Abstract
High intensity focused ultrasound (HIFU), is a promising, non-invasive modality for treatment of tumours in conjunction with magnetic resonance imaging or diagnostic ultrasound guidance. HIFU is being used increasingly for treatment of prostate cancer and uterine fibroids. Over the last 10 years a growing number of clinical trials have examined HIFU treatment of both benign and malignant tumours of the liver, breast, pancreas, bone, connective tissue, thyroid, parathyroid, kidney and brain. For some of these emerging indications, HIFU is poised to become a serious alternative or adjunct to current standard treatments – including surgery, radiation, gene therapy, immunotherapy, and chemotherapy. Current commercially available HIFU devices are marketed for their thermal ablation applications. In the future, lower energy treatments may play a significant role in mediating targeted drug and gene delivery for cancer treatment. In this article we introduce currently available HIFU systems, provide an overview of clinical trials in emerging oncological targets, and briefly discuss selected pre-clinical research that is relevant to future oncological HIFU applications.
Introduction
This year, high intensity focused ultrasound (HIFU), also called focused ultrasound surgery (FUS), enters its seventh decade as a clinical, interventional technology – dating back to the first demonstrations of its therapeutic potential in disorders of the central nervous system by Lindstrom [Citation1] and Fry [Citation2] in the 1950s. The most significant advances in clinical applications of HIFU have occurred over the last 20 years, as the development of modern magnetic resonance imaging (MRI) and diagnostic ultrasound allowed for accurate targeting and monitoring of HIFU interventions. At present there are both validated clinical applications of HIFU as well ongoing clinical trials examining new therapeutic opportunities. In this article we will provide a brief overview of HIFU delivery systems and discuss the current clinical status of HIFU in emerging oncological targets (excluding the more established targets of prostate cancer and uterine leiomyomas) as well as potential future applications.
HIFU systems
Ultrasound causes both thermal and non-thermal effects in biological tissues. Absorption of high intensity ultrasound energy results in elevated tissue temperatures and ablation at specific temperature thresholds. Non-thermal effects related to cavitation (the formation and collapse of gas-filled bubbles in an ultrasound field) or generation of boiling bubbles can also result in mechanical tissue disruption. To achieve these effects with HIFU, a piezoelectric transducer generates a pulse of ultrasound energy that propagates via a coupling medium (typically oil or degassed water) into the body, converging on a focal region. Within the focal region a small volume of tissue is exposed to high amplitude pressure waves. Depending on the protocol selected for HIFU delivery, the effect achieved at the focal point can be tailored to be predominantly thermal or non-thermal. The desired tissue effect occurs within the focal region, and the intervening tissue is not damaged. The minimally invasive nature of this ‘trackless’ therapy is an appealing alternative to percutaneous or surgical approaches ().
Figure 1. Simplified cross-sectional image of an MRI-guided focused ultrasound (MRgHIFU) system. The tissue between the HIFU beam focus within the target lesion and the HIFU transducer remains undamaged, as does the tissue distal to the target lesion. A temperature map within the target region can be obtained in near-real time in multiple imaging planes.
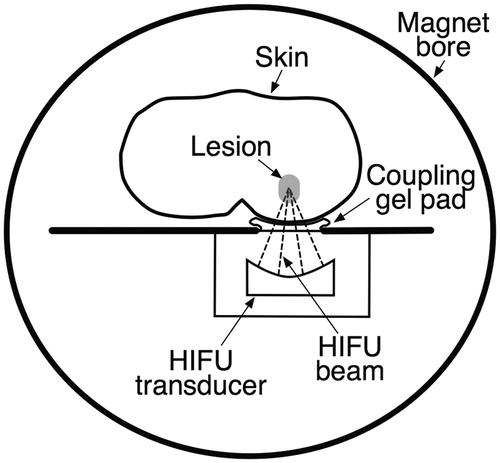
There are several commercially available HIFU devices that are manufactured worldwide for treatment of both benign and malignant tumours. In clinical practice both MRI and diagnostic ultrasound are used to safely guide HIFU therapy, and therapeutic HIFU transducers are designed for pairing with one of these imaging modalities. Ultrasound-guided HIFU (USgHIFU) systems allow real-time visualisation of gas bubbles caused by cavitation and boiling of the targeted soft-tissue [Citation3]. The active focal point appears as a hyperecho at the focus in the field of view of the diagnostic ultrasound probe. USgHIFU is relatively low cost and portable in comparison with MRI-guided HIFU (MRgHIFU) systems. There is active, preliminary research in the field of temperature mapping using diagnostic ultrasound [Citation4–6]; however, to date, a reliable and clinically accepted means of real-time temperature mapping with diagnostic ultrasound is not widely available. In contrast, MRgHIFU systems are large and expensive, but offer fine anatomic detail and high sensitivity for tumour detection (useful for therapy planning), as well as near real-time thermal monitoring of HIFU treatment with accuracy to approximately 1 °C [Citation7,Citation8] ().
Oncological applications of HIFU
HIFU in liver tumours
HIFU has been used for ablation of both primary and secondary liver tumours. More than 80% of patients with hepatocellular carcinoma (HCC) are poor candidates for curative surgical resection due to advanced underlying cirrhosis, and thus minimally invasive alternatives to surgery are desirable [Citation9–11]. Early trials conducted in the late 1990s in China demonstrated safety and feasibility of extracorporeal USgHIFU therapy for patients who either had unresectable HCC, or refused surgery [Citation12]. These results were subsequently confirmed in small trials in the UK [Citation13,Citation14]. USgHIFU ablation has also been successfully employed as adjunctive therapy to transarterial chemoembolisation (TACE) for non-surgical cases of HCC [Citation15–18] and hepatoblastoma [Citation19]. Chok et al. offered USgHIFU as a bridging therapy to liver transplant for patients with HCC in the context of Child-Pugh C cirrhosis who were not candidates for TACE or radiofrequency ablation (RFA), and successfully bridged a small cohort without significant adverse events [Citation20]. Other groups have successfully performed USgHIFU treatments for patients with liver metastases and observed complete lesion ablation, with minimal adverse events [Citation21,Citation22]. These preliminary data are promising and future randomised controlled trials will help to define survival benefit.
There are challenges to performing HIFU within the liver. Limited therapeutic windows through the ribs contribute to reflection of high energy ultrasound waves from the bone that can result in unintended burns to soft tissues between the transducer and its target [Citation23]. Respiratory motion is an additional complicating factor that can be mitigated via selective intubation and control of respiratory motion via mechanical ventilation, as well as saline injection to augment liver distance from the diaphragm [Citation24]. MRgHIFU for liver lesions remains in its infancy, but MR may offer additional solutions to current therapeutic obstacles encountered with ultrasound guidance. Active research continues in the development of motion gating for both MRgHIFU therapy and MR thermometry [Citation25,Citation26].
HIFU in breast tumours
The breast is ideally suited for HIFU therapy due to its superficial position, with no intervening vital structures. Ablation of both benign and malignant breast tumours has been performed using USgHIFU [Citation27,Citation28] and MRgHIFU [Citation29–33]. HIFU is a particularly attractive option for patients who desire breast conserving therapy since it is completely non-invasive, and could theoretically reduce the number of adverse events and improve cosmetic outcome compared to surgical intervention. Hynynen et al. [Citation32] conducted a pilot clinical trial in the USA using MRgHIFU for treatment of benign fibroadenomas of the breast, and demonstrated feasibility without significant adverse events. Overall tumour size was significantly smaller on follow up MRI, with subjective tissue softening over an average follow up of 6 months (range 1.5 months to 4 years). Early progress has also been made towards demonstrating non-inferiority of HIFU ablation to local surgical excision for solitary breast cancer. In 2005, Wu et al. [Citation28] reported a series of 22 patients with biopsy-proven, solitary breast cancer who refused surgery and underwent USgHIFU with adjuvant chemotherapy. Five-year disease-free survival and recurrence-free survival were 95% and 89%, respectively. Furusawa et al. [Citation29] reported results from a clinical trial in Japan involving 21 patients with biopsy-proven invasive or non-invasive ductal carcinoma. Patients were treated with MRgHIFU and followed over an average of 14 months (range 3–26 months), with one case of recurrence during that timeframe. Napoli et al. [Citation33] recently reported a small series of 10 patients in Italy with biopsy-proven invasive ductal breast cancer (stage T1M0N0) who underwent MRgHIFU of their tumours prior to routine breast conserving surgery. Surgical pathology revealed absence of residual cancer in 90% of cases with at least 5 mm of normal breast tissue around the target tissue. Further studies with long-term observation are necessary to draw definitive conclusions regarding the possibility of improved cosmesis, fewer adverse events, and non-inferior survival with HIFU vs. surgical standard of care in treatment of solitary breast cancer.
Of note, there is active research in pre-clinical, murine models examining MRgHIFU in delivery of adjuvant nanoparticle chemotherapy for breast cancer. Wong et al. reported on the use of a thermally activated chemotherapy agent as adjuvant treatment with MRgHIFU ablation of implanted breast cancer in mice [Citation34]. They found a significant increase in median survival time for mice who received the two therapies concurrently, vs. those who did not – suggesting the selective activation of the chemotherapy drug at the focus of the ultrasound treatment provides synergistic destruction of residual tumour cells, and may increase the rate of sustained remission.
HIFU in pancreatic cancer
Chemotherapy, radiotherapy and targeted drugs have all been relatively unsuccessful to date in significantly altering the course pancreatic cancer – which, untreated, carries a 16% 12-month survival rate [Citation35]. Most patients are inoperable at the time of diagnosis due to locally advanced disease or metastasis, and experience severe abdominal pain as the disease progresses [Citation36]. Ablative HIFU has been used as both primary and palliative therapy for pancreatic cancer. The majority of reported treatments have taken place in China since the late 1990s, and more recently case series have emerged from Korea, Japan, and a few European groups. Xiong et al. retrospectively examined 89 patients with inoperable pancreatic cancer (TNM stage II–IV) who underwent ablative USgHIFU for pain palliation – pain relief was achieved in 81% of patients [Citation37], similar to results achieved in other studies, including a small series from Italy [Citation38–41], with no significant adverse events. Zhao et al. [Citation42] conducted a phase II trial to evaluate the safety and efficacy of concurrent gemcitabine and USgHIFU therapy for 39 patients with locally advanced, unresectable pancreatic cancer. Pain was relieved in 79% of patients, the overall oncological response rate was 44% (determined by CT scan and the Response Evaluation Criteria in Solid Tumours), and four responders were subsequently able to undergo surgery with negative resection margins; however, the overall survival at 12 months was 51% – similar to the previously reported 53% 12-month survival rate derived from gemcitabine therapy alone [Citation43]. Further randomised controlled clinical trials are needed to evaluate any significant survival benefit achieved with ablative HIFU therapy alone. The mechanism of pain relief is not fully understood, but is hypothesised to involve direct, thermally induced damage of nerve fibres within the tumour, as well as indirect tumour shrinkage, removing mass effect from adjacent nerves [Citation44].
There are several areas of active HIFU research that are applicable to pancreatic cancer. Endoluminal HIFU transducers are currently in pre-clinical development for use with both ultrasound [Citation45] and MR [Citation46] guidance. These devices are particularly appealing for use in pancreatic cancer therapy, as they allow endoscopic placement of the HIFU transducer within the stomach or duodenum, directly adjacent to the pancreas. This allows for easier lesion targeting and lower risk of damage to overlying soft tissue structures in this delicate anatomical area. The use of HIFU-induced hyperthermia for localised chemotherapy drug release also shows great potential for treatment of pancreatic cancer [Citation47]. Conventional chemotherapy drugs are relatively ineffective in penetrating the fibrotic, hypovascular stroma of pancreatic adenocarcinoma, independent of the target specificity of the drug [Citation48]. Preliminary data from murine pancreatic cancer experiments utilising MRgHIFU hyperthermia to release doxorubicin from a temperature-sensitive liposome within the tumour resulted in a 2–8-fold increase in doxorubicin concentration within the tumour [Citation47].
HIFU in bone metastases
Bone metastases are the greatest contributor to cancer-related pain, and often severely diminish patient quality of life [Citation49]. Radiation therapy, in compliment to systemic and analgesic therapy, is currently the standard of care for localised, painful bony metastases. Overall response (pain reduction of ≥2 on the Numeric Rating Scale pain score at the treated site, without analgesic increase, or analgesic reduction of ≥25% without increased pain) to this treatment is approximately 60%, with complete response (a pain score of zero at the treated site) in approximately 25% [Citation50]. Patients with residual or recurrent pain following radiotherapy have limited treatment options, including: re-irradiation (resulting in some response, which may or may not be temporary, in approximately 60% of patients) [Citation51], surgical intervention (invasive, with complications observed in 13–20% of cases) [Citation52,Citation53], percutaneous cryoablation (minimally invasive, 49% of patients have at least partial response 1 week post-treatment, 69% at 24 weeks) [Citation54], and HIFU. Relevant advantages of HIFU over other modalities in this context include its non-invasive nature and the repeatability of the treatment until the clinical goal is reached – without issues related to radiation accumulated dose, or post-procedural tissue changes limiting access in repeated percutaneous intervention.
MRgHIFU is clinically approved in the European Union for palliative treatment of bone lesions, and is garnering clinical attention in western healthcare for treatment of radiotherapy-refractory painful bony metastases. Cortical bone has high acoustic resorption and low thermal conduction, and thus ultrasound energy is absorbed by the cortical surface, with minimal penetration into the medullary cavity [Citation55]. In cases of painful bone metastases, this relationship is advantageous for HIFU therapy – with most energy depositing over the periosteal surface of the bone, causing thermal injury to the periosteal nerves responsible for nociception [Citation56]. Preliminary clinical studies on the safety and efficacy of MRgHIFU for ablation of painful bone metastases demonstrated excellent response rates and safety – with 72–92% of patients reporting pain reduction and no significant adverse events [Citation56–58]. A recent phase III trial by Hurwitz et al. [Citation59] involving 147 patients from 17 medical centres across the USA, Canada, Israel, Italy and Russia found overall response rate in patients with painful bony metastases undergoing MRgHIFU ablation to be 64%. The most common adverse event was sonication pain, which occurred in 32% of patients, with 60% of adverse events resolving on the day of treatment. This study has established the safety and efficacy of MRgHIFU as a palliative treatment for painful bone metastases.
HIFU in primary bone tumours
HIFU ablation has been used in China to treat primary bone malignancy. Li et al. [Citation60] demonstrated safety of USgHIFU treatment for a small series of patients with osteosarcoma. Transient skin burns and local nerve damage were identified as the most common complications. Chen et al. conducted a prospective clinical study of limb-sparing USgHIFU ablation, in combination with appropriate chemotherapy, for 80 patients with primary bone tumours who refused or were not candidates for surgery [Citation61]. The treated tumours included osteosarcoma (78.8%), chondrosarcoma (12.5%), Ewing sarcoma (3.7%), giant cell tumour (1.3%) and unknown histological type (3.7%). 75% of patients had US Musculoskeletal Tumor Society stage IIb disease (high grade, extracompartmental, no metastases), 25% had stage III disease (metastatic). Complete tumour necrosis was achieved in 86% of patients (obstacles included tumour size >10 cm, or pelvic tumours with overlying bowel), using 1–2-cm margins of normal soft tissue and 3–5-cm of normal bone margins; 7% of patients with complete tumour necrosis had local progression at mean follow-up of 36.8 months (range 5–87 months), with 11.8% recurrence at 5 years (similar to results reported with limb-salvaging surgery). The disease-free survival rate was 86.4% for stage IIb patients at 5 years who had complete tumour ablation and completed their concurrent chemotherapy regimen. These results are comparable to those reported in western literature for limb-sparing surgical intervention combined with radiotherapy [Citation62,Citation63]; however, well-powered randomised control trials have yet to be published to compare HIFU ablation with limb-sparing surgery.
Osteoid osteoma (a benign bone tumour most common in young men), when symptomatic, is treated with CT-guided RFA with reported success rates of 89–95%, as reviewed by Motamedi et al. [Citation64]. Two recent studies from Italy examined MRgHIFU as an alternative therapy for non-spinal osteoid osteoma. Napoli et al. [Citation65] reported a small series (n = 6) with good efficacy and without major complications. Geiger et al. [Citation66] performed a prospective, multicentre clinical trial (n = 29) and demonstrated complete clinical success (pain score of 0) in 90% of patients over 1 year follow-up, without adverse events. These preliminary results support the possibility of comparable clinical efficacy between CT-guided RFA and MRgHIFU. The latter is an appealing alternative given its non-invasive nature and lack of associated ionising radiation exposure for a predominantly young patient population. Additional randomised clinical studies will further help to define the role of MRgHIFU in treatment of osteoid osteoma.
HIFU in connective tissue/extra-abdominal desmoid tumours
Extra-abdominal desmoid tumours lack the capacity for metastasis, but can be locally aggressive, and are prone to recur even after complete resection [Citation67]. Symptomatic, recurrent tumours after primary surgical resection are often treated with radiation therapy, with or without neoadjuvant chemotherapy, to avoid the morbidity of repeated surgical resections [Citation68]. Both the non-invasive nature and the repeatability of HIFU therapy make it an appealing modality in this context. Wang et al. [Citation69] reported results of USgHIFU ablation for eight patients with multiple recurrent extra-abdominal desmoid tumours, with overall >50% decrease in tumour volume over mean follow-up of 30 months. Preliminary data reported by Ghanouni et al. [Citation70] using MRgHIFU to treat five patients with extra-abdominal desmoid tumours showed no serious adverse events. Further studies are needed to evaluate long-term outcomes and success in local disease control vs. conventional radiotherapy for non-surgical or recurrent disease.
HIFU in thyroid and parathyroid tumours
The cost and potential morbidity of surgery for patients with benign, but enlarging thyroid nodules or symptomatic parathyroid adenomas has inspired a number of minimally invasive approaches to therapy – including percutaneous ethanol injections, laser ablation, or RFA [Citation71,Citation72]. In Europe HIFU has also recently been explored as a non-invasive means to treat these conditions. Esnault et al. [Citation73] reported a feasibility study of 25 patients with multinodular goitre who underwent USgHIFU ablation therapy prior to surgery. Successful ablation was seen in 95% of treated nodules demonstrated by either coagulative necrosis or cavitation on histopathological analysis. HIFU treatments were well tolerated with good patient safety – transient local pain and minor skin burns were the most common adverse events. Kovatcheva et al. [Citation74] described USgHIFU ablation therapy for four patients with symptomatic primary hyperparathyroidism who declined surgery. Serum parathyroid hormone level normalised in two of the four patients post-treatment. Mild to moderate transient subcutaneous oedema was the most common adverse effect observed. These initial studies are promising, and further studies, including randomised trials examining long-term outcomes vs. standard of care, will help to define the role of HIFU in thyroid and parathyroid disease.
HIFU in kidney tumours
Compared to the 1990s, renal cancers are currently diagnosed at a significantly earlier stage and smaller overall size in the USA [Citation75,Citation76]. Approximately 38% of these tumours are less than 3 cm at time of diagnosis [Citation76] and 60% are asymptomatic [Citation77]. For peripheral, radiologically suspicious lesions 1–4 cm in size, partial nephrectomy is currently the treatment of choice [Citation78]. However, reported surgical complication rates range from 9–33%, due in large part to the many medical co-morbidities commonly encountered in this patient population [Citation79], and thus minimally invasive alternatives are desirable. Percutaneous and laparoscopic thermal ablation (e.g. radiofrequency, microwave, or cryoablation) have been employed for these small tumours in recent clinical trials demonstrating 93–95% recurrence free survival at 5 years [Citation80,Citation81]. In Europe, pilot clinical trials of laparoscopic HIFU devices (trochar-driven, ultrasound-guided HIFU transducers directly approximated to the renal tumour) have demonstrated successful tumour ablation, with the potential benefit of no required tumour puncture vs. other minimally invasive ablative techniques [Citation82,Citation83]. Further studies are needed to clarify the oncological success and reliability of laparoscopic HIFU.
Attempts at extracorporeal HIFU ablation of kidney tumours have shown unsatisfactory tumour destruction with a high rate of incomplete ablation due to respiratory motion and acoustic impedance from overlying ribs and perinephric fat [Citation21,Citation84–86]. As mentioned above, there is ongoing research in the development of motion gating for MRgHIFU therapy [Citation25] and MR thermography [Citation26] that will theoretically allow for more effective and accurate targeting of organs prone to respiratory movement – including the liver and kidneys.
HIFU in brain tumours
Trans-cranial HIFU is an appealing, non-invasive treatment modality for neurosurgical interventions. Efficient ultrasound transduction through the skull, however, has posed a significant obstacle that has prevented this therapy from entering clinical practice since early experiments in the 1950s [Citation87]. In the last 10 years, the advent of MR thermography and phased-array HIFU transducers paved the way for a pilot clinical trial in the USA that examined ablative MRgHIFU for treatment of glioblastoma multiforme [Citation88]. McDannold et al. [Citation88] attempted MRgHIFU therapy in three patients with glioblastoma using a hemispherical phased-array HIFU transducer. They successfully focused the transcranial ultrasound beam within the brain parenchyma and generated a heat signature detectable with MR thermography, but unfortunately were limited by the device power available at the time, and were unable to achieve thermal coagulation at the targeted lesions. Subsequent studies have successfully utilised MRgHIFU with hemispheric phased-array transducers and computed tomographic correction algorithms to generate targeted intracranial foci of thermal coagulation for treatment of chronic neuropathic pain [Citation89,Citation90], essential tremor [Citation91] and Parkinson’s disease [Citation92]. These results are promising in anticipation of forthcoming oncological feasibility studies.
In addition to ablation, HIFU combined with systemically administered microbubbles has been shown to locally and transiently disrupt the blood–brain barrier (BBB) at discrete targets in pre-clinical studies [Citation93]. Over the last 10 years, pre-clinical studies in murine cancer models of both primary and metastatic brain tumours have shown successful delivery of multiple systemic therapies to the brain with FUS-mediated BBB opening. Successful immunotherapies include Herceptin [Citation94], and targeted delivery of natural killer cells to breast tumours metastasised to the brain [Citation95]. Chemotherapy agents, including 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) [Citation96,Citation97], epirubicin [Citation98] and doxorubicin [Citation99–101] have also been successfully transported across the BBB following FUS exposure, with associated decrease in tumour growth and improvement in animal survival. These results are encouraging, and future human trials of these techniques will help to elucidate their role in clinical treatment of brain cancer.
Conclusion and future applications
High intensity focused ultrasound is an effective, non-invasive technique to achieve tumour ablation and to facilitate targeted medical therapy to tumours. Although HIFU technology has been in clinical use since the 1950s, limited complimentary technologies have hindered its advancement into clinical oncology beyond a small spectrum of disease. Today, HIFU has reached an exciting threshold of both transducer and guidance-system development. The advent of MRI guidance systems with real-time thermal mapping, phased-array transducers with computed tomographic correction algorithms, endoluminal transducers, motion gating for both MRgHIFU therapy and MR thermometry, temperature mapping for diagnostic ultrasound, and numerous innovations in medical nanotechnology (such as microbubble-assisted BBB disruption, and encapsulated chemotherapy and gene therapy agents capable of targeted, thermally induced delivery) have paved the way for oncological applications that were previously impractical in western medicine. HIFU is poised to bridge the promising data from preliminary trials to more robust, randomised clinical trials for treatment of liver, breast, pancreatic, bone, desmoid, thyroid, parathyroid, kidney and brain tumours. Although completion of such trials is challenging and time-consuming for relatively uncommon diseases, the results will help to better define the emergent role of HIFU in management of a broad spectrum of oncology.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lindstrom PA. Prefrontal ultrasonic irradiation – A substitute for lobotomy. Am Med Assoc Arch Neurol Psychiatry 1954;72:399–425
- Fry WJ, Barnard JW, Fry EJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science 1955;122(3168):517–8
- Vaezy S, Shi X, Martin RW, Chi E, Nelson PI, Bailey MR, et al. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol 2001;27:33–42
- Huang CW, Lien DH, Chen BT, Shieh J, Tsui PH, Chen CS, et al. Ultrasound thermal mapping based on a hybrid method combining cross-correlation and zero-crossing tracking. J Acoust Soc Am 2013;134:1530–40
- Maleke C, Konofagou EE. Harmonic motion imaging for focused ultrasound (HMIFU): A fully integrated technique for sonication and monitoring of thermal ablation in tissues. Phys Med Biol 2008;53:1773–93
- Hou GY, Marquet F, Wang S, Konofagou EE. Multi-parametric monitoring and assessment of high-intensity focused ultrasound (HIFU) boiling by harmonic motion imaging for focused ultrasound (HMIFU): An ex vivo feasibility study. Phys Med Biol 2014;59:1121–45
- De Poorter J, De Wagter C, De Deene Y, Thomsen C, Stahlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: In vivo results in human muscle. Magn Reson Med 1995;33:74–81
- Kohler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009;36:3521–35
- Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: A 31-year experience. Br J Surg 1987;74:839–42
- Zibari GB, Riche A, Zizzi HC, McMillan RW, Aultman DF, Boykin KN, et al. Surgical and nonsurgical management of primary and metastatic liver tumors. Am Surg 1998;64:211–20, discussion 20–1
- Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 1997;12:S319–28
- Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol 2004;11:1061–9
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931–5
- Leslie T, Ritchie R, Illing R, Ter Haar G, Phillips R, Middleton M, et al. High-intensity focused ultrasound treatment of liver tumours: Post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br J Radiol 2012;85:1363–70
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659–67
- Li CX, Wu PH, Fan WJ, Huang JH, Zhang FJ, Zhang L, et al. [Clinical effect of transcatheter arterial chemoembolization combined with high intensity focused ultrasound ablation in treatment of large hepatocellular carcinoma]. Zhonghua Yi Xue Za Zhi 2009;89:754–7
- Kim J, Chung DJ, Jung SE, Cho SH, Hahn ST, Lee JM. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma <5 cm: Comparison with transarterial chemoembolisation monotherapy – Preliminary observations. Br J Radiol 2012;85:e940–6
- Cheung TT, Poon RT, Jenkins CR, Chu FS, Chok KS, Chan AC, et al. Survival analysis of high-intensity focused ultrasound therapy vs transarterial chemoembolization for unresectable hepatocellular carcinomas. Liver Int 2014;34:e136–43
- Wang S, Yang C, Zhang J, Kong XR, Zhu H, Wu F, et al. First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 2014;59:170–7
- Chok KS, Cheung TT, Lo RC, Chu FS, Tsang SH, Chan AC, et al. Pilot study of high-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients wait-listed for liver transplantation. Liver Transpl 2014;20:912–21
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a western population. Br J Cancer 2005;93:890–5
- Sung HY, Cho SH, Kim JI, Cheung DY, Han JY, Kim JK, et al. High intensity focused ultrasound therapy resulted in a complete response in a patient with advanced gastric cancer with liver metastases: A case report. Eur J Gastroenterol Hepatol 2008;20:707–9
- Hwang JH, Crum LA. Current status of clinical high-intensity focused ultrasound. 31st Annual International Conference of the IEEE EMBSMinneapolis, Minnesota, USA, September 2-6. Eng Med Biol Soc 2009;2009:130–3
- Wijlemans JW, de Greef M, Schubert G, Moonen CT, van den Bosch MA, Ries M. Intrapleural fluid infusion for MR-guided high-intensity focused ultrasound ablation in the liver dome. Acad Radiol 2014. doi:j.acra/j.acra.2014.06.015. [Epub ahead of print]
- Auboiroux V, Petrusca L, Viallon M, Muller A, Terraz S, Breguet R, et al. Respiratory-gated MRgHIFU in upper abdomen using an MR-compatible in-bore digital camera. Biomed Res Int 2014;2014:421726
- Celicanin Z, Auboiroux V, Bieri O, Petrusca L, Santini F, Viallon M, et al. Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs. Magn Reson Med 2014;72:1087–95
- Wu F, Wang ZB, Cao YD, Zhu XQ, Zhu H, Chen WZ, et al. ‘Wide local ablation’ of localized breast cancer using high intensity focused ultrasound. J Surg Oncol 2007;96:130–6
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res Treat 2005;92:51–60
- Furusawa H, Namba K, Nakahara H, Tanaka C, Yasuda Y, Hirabara E, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer 2007;14:55–8
- Huber PE, Jenne JW, Rastert R, Simiantonakis I, Sinn HP, Strittmatter HJ, et al. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res 2001;61:8441–7
- Zippel DB, Papa MZ. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer 2005;12:32–8
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study. Radiology 2001;219:176–85
- Napoli A, Anzidei M, Ciolina F, Marotta E, Cavallo Marincola B, Brachetti G, et al. MR-guided high-intensity focused ultrasound: Current status of an emerging technology. Cardiovasc Intervent Radiol 2013;36:1190–203
- Wong A, Watson KD, Fite BZ, Liu Y, Seo JW, Mahakian LM, et al. Enhanced accumulation of Cu-doxorubicin nanoparticles with MR guided focused ultrasound thermal ablation. Paper presented at the International Symposium on Therapeutic Ultrasound, Las Vegas, NV, 3 April 2014
- Faivre J, Forman D, Esteve J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe. EUROCARE Working Group. Eur J Cancer 1998;34:S2184–90
- Nakakura EK, Yeo CJ. Periampullary and pancreatic cancer. In: Blumgart LH, editor. Surgery of the Liver, Biliary Tract and Pancreas. 4th ed. Philadelphia: Saunders; 2007
- Xiong LL, Hwang JH, Huang XB, Yao SS, He CJ, Ge XH, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009;10:123–9
- Wang K, Chen Z, Meng Z, Lin J, Zhou Z, Wang P, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia 2011;27:101–7
- Li PZ, Zhu SH, He W, Zhu LY, Liu SP, Liu Y, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int 2012;11:655–60
- Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. Am J Roentgenol 2010;195:W245–52
- Sung HY, Jung SE, Cho SH, Zhou K, Han JY, Han ST, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011;40:1080–6
- Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010;21:447–52
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 2008;19:1592–9
- Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract 2014;2014:205325
- Hwang JH, Farr N, Morrison K, Wang Y, Khokhlova T, Ko B, et al. Development of an EUS-guided high-intensity focused ultrasound endoscope. Gastrointestinal Endoscopy 2011;73:AB155
- Adams M, Salgaonkar VA, Jones P, Plata JC, Chen HY, Pauly KB, et al. Experimental investigations of an endoluminal ultrasound applicator for MR-guided thermal therapy of pancreatic cancer. Paper presented at the International Symposium on Therapeutic Ultrasound, Las Vegas, NV, 3 April 2014
- Farr N, Wang Y, D’Andrea S, Starr F, Lee D, Hwang JH. Hyperthermia triggered drug delivery in pancreatic cancer mouse model using MR-guided focused ultrasound. Paper presented at the International Symposium on Therapeutic Ultrasound, Las Vegas, NV, 3 April 2014
- Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011;60:861–8
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:S6243–9
- Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol 2007;25:1423–36
- Huisman M, van den Bosch MA, Wijlemans JW, van Vulpen M, van der Linden YM, Verkooijen HM. Effectiveness of reirradiation for painful bone metastases: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2012;84:8–14
- Ratasvuori M, Wedin R, Keller J, Nottrott M, Zaikova O, Bergh P, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol 2013;22:132–8
- Weiss RJ, Wedin R. Surgery for skeletal metastases in lung cancer. Acta Orthop 2011;82:96–101
- Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: Multicenter trial. Cancer 2013;119:1033–41
- Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med 2009;60:417–30
- Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases – Preliminary clinical experience. Ann Oncol 2007;18:163–7
- Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicenter study. Ann Surg Oncol 2009;16:140–6
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging-guided focused ultrasound. Radiology 2008;249:355–63
- Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J Natl Cancer Inst 2014;106:1–9. doi: 10.1093/jnci/dju082
- Li C, Wu P, Zhang L, Fan W, Huang J, Zhang F. Osteosarcoma: Limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol Ther 2009;8:1102–8
- Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: Effective treatment with high-intensity focused ultrasound ablation. Radiology 2010;255:967–78
- Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68
- Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203
- Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, et al. Thermal ablation of osteoid osteoma: Overview and step-by-step guide. Radiographics 2009;29:2127–41
- Napoli A, Mastantuono M, Cavallo Marincola B, Anzidei M, Zaccagna F, Moreschini O, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013;267:514–21
- Geiger D, Napoli A, Conchiglia A, Gregori LM, Arrigoni F, Bazzocchi A, et al. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: A prospective multicenter evaluation. J Bone Joint Surg Am 2014;96:743–51
- Mullen JT, Delaney TF, Kobayashi WK, Szymonifka J, Yeap BY, Chen YL, et al. Desmoid tumor: Analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol 2012;19:4028–35
- Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: Prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol 1999;17:158–67
- Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: Preliminary results. Int J Hyperthermia 2011;27:648–53
- Ghanouni P, Bitton R, Bucknor M, Butts Pauly K, Avedian R. Treatment of extra-abdominal desmoid tumors using MR guided high intensity focused ultrasound: Preliminary results after five patients. Paper presented at the International Symposium on Therapeutic Ultrasound, Las Vegas, NV, 3 April 2014
- Papini E, Pacella CM, Misischi I, Guglielmi R, Bizzarri G, Dossing H, et al. The advent of ultrasound-guided ablation techniques in nodular thyroid disease: Towards a patient-tailored approach. Best Pract Res Clin Endocrinol Metab 2014;28:601–18
- Iglesias P, Diez JJ. Current treatments in the management of patients with primary hyperparathyroidism. Postgrad Med J 2009;85(999):15–23
- Esnault O, Franc B, Menegaux F, Rouxel A, De Kerviler E, Bourrier P, et al. High-intensity focused ultrasound ablation of thyroid nodules: First human feasibility study. Thyroid 2011;21:965–73
- Kovatcheva RD, Vlahov JD, Shinkov AD, Borissova AM, Hwang JH, Arnaud F, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism: A feasibility study in four patients. Am J Roentgenol 2010;195:830–5
- Cooperberg MR, Mallin K, Ritchey J, Villalta JD, Carroll PR, Kane CJ. Decreasing size at diagnosis of stage 1 renal cell carcinoma: Analysis from the National Cancer Data Base, 1993 to 2004. J Urol 2008;179:2131–5
- Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: Analysis of the National Cancer Data Base. Cancer 2008;113:78–83
- Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203–5
- Heuer R, Gill IS, Guazzoni G, Kirkali Z, Marberger M, Richie JP, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol 2010;57:223–32
- Porpiglia F, Volpe A, Billia M, Scarpa RM. Laparoscopic versus open partial nephrectomy: Analysis of the current literature. Eur Urol 2008;53:732–42, discussion 42–3
- Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63:486–92
- Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: Experience from treating 243 small renal masses over 7.5 years. Cancer 2010;116:3135–42
- Klingler HC, Susani M, Seip R, Mauermann J, Sanghvi N, Marberger MJ. A novel approach to energy ablative therapy of small renal tumours: Laparoscopic high-intensity focused ultrasound. Eur Urol 2008;53:810–16, discussion 7–8
- Ritchie RW, Leslie TA, Turner GD, Roberts IS, D’Urso L, Collura D, et al. Laparoscopic high-intensity focused ultrasound for renal tumours: A proof of concept study. BJU Int 2011;107:1290–6
- Marberger M, Schatzl G, Cranston D, Kennedy JE. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int 2005;95:S52–5
- Hacker A, Michel MS, Marlinghaus E, Kohrmann KU, Alken P. Extracorporeally induced ablation of renal tissue by high-intensity focused ultrasound. BJU Int 2006;97:779–85
- Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol 2003;170:2237–40
- Fry WJ, Mosberg WH Jr, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954;11:471–8
- McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010;66:323–32, discussion 32
- Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus 2012;32:E1
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol 2009;66:858–61
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640–8
- Magara A, Buhler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J Therapeut Ultrasound 2014;2:1–8
- Bing C, Ladouceur-Wodzak M, Wanner CR, Shelton JM, Richardson JA, Chopra R. Trans-cranial opening of the blood–brain barrier in targeted regions using a stereotaxic brain atlas and focused ultrasound energy. J Therapeut Ultrasound 2014;2:1–11
- Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood–brain/blood–tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release 2012;163:277–84
- Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 2013;73:1892–9
- Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, et al. Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010;255:415–25
- Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, et al. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012;33:704–12
- Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA 2010;107:15205–10
- Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer 2007;121:901–7
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood–brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 2012;38:1716–25
- Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J Control Release 2013;169:103–11
