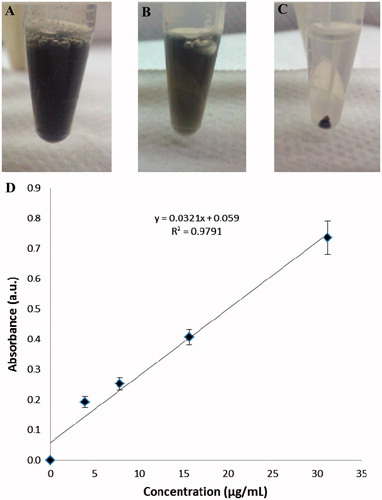
To quantify the amount of GAS-Ab-MWNT attached to bacteria, the solutions were centrifuged, washed with PBS buffer and resuspended in THY broth. Specifically, a much higher centrifugation speed is required to pellet the nanotubes (14,000 rpm for 30 min) whereas 2000 rpm for 2 min will pellet the bacteria. Thus, nanotubes that did not bind to GAS were retained in the supernatant, and the bacteria to which nanotubes had attached were captured in the pellet. This removed the vast majority of MWNT-COOH or GAS-Ab-MWNTs that did not bind to bacteria. As shown in ), the suspension of equal volumes of GAS-Ab-MWNT and GAS is quite dark, indicative of the high concentration of nanotubes. After centrifuging the GAS with bound nanotubes, the resulting supernatant, as shown in ), is lighter in colour but still fairly dark, indicating that the majority of GAS-Ab-MWNT were not bound to bacteria. ) is a representative image of pelleted GAS to which GAS-Ab-MWNT had attached. The supernatant of GAS incubated with non-functionalised MWNT-COOH was also quite dark (not shown), indicative of the non-attachment of MWNT-COOH to bacteria. The supernatants of non-bound GAS-Ab-MWNT and MWNT-COOH were measured by UV-Vis absorption spectroscopy to determine the concentration of nanotubes that were not bound to bacteria. A calibration curve was formulated using known concentrations of MWNT-COOH, as shown in ). The supernatant absorbance value corresponds to a concentration of MWNTs. GAS was incubated with 100 µg of either GAS-Ab-MWNT or MWNT-COOH. The amount of MWNT-COOH bound to GAS after incubation was 4 µg whereas for GAS-Ab-MWNT the amount bound was 14 µg.
Figure 3 (A) A suspension of equal parts GAS and GAS-Ab-MWNT. (B) The resulting supernatant after centrifuging GAS-Ab-MWNT that had bound to GAS. (C) The resulting pellet of GAS with attached GAS-Ab-MWNT after incubation. (D) A calibration curve of known concentrations of f-MWNTs and GAS bacteria grown to an OD600 = 0.6 in 1:1 PBS:THY broth.
Photothermal ablation of planktonic and biofilm GAS
Photothermal ablation is possible using both MWNT-COOH and GAS-Ab-MWNT, as shown in ; with the GAS-Ab-MWNT being much more successful at killing GAS bacteria both in planktonic culture and in biofilms, as measured by the number of colony forming units (CFUs) that grow after treatment. There is a statistically significant decrease in bacterial counts for planktonic GAS bacteria incubated with MWNT-COOH (47%) or GAS-Ab-MWNT (71%), without exposure to infrared light (). The control group, which received no MWNT but was exposed to infrared light (1.3 W/cm2, 800 nm), showed a progressive decrease in CFUs with increasing time of exposure, correlating to a 16, 36, and 47% reduction with 10, 20, and 30 s of light exposure. In comparison, MWNT-COOH provided a 60, 68, and 86% decrease in CFUs for 10, 20 and 30 s, respectively (). The results of the GAS-Ab-MWNT group demonstrate the greatest reduction in CFUs, beginning with a 97% reduction upon exposure to 10 s of 800 nm light, 99.98% after 20 s, and 100% killing of planktonic GAS at 30 s of infrared exposure (). To correlate temperature with a decrease in CFUs, 10 s of laser exposure results in a temperature of 46 °C using functionalised or non-functionalised MWNT. GAS subjected to this treatment using MWNT-COOH resulted in a 5-fold reduction of CFUs whereas GAS-Ab-MWNT provided a 100-fold reduction in GAS CFUs. Laser exposure of 20 s delivered a temperature of 55 °C to the solution surrounding the GAS, but MWNT-COOH had only a 5-fold reduction in CFUs and GAS-Ab-MWNT had a 10,000-fold reduction in CFUs; 30 s of 800-nm light resulted in a bulk solution temperature of 62 °C, but GAS-Ab-MWNT had completed reduction of all GAS CFUs, whereas MWNT-COOH had a 10-fold reduction in CFUs.
Figure 4. The number of viable bacteria after treatment with MWNT-COOH and GAS-Ab-MWNT exposed to 800-nm light, 1.3 W/cm2: (A) planktonic GAS, or (B) GAS biofilm. (C) A plot of the time of laser exposure versus the change in temperature of a 200 µL volume of 100 µg/mL of MWNT-COOH or GAS-Ab-MWNT. (D) The reduction of viable bacteria normalised to the controls for the various treatment groups.
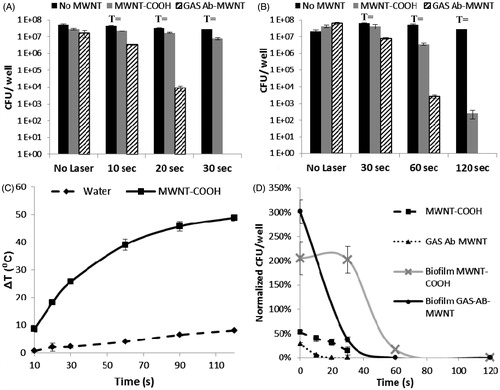
Biofilms are robust communities of bacteria and are less sensitive to damaging hyperthermia than planktonic bacteria, as demonstrated in ). Although planktonic bacteria with GAS-Ab-MWNT bound to their surfaces were easily killed within 30 s of laser exposure, biofilms treated with the same concentration of GAS-Ab-MWNT (100 µg/mL) saw only a 63% decrease in bacterial counts. However, at 60 and 120 s of laser exposure, GAS-Ab-MWNT produced a 99.99% and 100% killing of bacteria in biofilm, respectively. In comparison, MWNT-COOH resulted in an 83% and 99.999% decrease in bacterial counts from the biofilm after 60 and 120 s of infrared exposure, respectively. A significantly different trend occurred in the GAS biofilm group treated with MWNT-COOH, whereas 106% increase in CFUs can be observed after just 30 s of laser exposure. Similarly, GAS treated with no nanotubes had a 213%, 149%, and 27% increase in bacterial counts from biofilms exposed to 1.3 W/cm2 of 800-nm light for 30, 60 and 120 s, respectively. To correlate temperature with a decrease in biofilm-residing GAS CFUs, 30 s of laser exposure results in a temperature of 62 °C using functionalised or non-functionalised MWNT. Biofilm-residing GAS subjected to this treatment using MWNT-COOH resulted in a 4-fold reduction of CFUs whereas GAS-Ab-MWNT provided a 12-fold reduction in CFUs. Laser exposure of 60 s delivered a temperature of 77 °C to the solution surrounding the GAS in biofilms, and MWNT-COOH had a 15-fold reduction in CFUs while GAS-Ab-MWNT had a 10,000-fold reduction in CFUs; 120 s of 800-nm light resulted in a bulk solution temperature of 85 °C, and GAS-Ab-MWNT had completed reduction of all GAS CFUs, while MWNT-COOH had an 80,000-fold reduction in CFUs.
The results of indicate that 100 µg/mL of MWNT should be an ideal concentration to generate sufficient heat to irreversibly damage GAS in photothermal ablation procedures. The results of time for infrared light exposure at 1.3 W/cm2 versus temperature change in a 200 µL volume of MWNT-COOH (or GAS-Ab-MWNT – data not shown because it directly overlaps with MWNT-COOH) at this concentration were plotted in ) to demonstrate the temperatures to which the planktonic or biofilm samples might be subjected. Bacteria samples were kept at 37 °C prior to and during exposure to 800 nm light; therefore, based on the results from ), maximum temperatures in the bulk fluid around planktonic GAS or bacteria within biofilms would be 37° + 25° = 62 °C for 30 s, 37°+40° = 77 °C for 60 s, and 37°+48° = 85 °C for 120 s of laser exposure. ) further demonstrates that significantly less energy is required for ablation of planktonic bacteria compared to GAS in biofilms. The results also show that less time is needed to ablate both planktonic and biofilm embedded GAS using GAS-Ab-MWNT compared to MWNT-COOH. An additional result is the correlation between the heating curve in ) and the decrease in bacterial counts for GAS treated with GAS-Ab-MWNT in ). The increase in temperature change fits a positive second order polynomial (), whereas the decrease in CFUs for both planktonic and biofilm GAS fit a negative second order polynomial equation (
). However, neither of the MWNT-COOH treated groups correlate directly with the increase in temperature change.
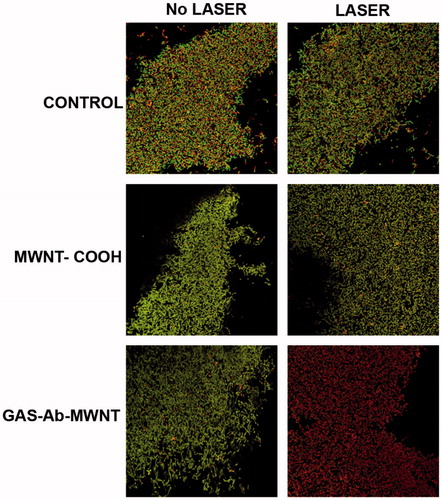
Confocal laser scanning microscopy was done on biofilms treated with and without laser and subjected to MWNT-COOH, GAS-Ab-MWNT, or no nanotubes, as represented by the images presented in . Live cells are stained green, while dead cells are stained red. The control group without nanotubes shows a vast population of live bacteria with very little difference between the no laser treatment and laser treatment groups. The high level of green fluorescence indicates that there are many viable bacteria in all of the groups, except biofilms treated with GAS-Ab-MWNT and laser. The percentage of dead cells in each image was quantified by determining the relative percentage of live/green GAS to dead/red GAS in three different locations on the biofilm. The control without nanotubes or laser had a dead cell population of 48.9%, whereas the control group with laser had a dead population of 46.3%. Addition of MWNT-COOH without laser resulted in 55.2% of bacteria in the biofilm being dead. However, the biofilms treated with MWNT-COOH and laser had 47.4% of the population being dead GAS. Biofilms treated with GAS-Ab-MWNT and no laser had 44.6% dead bacteria, and laser exposure increased this to 78.7% of dead GAS in the biofilm. The confocal results help to corroborate through visual representation the results obtained measuring the number of viable CFUs in the planktonic culture and biofilm exposed samples ().
Figure 5. Confocal microscopy of the biofilms treated with MWNT and/or laser. Green indicates live cells and red indicates dead or dying cells. There was very little death of bacterial cells within the biofilms except for the group exposed to both GAS-Ab-MWNT and laser, where the majority of cells within the biofilm were unviable.
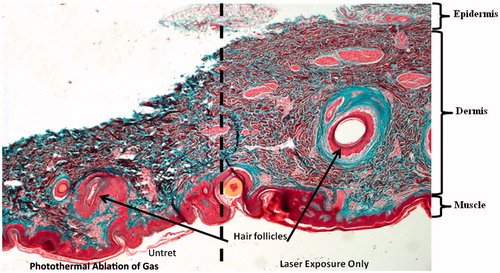
A suspension of GAS with attached nanotubes in direct contact with ex vivo porcine skin was photothermally ablated and the histological view of damage to the skin is depicted in . GAS contained within the cylindrical container was completely killed (data not shown), similarly to the results demonstrated in . The left side of the image was where the cylindrical container with GAS was placed with the addition of the nanotubes. Skin to the right side of the dashed line was exposed to infrared light, in the absence of GAS and nanotubes. The left side of this image shows that tissue damage occurred, as noted by shrinkage of the tissue in the vertical direction, including the collapse of a hair follicle. There was also coagulation of collagen and other proteins, as observed by areas of dark red/maroon staining. In contrast, the right side of the image shows healthy viable dermis with no obvious signs of irreversible tissue damage and an open hair follicle.
Figure 6. Ex vivo porcine skin to which a cylindrical volume of GAS and nanotubes was secured on the left hand side of the image and the GAS photothermally ablated. Dermal skin cells are observed as light pink and red, while collagen fibres were stained blue. Tissue shrinkage and coagulation of collagen and cell death are observed on the left side adjacent to photothermal ablation. Thermally denatured collagen stains deep red instead of blue and is indicative of tissue damage.
Discussion
In this work we have aimed to covalently functionalise a GAS antibody to the surface of a MWNT to provide a means of targeting the MWNT to the bacteria for photothermal ablation. Both the FTIR and Raman spectroscopy results () suggest that the synthesis for attachment of the GAS antibody is via covalent bonding. The slight broadening of the FTIR band near 3300 cm−1 indicates bond stretching which is indicative of the antibody being bound to the MWNT sidewall. This slight shift to higher wavenumber is due to covalent functionalisation of the antibody to the nanotube [Citation38]. Quenching of the peak near 465 cm−1 in the Raman spectra further supports the premise that the GAS antibody and MWNT are covalently bonded. The characteristic D- and G-bands for the MWNT-COOH and GAS-Ab-MWNTs are shown near 1300 and 1585 cm−1, respectively. The D-band is derived from the defects along the walls of the tube [Citation39]. The ratio of the D-band to the G-band (ID/IG) indicates the degree of disorder along the tube. An increase in the ID/IG ratio signifies an increase in the degree of chemical functionalisation on the nanotube walls. The intensity of the D-band for the GAS-Ab-MWNTs is slightly stronger compared to the D-band for MWNT-COOH, indicating covalent functionalisation ().
Bulk temperatures above 70 °C are needed for thermal disinfection using MWNT, but lower bulk temperatures may be acceptable because the heat source is localised to the bacterial surface, causing localised high temperature that dissipates quickly over a short distance. Considering that in vivo human temperature is at least 37 °C, then temperature increases of greater than 33 °C are needed. The results of the MWNT concentration and corresponding temperature change shown in demonstrate that MWNT concentrations above 50 µg/mL are acceptable to induce sufficient heat for ablation of GAS. This is because the baseline temperature in the measured sample was 37 °C, plus 38 °C from the heat generated by 50 µg/mL of nanotubes stimulated by NIR nanotube heating, for a total of 70 °C, which is over the thermal threshold for bacterial ablation. Previous results have shown minimal toxicity of nanotubes at 100 µg/mL against Escherichia coli and S. aureus, and therefore this amount was used for the incubation with GAS for the photothermal ablation experiments [Citation40]. The concentration and corresponding temperature change are related through a logarithmic equation (), indicating that concentrations significantly higher than 100 µg/mL will not provide greatly enhanced heating performance for photothermal ablation. One potential drawback to the current study on temperature increases is that the overall temperature increase in a 200 µL volume was used to determine the extent of elevated temperatures that GAS might be subjected to. Using MWNT-COOH there is a 10-fold decrease in planktonic GAS whereas GAS-Ab-MWNT causes complete GAS killing (even though the temperature of the bulk solution around the GAS only reaches 62 °C). This result supports our hypothesis that GAS-Ab-MWNT are most likely attached to the surface of GAS via the GAS antibody and further alludes to the idea that temperatures higher than 62 °C occur at the bacterial cell wall, leading to GAS death. We are not able to experimentally measure the heat generated by a single MWNT attached to GAS. However, other authors indicate that indeed the temperatures generated (by SWNT) are much higher than we are measuring in the bulk solution around bacteria, even up to hundreds of degrees Celsius by some predictions [Citation41].
The amount of GAS antibody bound per mg of MWNT was 10 µg, as determined from the BCA assay. The procedures for incubating specific amounts of antibody with nanoparticles were done in accordance with many published references that use between 5–50 µg of antibody per mg of nanoparticle [Citation42–45]. Of the delivered GAS antibody, 40% was able to bind to MWNT during incubation to facilitate attachment. This percentage of antibody attachment is better than previous work which had 12% attachment of antibody to nanotubes [Citation21,Citation46]. However, the amount of antibody used in our current system was relatively low. The binding of GAS-Ab-MWNT could be improved by loading the nanotubes with much higher amounts of antibody, although this becomes somewhat expensive when commercially purchased antibodies are used. In the current work, MWNT have been utilised, which provide an overall lower amount of surface area (and hence fewer binding locations for GAS antibody) than SWNT. Binding of GAS antibody-functionalised nanotubes for photothermal ablation might therefore be improved if SWNT were used instead of MWNT. The amount of nanotubes bound to GAS demonstrates that 14% of the GAS-Ab-MWNT attach to the bacteria while the majority are easily removed (). The photothermal results as shown in demonstrate that even though a low percentage of GAS-Ab-MWNT associate with the GAS, the numbers are sufficient for GAS killing. Localisation of the heat-generating nanoparticles adjacent to the bacterial cell wall may be advantageous for inducing sufficient heat for directly killing the bacterium, or for potentially inducing mild heating to make the cell wall more permeable for enhanced transport of antibiotics.
Previous studies have shown that photothermal ablation of planktonic bacteria is feasible using gold nanoparticles, carbon nanotubes, or graphene [Citation9,Citation10,Citation12,Citation13,Citation40,Citation47–49]. However, the current study is the first to highlight how antibody-functionalised MWNTs significantly enhance photothermal ablation of a clinically derived invasive strain of GAS in both planktonic cultures and within a biofilm. The results of this study suggest the potential for translational treatments of GAS in soft tissue and dermal infections. Because clinical infections show evidence suggesting that the associated bacteria reside within a biofilm state, it is vital to understand the parameters for biofilm disruption and bacterial killing. Although there was 100% eradication of planktonic GAS treated with GAS-Ab-MWNT within 30 s of exposure to 800 nm light, the parameters do not provide the same effectiveness for killing GAS within a biofilm (). Doubling the duration of laser exposure is required to eradicate 99.99% of GAS in biofilm, and 100% GAS killing is achieved using 120 s of irradiation. ), a graph of laser time versus temperature change for a 100 µg/mL concentration, shows only about 5 °C temperature increase between 60 and 120 s of laser exposure. Since 60 s provides an almost complete eradication of GAS in biofilm, this might be the ideal parameter to reduce the bacterial load enough for a patient’s immune system to fight off any remaining infection. Reducing the duration of laser exposure should also minimise heat dissipation to underlying skin tissue. It is clear that in both planktonic and biofilm samples, nanotubes targeted directly to the GAS surface offer a significant advantage for photothermal ablation. Since either MWNT-COOH or GAS-Ab-MWNT eradicate GAS in biofilms treated at 120 s, it can be postulated that the treatment time is too long, and shorter times may be equally advantageous when targeted MWNT are employed. As shown in ), there are differences in the rate of GAS killing between GAS-Ab-MWNT and MWNT-COOH use. For example, both planktonic and biofilm bacteria treated with GAS-Ab-MWNT and laser exposure have a decrease in CFUs corresponding to a logarithmic function where the temperature is time dependent (non-isothermal). This is in contrast to planktonic GAS treated with MWNT-COOH, which follows an almost linear decrease in CFUs, and has a much slower decrease in CFUs; this trend is indicative that bulk heating of the media around GAS is needed to ensure bacterial kill.
) also highlight an unusual finding, namely the significant increase in CFUs for GAS biofilms treated with nanotubes and no laser exposure. Biofilm-residing GAS treated with MWNT-COOH had a 2-fold increase in the number of CFUs compared to the control group which did not receive any nanotubes. A 3-fold increase was observed for biofilms treated with GAS-Ab-MWNT. The method for quantifying CFUs was by serial dilution and triplicate plating of bacteria onto agar plates. Therefore the increase in CFUs appears to be a real trend, and correlates well with a previous study using SWNT. In that study Rodrigues et al. [Citation50] showed that 100 µg/mL of SWNT in suspension incubated with E. coli for 48 h resulted in a 1.4-fold increase in bacterial cell growth. In contrast, ) shows that MWNT-COOH yields a 1.89-fold decrease, and GAS-Ab-MWNT yields a 3.45-fold decrease in planktonic bacteria when there is no laser exposure. The difference between our planktonic results and those by Rodrigues et al. could be justified by a hypothesis posited by Rodrigues et al., who evaluated the impact of SWNT on E. coli biofilms (in the absence of infrared light) [Citation50]. In that study as well as ours, nanotubes were found to be extracellular. The authors determined that it is possible for SWNT to inherently kill bacteria through either oxidative mechanisms or by directly piercing the bacteria. They hypothesise that bacteria interacting with the nanotubes or nanotube aggregates die and coat the nanotubes with their intracellular nutrients which other bacteria use to proliferate and bind to more nanotubes. This mechanism was further supported by demonstrating that extrapolysaccharide substances (EPS) from mature biofilms are needed for proliferation and resistance to SWNT disruption. In the current work we observed a slight decrease in CFUs from planktonic GAS (no or minimal EPS) upon exposure to MWNT-COOH alone, with a statistically significant decrease from GAS-Ab-MWNT. Biofilm-residing GAS (presence of EPS) has an increase in CFUs following exposure to either MWNT-COOH or GAS-Ab-MWNT. This increase might be due to some of the bacteria in the biofilm dying in response to either a lack of nutrients or presence of MWNT, and as some of the GAS die, the release of their intracellular contents provides increased binding to the nanotubes while simultaneously providing more nutrients for enhanced proliferation and growth of the biofilm. The confocal images presented in show that even in the absence of nanotubes there is a significant percentage of dead cells which are available to interact with nanotubes in the biofilm. Biofilms treated with and without MWNTs and laser exposure further support the graphical data in . These visual representations indicate that there is minimal damage to the biofilms unless GAS-Ab-MWNT and laser exposure are simultaneously applied.
Throughout the literature, many different types of nanoparticles have been used for photothermal ablation, mainly for treating cancer, including metal nanoparticles like gold nanorods and gold nanoshells, silver, conjugated polymers, and carbon, including SWNT, MWNT and graphene [Citation12,Citation13,Citation17,Citation18,Citation25,Citation51–53]. Nanotubes offer distinct advantages over some of the metal nanoparticles because 1) nanotubes do not have to be modified in order to have excellent absorption in the infrared, 2) they are very efficient at generating heat when stimulated with infrared light, 3) they have a high aspect ratio which may allow for enhanced mobility through biofilms and the potential to interface with more bacteria at once than spherical nanoparticles can, 4) they do not suffer from thermal deformation like metal nanoparticles can when exposed to laser irradiation. In addition, nanotubes have a broad absorption peak in the infrared so they can be stimulated using multiple infrared sources (800 nm and/or 1064 nm for example).
The majority of publications on biological interactions, toxicity and applications such as photothermal ablation of carbon nanotubes evaluate SWNT. Although there is still a lot of conflicting literature on the toxicity of nanotubes, a few characteristics for reducing toxic effects are important, including the aggregation state of the nanotubes as well as the aspect ratios and sidewall functionalization [Citation54–56]. Because SWNT have a small diameter and long length (high aspect ratio), they have been shown to have an exceptional ability to pierce bacteria, leading to significant membrane damage and death [Citation55]. Both SWNT and MWNT have been shown capable of generating reactive oxygen species which can damage bacteria, leading to death [Citation4,Citation57]. Another important characteristic for undesirable toxic response is the electronic nature of the nanotube. The chirality of nanotubes affords the possibility of having either metallic or semi-conducting potential. In fact, Vecitis et al. [Citation58] have shown specifically that metallic SWNT are more toxic to E. coli than semi-conducting SWNT, most likely because metallic tubes cause more oxidative stress. In the present work, MWNT have been used partially to avoid the phenomenon where bacterial cell death occurs due to membrane damage from SWNT piercing the bacteria. MWNT are composed of many sidewalls, and can therefore be either metallic or semi-conducting; however, it is much more challenging to separate these populations for MWNT than for SWNT, and in the present work it is not known if the MWNT are metallic or semi-conducting, although they are most likely an impure mixture of both types. A unique benefit that MWNT may offer over SWNT is that because of their multiple layers, they have higher absorption coefficients in the near-infrared [Citation59].
A vital concern when utilising nanoparticle-induced photothermal ablation to treat GAS infections is what the effect will be on adjacent host tissue. To help relieve this concern an ex vivo study was undertaken using a suspension of nanotubes and GAS held in a chamber attached to fresh porcine skin. The set-up allowed for both nanotubes and GAS to be in direct contact with the epidermal layer of the skin. Damage to the skin was observed by staining the skin after laser exposure to highlight tissue shrinkage, cell death, and coagulation of collagen fibres. Although the tissue remained intact, notable tissue damage was seen in the portion of skin (left side of ) in direct contact to where GAS had been photothermally ablated. An independent pathologist evaluated the tissue, and confirmed that the tissue damage is indicative of a second-degree burn, from which the tissue would be expected to recover. However, the adjacent tissue was quite healthy (right side of ), with no obvious tissue damage. This result further supports the premise that localised photothermal ablation therapy using targeted carbon nanotubes may lead to a beneficial treatment option for rampant GAS infections. One challenge with using photothermal ablation to eradicate bacterial infections is that high temperatures are generated and this may cause undesirable damage to surrounding tissues. However, one of the major problems with GAS dermal infections is the spread of GAS along the fascial planes. Common clinical treatment routinely involves surgical removal of skin adjacent to the GAS-infected area. One of the end goals for using targeted MWNT to treat GAS infections is to stem the spread of the infection. Further refinement of the GAS-Ab-MWNT photothermal ablation, including better targeting of the nanotubes to the bacterial cell surface and the use of pulsed lasers are expected to further localise the heat generated and reduce thermal damage. A number of groups have studied the potential negative impacts of nanotubes on health; MWNT have been identified as possible candidates for some dermal wound healing applications [Citation34]. A few published works have shown that MWNT, when functionalised appropriately such as with ammonium groups, do not appear to damage skin, but do illicit a mild inflammatory response in both human epidermal keratinocytes and fibroblasts [Citation60]. Orecchoni et al. [Citation60] showed that MWNT-COOH functionalised with ammonium increased the expression of IL-1B, IL6, THF, IL10 as well as the CXCR3 and CCR5 pathways. Other groups have shown that both raw and oxidised MWNT induce inflammatory pathways in monocytes that parallel those of toll-like receptors [Citation61]. Further work done in macrophages show that this cell line also has a significant increase in the expression of cytokines and chemokines that facilitate a fibrotic response, namely IL-6, IL-10, TNFα, IL-1β, IFN-γ, TGF-β1, αSMA, and PDGF [Citation57]. A challenge with fibrosis is that it can lead to tissue contracture and scarring; however, a recent study by Wailes et al. [Citation62] shows that MWNT can inhibit undesirable contraction of mesenchymal cells, and that MWNT (not oxidised or functionalised) serve as an antioxidant agent which in turn down-regulates cell contraction. Thus far, these results have only been demonstrated for MWNT, and not for SWNT. Dermal fibrosis is beneficial in wound healing, especially in closure of wounds after infection or injury, therefore MWNT may be beneficial if they remain in the treated area after photothermal ablation. Based on the lack of significant toxic potential from MWNT, as well as their probable benefits in wound healing, they appear to have vast promise as photothermal agents for applications in treating dermal infections caused by pathogenic bacteria.
Conclusion
The results of this study demonstrate that MWNT can be used as effective NIR-inducible agents for photothermal ablation of both planktonic as well as biofilm-residing GAS. The elevated temperatures induced by infrared-stimulated nanotubes were shown to be capable of killing GAS. Immediately after exposure to 800 nm light, MWNTs were found to be capable of generating temperatures in excess of 25–48 °C above the initial starting temperature of 37 °C to locally kill GAS. Based on the number of CFUs after photothermal ablation, one major result is that GAS-Ab-MWNT provides enhanced GAS killing compared to un-functionalised MWNT-COOH. In fact, only 14% of GAS-Ab-MWNT attach to the bacteria, and this amount is still capable of yielding sufficient photothermal ablation. The present work demonstrates that GAS-Ab-MWNT can effectively eradicate GAS bacteria within biofilms via photothermal mechanisms. The ex vivo data supports our hypothesis that localised heat using targeted nanotubes should not cause thermal damage in tissues adjacent to the infected tissue. Further refinements using bacteria-targeted carbon nanotubes include adding higher loadings of antibodies to the sidewall, or using SWNT instead of MWNT. Photothermal inactivation of bacteria using targeted nanoparticles may provide a promising solution for helping to stem the spread of disease caused by pathogenic bacteria. We anticipate that the results presented here will promote more research in this area of clinical need.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jarvis WR. The United States approach to strategies in the battle against healthcare-associated infections, 2006: Transitioning from benchmarking to zero tolerance and clinician accountability. J Hosp Infect 2007;65:3–9
- Centers for Disease Control and Prevention. Core Surveillance Report, Emerging Infections Program Network, Group A Streptococcus, 2009
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: Methods and literature. Int J Nanomed 2012;7:2767
- Vatanpour V, Madaeni SS, Moradian R, Zinadini S, Astinchap B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J Membr Sci 2011;375:284–94
- Upadhyayula VK, Deng S, Mitchell MC, Smith GB. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci Total Environ 2009;408:1–13
- Brady-Estévez AS, Kang S, Elimelech M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008;4:481–4
- Huang WC, Tsai PJ, Chen YC. Multifunctional Fe3O4@Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic-resistant bacteria. Small 2009;5:51–6
- Jo W, Kim MJ. Influence of the photothermal effect of a gold nanorod cluster on biofilm disinfection. Nanotechnology 2013;24:195104
- Kim CB, Yi DK, Kim PSS, Lee W, Kim MJ. Rapid photothermal lysis of the pathogenic bacteria, Escherichia coli using synthesis of gold nanorods. J Nanosci Nanotech 2009;9:2841–5
- Norman RS, Stone JW, Gole A, Murphy CJ, Sabo-Attwood TL. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett 2008;8:302–6
- Wang YW, Fu YY, Wu LJ, Li J, Yang HH, Chen GN. Targeted photothermal ablation of pathogenic bacterium, Staphylococcus aureus, with nanoscale reduced graphene oxide. J Mater Chem B 2013;1:2496–501
- Wu MC, Deokar AR, Liao JH, Shih PY, Ling YC. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 2013;7:1281–90
- Zharov VP, Mercer KE, Galitovskaya EN, Smeltzer MS. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J 2006;90:619–27
- Kotagiri N, Lee JS, Kim J-W. Selective pathogen targeting and macrophage evading carbon nanotubes through dextran sulfate coating and PEGylation for photothermal theranostics. J Biomed Nanotechnol 2013;9:1008–16
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol 2001;19:316–17
- Kennedy J, Blair I, McDowell D, Bolton D. An investigation of the thermal inactivation of Staphylococcus aureus and the potential for increased thermotolerance as a result of chilled storage. J Appl Microbiol 2005;99:1229–35
- Kam NWS, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 2005;102:11600–5
- Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA 2009;106:12897–902
- Van Asselt ED, Zwietering MH. A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int J Food Microbiol 2006;107:73–82
- Hossain MS, Balakrishnan V, Rahman NNNA, Sarker MZI, Kadir MOA. Treatment of clinical solid waste using a steam autoclave as a possible alternative technology to incineration. Int J Environ Res Public Health 2012;9:855–67
- Marches R, Mikoryak C, Wang R-H, Pantano P, Draper RK, Vitetta ES. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnology 2011;22:095101
- Maksimenko SA, Slepyan GY, Nemilentsau AM, Shuba MV. Carbon nanotube antenna: Far-field, near-field and thermal-noise properties. Physica E Low Dimens Syst Nanostruct 2008;40:2360–4
- Boldor D, Gerbo NM, Monroe WT, Palmer JH, Li Z, Biris AS. Temperature measurement of carbon nanotubes using infrared thermography. Chem Mater 2008;20:4011–16
- Fabbro C, Ali-Boucetta H, Da Ros T, Kostarelos K, Bianco A, Prato M. Targeting carbon nanotubes against cancer. Chem Commun 2012;48:3911–26
- Graham EG, MacNeill CM, Levi-Polyachenko NH. Quantifying folic acid-functionalized multi-walled carbon nanotubes bound to colorectal cancer cells for improved photothermal ablation. J Nanopart Res 2013;15:1–12
- Lee P-C, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials 2013;34:8756–65
- Lu Y-J, Wei K-C, Ma C-CM, Yang S-Y, Chen J-P. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B 2012;89:1–9
- Xiao Y, Gao X, Taratula O, Treado S, Urbas A, Holbrook RD, et al. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer 2009;9:351
- Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009;30:6041–7
- Reid SD, Hoe NP, Smoot LM, Musser JM. Group A Streptococcus: Allelic variation, population genetics, and host-pathogen interactions. J Clin Invest 2001;107:393–9
- Musser JM, Krause RM. The revival of group A streptococcal diseases, with a commentary on staphylococcal toxic shock syndrome. In: Krause RM, editor. Emerging Infections. New York: Academic Press, 1998. pp 185–218
- Huang W, Taylor S, Fu K, Lin Y, Zhang D, Hanks TW, et al. Attaching proteins to carbon nanotubes via diimide-activated amidation. Nano Lett 2002;2:311–14
- Gu H, Rapole SB, Huang Y, Cao D, Luo Z, Wei S, Guo Z. Synergistic interactions between multi-walled carbon nanotubes and toxic hexavalent chromium. J Mater Chem A 2013;1:2011–21
- Zhang Y, Wang B, Meng X, Sun G, Gao C. Influences of acid-treated multiwalled carbon nanotubes on fibroblasts: Proliferation, adhesion, migration, and wound healing. Ann Biomed Eng 2011;39:414–26
- Oraki Kohshour M, Mirzaie S, Zeinali M, Amin M, Said Hakhamaneshi M, Jalaili A, et al. Ablation of breast cancer cells using trastuzumab-functionalized multi-walled carbon nanotubes and trastuzumab-diphtheria toxin conjugate. Chem Biol Drug Design 2014;83:259–65
- DiLeo RA, Landi BJ, Raffaelle RP. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J Appl Phys 2007;101:064307
- Zhao X, Ando Y, Qin L-C, Kataura H, Maniwa Y, Saito R. Radial breathing modes of multiwalled carbon nanotubes. Chem Phys Lett 2002;361:169–74
- Li R, Wu RA, Zhao L, Wu M, Yang L, Zou H. P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano 2010;4:1399–408
- Osswald S, Flahaut E, Ye H, Gogotsi Y. Elimination of D-band in Raman spectra of double-wall carbon nanotubes by oxidation. Chem Phys Lett 2005;402:422–7
- Levi-Polyachenko N, Braden A, Rosenbalm T, Wagner W, Morykwas M, Argenta L, et al. Electrically conductive polymer nanotubes with anti-bacterial properties. NanoLife 2012;2:1241002
- Panchapakesan B, Lu S, Sivakumar K, Taker K, Cesarone G, Wickstrom E. Single-wall carbon nanotube nanobomb agents for killing breast cancer cells. Nanobiotechnology 2005;1:133–9
- Rezaeipoor R, John R, Adie SG, Chaney EJ, Marjanovic M, Oldenburg AL, et al. Fc-directed antibody conjugation of magnetic nanoparticles for enhanced molecular targeting. J Innov Opt Health Sci 2009;2:387–96
- Kouchakzadeh H, Shojaosadati SA, Tahmasebi F, Shokri F. Optimization of an anti-HER2 monoclonal antibody targeted delivery system using PEGylated human serum albumin nanoparticles. Int J Pharm 2013;447:62–9
- Hilger I, Trost R, Reichenbach JR, Linss W, Lisy M-R, Berndt A, Kaiser WA. MR imaging of HER-2/neu protein using magnetic nanoparticles. Nanotechnology 2007;18:135103
- Shamsipour F, Zarnani AH, Ghods R, Chamankhah M, Forouzesh F, Vafaei S, et al. Conjugation of monoclonal antibodies to super paramagnetic iron oxide nanoparticles for detection of HER2/neu antigen on breast cancer cell lines. Avicenna J Med Biotechnol 2009;1:27–31
- Marches R, Chakravarty P, Musselman IH, Bajaj P, Azad RN, Pantano P, et al. Specific thermal ablation of tumor cells using single-walled carbon nanotubes targeted by covalently-coupled monoclonal antibodies. Int J Cancer 2009;125:2970–7
- Chen WY, Lin JY, Chen WJ, Luo LY, Diau EWG, Chen YC. Functional gold nanoclusters as antimicrobial agents, for antibiotic-resistant bacteria. Nanomedicine 2010;5:755–64
- Mamouni J, Tang YA, Wu M, Vlahovic B, Yang LJ. Single-walled carbon nanotubes coupled with near-infrared laser for inactivation of bacterial cells. J Nanosci Nanotechnol 2011;11:4708–16
- Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol 2010;28:207–13
- Rodrigues DF, Elimelech M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environ Sci Technol 2010;44:4583–9
- Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol 2009;4:688–94
- MacNeill CM, Coffin RC, Carroll DL, Levi-Polyachenko NH. Low band gap donor-acceptor conjugated polymer nanoparticles and their NIR-mediated thermal ablation of cancer cells. Macromol Biosci 2013;13:28–34
- Thompson EA, Graham E, MacNeill CM, Young M, Donati G, Wailes EM, et al. Differential response of MCF7, MDA-MB-231, and MCF 10A cells to hyperthermia, silver nanoparticles and silver nanoparticle-induced photothermal therapy. Int J Hyperthermia. 2014;30:312–23
- Yang C, Mamouni J, Tang Y, Yang L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010;26:16013–19
- Liu S, Wei L, Hao L, Fang N, Chang MW, Xu R, et al. Sharper and faster ‘nano darts’ kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009;3:3891–902
- Pasquini LM, Hashmi SM, Sommer TJ, Elimelech M, Zimmerman JB. Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ Sci Technol 2012;46:6297–305
- He X, Young SH, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol 2011;24:2237–48
- Vecitis CD, Zodrow KR, Kang S, Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010;4:5471–9
- Kim JW, Shashkov EV, Galanzha EI, Kotagiri N, Zharov VP. Photothermal antimicrobial nanotherapy and nanodiagnostics with self-assembling carbon nanotube clusters. Lasers Surg Med 2007;39:622–34
- Orecchioni M, Bedognetti D, Sgarrella F, Marincola F, Bianco A, Delogu LG. Impact of carbon nanotubes and graphene on immune cells. J Transl Med 2014;12:138–149
- Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis 2010;20:S65–72
- Wailes EM, Levi-Polyachenko NH. Inhibition of mesenchymal cell contraction using carbon nanotubes. Nanomater Tissue Regen 2013;1:1–13