Abstract
The clinical role of magnetic resonance image-guided high-intensity focused ultrasound (MR-HIFU) is rapidly expanding due to its merit of non-invasiveness. MR thermometry based on a proton resonance frequency shift technique is able to accurately measure HIFU-induced temperature changes, which provides considerable advantages over ultrasonography-guided HIFU in terms of safety and therapeutic efficacy. Recent studies and the resulting technological advances in MR-HIFU such as MR thermometry for moving organs, MR-acoustic radiation force imaging, and a volumetric mild hyperthermia technique further will expand its clinical roles from mere ablation therapy to targeted drug delivery and chemo- or radio-sensitisation for cancer treatment. In this article, MR-HIFU therapy is comprehensively reviewed with an emphasis on the roles of MR imaging in HIFU therapy, techniques of MR monitoring, recent advances in clinical MR-HIFU systems, and potential future applications of MR-HIFU therapy. In addition, the pros and cons of MR-HIFU when compared with ultrasonography-guided HIFU are discussed.
Introduction
High-intensity focused ultrasound (HIFU) is an emerging therapeutic modality which is actively utilised for the treatment of various types of benign or malignant diseases such as prostate cancer, uterine fibroids and adenomyosis, liver cancer, and metastatic bone tumour. In HIFU therapy, high energy ultrasound waves are focused into one small spot in the target tissue, which results in a thermal or mechanical effect. Because ultrasound waves are able to propagate through the body, HIFU therapy can be performed in a completely non-invasive manner, which is its greatest advantage over other surgical or minimally invasive therapeutic modalities. This non-invasive nature of HIFU therapy bears a larger significance in view of the major trend in modern medicine of developing and using less invasive modalities of diagnosis and treatment [Citation1].
As in other minimally invasive therapies, image guidance and monitoring are essential parts of HIFU therapy in order to secure safe and effective treatment. Currently, either magnetic resonance (MR) imaging or ultrasonography (US) is used by clinical HIFU systems. Each method has pros and cons, and, in summary, while US is more cost effective and provides higher time resolution, MR imaging is superior in terms of better imaging quality and the availability of temperature measurement. In general, imaging as a guiding or monitoring tool should be capable of identifying the target as well as the structures which are at possible risk around the target or in the treatment path, monitoring the treatment process, assessing the therapeutic responses, and detecting possible procedure-related complications. MR imaging seems to satisfy these requisites better than US.
In this review, MR image-guided HIFU (MR-HIFU) therapy will be comprehensively reviewed emphasising the roles of MR imaging in HIFU therapy, the techniques of MR monitoring in HIFU therapy, the recent advances in clinical MR-HIFU systems and the potential future applications of MR-HIFU therapy. In addition, the pros and cons of MR-HIFU compared to US- HIFU will be discussed.
Roles of MR imaging in HIFU therapy
Due to its unique capabilities, MR imaging plays an important role in every step of HIFU therapy from the screening and planning stage to post-therapeutic assessment.
Pre-therapeutic assessment
MR imaging provides crucial information not only about anatomy but also about likely therapeutic responses, which enables practitioners to decide whether or not and how the HIFU therapy should be performed. Anatomical information that should be analysed in pre-therapeutic MR examination includes 1) the size and location of the target; 2) the thickness of the subcutaneous fat layer; and 3) the presence or absence of obstacles in the HIFU beam path. The size of the target treatable in one session is limited based on the treatment time allowed and the speed of the HIFU system. Treatment time is commonly limited to 3–4 h because of the risk of deep vein thrombosis of the lower extremities caused by immobilisation. Considering this time limit and the treatment speed of the currently available MR-HIFU systems [Citation2], the size limit of the target tumour should not be over 10–12 cm in diameter [Citation3,Citation4]. The location of the target should be evaluated in consideration of the limit in treatable depth which is dependent on the maximal focal length of the HIFU transducer, as well as the surrounding organs that may be susceptible to collateral thermal injury. The thickness of the subcutaneous fat layer is also important because a fat layer that is too thick attenuates acoustic energy and reduces the treatable depth [Citation5]. On the other hand, a too thin fat layer may not always be beneficial. For instance, in the treatment of bony lesions, if the target tumour is located very superficially, the skin becomes more susceptible to burns due to a higher energy density. The presence or absence of obstacles or heat-susceptible tissues (e.g. a surgical scar, the gastrointestinal tract, or a nerve bundle) in the sonication path should also be evaluated. If present, it is important to know whether or not it can be avoided by tilting the sonication angle or using other manipulation techniques [Citation6].
Predicting the therapeutic response is also an important role of the pre-treatment MR exam. This role is best illustrated in the treatment of uterine fibroids. High signal intensity on T2-weighted images [Citation7,Citation8] is associated with high cellularity relative to fibrous component (i.e. increased cell water component), and a high volume transfer constant (Ktrans) in dynamic contrast-enhanced MR/a high relative peak enhancement in semiquantitative perfusion MR [Citation5,Citation9] is associated with increased vascularity, both of which are well-known predictors for poor therapeutic responses to HIFU therapy. A thick subcutaneous fat layer appears to be an influential factor as well [Citation5]. Uterine fibroids with these poor prognostic factors should be excluded from this treatment. However, there have been no well-established exclusion criteria that account for all of the known influential factors.
Guiding and monitoring
Guiding and monitoring are key roles of MR imaging in MR-HIFU therapy. If the location of the target tumour is identified on MR imaging taken after positioning of the patient, the MR-HIFU system adjusts the location of HIFU focus by mechanical and electronic methods to synchronise it with the target coordinate indicated by the operator. Before therapy sonication, test sonication of low energy is performed to verify the synchronisation between the intended and the actual foci. Once verified, therapy sonication of high energy is done. During sonications, MR imaging monitors any temperature change at the target area using a proton resonance frequency (PRF) shift technique explained below. The patient’s motion can be detected easily by comparing the continuously updated anatomical images with the baseline image or fiducial markers placed at easily recognisable points such as margins of the organ or the tumour, and is important for safety. After sonication the MR-HIFU system calculates the thermal dose online and shows the area where the lethal thermal dose has been delivered. By this process it becomes apparent whether sufficient heat has been delivered to the target. A temperature change in the areas at risk of thermal injury can be monitored as well. The presumed sonication path is overlaid on a MR image so the operator is able to identify whether there is an obstacle or heat-susceptible tissue in the sonication path, and, if necessary, appropriate manoeuvres can be performed to avoid them. For instance, in uterine fibroid therapy, an MR image-guided bowel manipulation technique [Citation6] can be performed easily, but the same manoeuvre would be difficult under US-only guidance. Taking all of these features into consideration, MR-HIFU appears obviously superior to US-HIFU in terms of safety and therapeutic efficacy.
Post-therapeutic assessment
Post-therapeutic assessment can be conducted by MR imaging on the spot, immediately after the treatment. Contrast-enhanced T1-weighted imaging is a standard method to show an area of coagulation necrosis induced by MR-HIFU ablation therapy which is manifested as a void of contrast enhancement due to eradication of blood flow. At the same time, the occurrence of procedure-related complications can be evaluated by MR imaging as well.
Techniques of MR monitoring in HIFU therapy
Although US is able to measure temperature based on the difference in speeds of sound in different temperature environments, its capability is limited from a clinical viewpoint because an exact measurement is available only at the low temperature range up to approximately 50 °C [Citation10], which is not useful for thermal ablation therapy. On the other hand, performance of MR-based temperature measurement (i.e. MR thermometry) satisfies the typical clinical requirements. Feasibility of temperature measurement during therapy is a fundamental difference of MR-HIFU compared to US-HIFU.
MR thermometry technique
There are several MR parameters known to be sensitive to temperature changes, which include proton density, T1 and T2 relaxation times, the diffusion coefficient, magnetisation transfer, and PRF shift. There are temperature-sensitive MR contrast agents as well. Hence, several kinds of MR techniques are actually capable of measuring temperature. Among them, the PRF shift-based method has the most advantages for clinical use in terms of acquisition time and linearity with temperature and sensitivity, for example [Citation11,Citation12].
The principle of PRF shift-based MR thermometry is as follows. If the temperature increases, hydrogen bonds of water molecules stretch, bend, and break, which increases the electron screening (or shielding) effect. Because the local magnetic field is attenuated by the screening constant, an increase in the screening effect decreases the local magnetic field. The decreased local magnetic field in turn decreases the PRF because the PRF is determined by a product of the gyromagnetic ratio and the local magnetic field [Citation11,Citation12]. For this reason, an increase in temperature decreases the water PRF at a ratio of 0.0094 ppm/°C (α). Temperature maps can be constructed by measuring the phase change in resonance frequency according to Equation Equation1(1)
(1)
where φ(T) = phase in the current image, φ(T0) = phase of a baseline image, γ = gyromagnetic ratio, α = PRF change coefficient, B0 = magnetic field strength, and TE = echo time. As the equation implies, PRF shift-based MR thermometry is basically a subtraction imaging technique. The MR pulse sequence used is a gradient echo planar image (EPI), or a segmented EPI with parallel imaging [Citation11,Citation12].
PRF shift-based MR thermometry () is the preferred choice in the clinical MR-HIFU systems currently available because of its high precision and sensitivity, excellent linearity with temperature over a wide range (from −15 °C to 100 °C), near independence from tissue type, no dependence on tissue change (i.e. cooked vs. uncooked), and satisfactory spatial and temporal resolutions [Citation13]. However, this technique also has drawbacks. It is less sensitive at low field strengths, thus a high field MR scanner over 1.0 T is necessary. This is not a very critical issue because 1.5- or 3-T MR scanners are popularly adopted for diagnostic imaging as well. The PRF-based technique is unable to measure the temperature of fat tissue due to a lack of hydrogen bonds. This is clinically a fairly important limitation because the subcutaneous fat layer is one of the susceptible areas of near-field heat accumulation-induced injury. To overcome this limitation, T2 relaxation time-based MR thermometry was recently tried and successfully performed in clinical uterine fibroid treatment [Citation14]. T2 thermometry was able to measure temperature in subcutaneous fat tissue with a time resolution of 16 s, which, while much lower than the PRF shift-based technique, was still clinically acceptable. T2 relaxation time has a sigmoidal, rather than linear relationship with temperature change [Citation12]. Another clinically important limitation is its inability to monitor bony cortex (due to a lack of mobile protons) as well as fatty bone marrow when treating bony lesions. Instead, the soft tissue immediately adjacent to the cortex responds linearly to bone temperature change, thus allowing thermal monitoring [Citation15].
Figure 1. Example of PRF shift-based MR thermometry during MR-HIFU ablation therapy of a uterine fibroid. (A) Coronal (left) and sagittal (right) MR temperature maps obtained during HIFU sonication with a 16-mm treatment cell. The HIFU target can be seen to be heated to 68 °C (arrows) which was successful. Colour changes remote from the HIFU target are artefacts caused by motion (arrowheads). (B) Coronal (left) and sagittal (right) thermal dose maps immediately after HIFU sonication. Thermal dose contours for 240 EM (white line) and 30 EM (orange line) are demonstrated together (arrows). The semi-opaque brown ellipsoids represent the already ablated treatment cells.
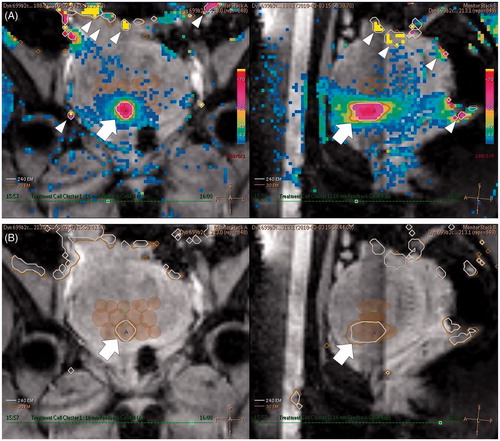
Utility of MR thermometry
The thermal dose is defined as an estimate of the actual heat calculated as an exposure time at the reference temperature of 43 °C, and its unit is EM (equivalent minutes at 43 °C). This is an important concept because tissue damage not only of the target tumour but also of normal tissue depends on both temperature and exposure time. Lethal thermal doses of various types of biological tissues have been investigated, and the range is known to be 25–240 EM [Citation16,Citation17]. Based on these results, the MR-HIFU system calculates a thermal dose map during the treatment according to the Sapareto–Dewey equation [Citation18] and demonstrates the area where the thermal dose exceeds 240 EM ().
MR thermometric information can be used for closed-loop feedback control. Closed-loop feedback control means that the sonication duration and acoustic power are adjusted based on the temperature and thermal dose information from MR thermometry on a real-time basis. This technique enables more stable and homogeneous heating even in heterogeneous tissue [Citation19].
MR thermometry information can also be utilised for predicting the therapeutic response. The extent of tissue where a lethal thermal dose is delivered can be regarded as the ablation zone before contrast enhancement [Citation20]. In one recent clinical study on uterine fibroids [Citation21], the post-sonication temperature decay rate was found to effectively predict the therapeutic response to MR-HIFU ablation therapy. Slower temperature decay was associated with better ablation results probably because with complete ablation temperature decay is determined only by heat diffusion, while temperature decay is determined by both heat diffusion and residual perfusion in incomplete ablation.
MR thermometry and motion
MR thermometry is very susceptible to motion because it is a subtraction imaging technique. There are many sources of motion during HIFU therapy including respiration, peristalsis, muscle tension, the patient’s movement, or even swelling of treated tissue. Among them, respiratory motion is most important, especially in the treatment of the abdominal organs such as the liver or the kidney. This is the main reason why MR-HIFU therapy is difficult to apply clinically. To overcome this drawback, several strategies have been suggested. Most simply, MR-HIFU ablation during respiratory gating under general anaesthesia with mechanical ventilation or during regular free breathing was tried in the clinical treatment of liver cancer [Citation22,Citation23]. Other more sophisticated methods include a triggered, navigated, multi-baseline method, a referenceless or self-referenced method, and a hybrid of both methods. In the triggered, navigated, multi-baseline method, the diaphragm position is determined with a navigator echo after collecting multiple baseline images, and then a baseline image corresponding to the determined diaphragm position is chosen and used for image subtraction [Citation24]. The referenceless or self-referenced method estimates the background phase by fitting a polynomial function to the unwrapped background phase or a complex valued polynomial to the complex image outside the heating region using a weighted least-square fit. This technique requires a heating area at least partially surrounded by a non-heated region with a high signal to noise ratio [Citation25,Citation26]. These techniques have not yet been adopted by the clinical MR-HIFU system. However, continuous MR thermometry and tracking of HIFU focus for the target with periodic motion was realised by an animal study using the clinical MR-HIFU system [Citation27].
MR-acoustic radiation force imaging
MR-acoustic radiation force imaging (MR-ARFI) is a new technique to monitor MR-HIFU therapy. Acoustic radiation force refers to a steady, time-averaged force that acts upon objects in an acoustic field. Radiation force is formed by a transfer of momentum from the ultrasound field [Citation28]. When HIFU is applied, micron displacement of tissue is induced at the HIFU focus towards the direction of US propagation. In MR-ARFI, a pulse sequence capable of detecting such micron displacements encodes motion into the phase of the MR image, and therefore MR-ARFI is able to visualise the location of a focus of low-energy US that induces no heating [Citation29]. This MR technique shares an essentially similar principle with diffusion weighted imaging with respect to the use of a motion encoding gradient. Both gradient-recalled echo and spin echo MR-ARFI sequences have been implemented in combination with line scans or 2D Fourier transformation readouts [Citation30,Citation31]. Recently, an in vivo animal application of MR-ARFI under free breathing was reported to be feasible [Citation32]. The MR-ARFI technique is not clinically available yet. However, it has potential clinical roles such as validation of focal location prior to HIFU ablation sonication, adaptive focusing to improve focusing quality which may be useful for heterogeneous tissue or transcranial HIFU, and monitoring of low-powered sonication for the purpose of targeted drug delivery, blood–brain barrier opening, or thrombolysis [Citation31,Citation33].
Recent advances in clinical MR-HIFU system
Clinical MR-HIFU systems
At the time of writing there are two major vendors of clinical MR-HIFU systems: InSightec (Tirat Carmel, Israel) and Philips Healthcare (Vantaa, Finland) in the market, producing the ExAblate® and Sonalleve® systems, respectively. The clinical results for ExAblate and Sonalleve systems were first reported in 2003 and 2011, respectively, both of which dealt with uterine fibroid ablation therapy [Citation34,Citation35].
While both systems share common features of a multi-elemented HIFU transducer enabling both mechanical and electronic adjustments of HIFU focus, and MR thermometric temperature monitoring with closed-loop feedback control, sonication strategies differ between the two systems. The ExAblate system adopts a so-called ‘point-by-point’ sonication method which is able to generate ablation zones of only a relatively small size, about 5 mm in axial diameter, per sonication [Citation36] by using a stationary HIFU focus unless variable length sonication technique (discussed later) is adopted. On the other hand, the Sonalleve system adopts a ‘volumetric’ sonication method in which the HIFU focus is electronically steered along a trajectory comprised of multiple outward-moving concentric circles in order to increase the volume of tissue ablated per sonication. The number of such circles which are aligned perpendicular to the HIFU beam direction determines the sizes of the ablation zone with an axial diameter ranging from 4 to 16 mm [Citation37].
In the volumetric sonication method, the energy efficiency of HIFU sonication was found to improve as the size of treatment cell increased in an in vivo animal study as well as in a clinical study [Citation35,Citation37]. If this result is extrapolated in a comparison to the point-by-point sonication method, it is not difficult to speculate that the energy efficiency of the volumetric sonication method would be superior. Consistent with this speculation, the treatment speed by volumetric MR-HIFU ablation was found to be substantially enhanced in clinical uterine fibroid therapy when compared to previous publications using a point-by-point sonication method [Citation2]. This improved treatment speed contributes to an increase of the tumour size treatable in one session, thus widening the range of patients who can benefit from this therapeutic modality.
In the ExAblate system, a variable length sonication technique recently became available. In this technique the HIFU focus is mechanically shifted forward during a single burst sonication, therefore variable lengths (10–70 mm) of an elongated ablation zone can be formed (ExAblate OR) [Citation38]. This is also a kind of volumetric sonication method in that HIFU focus is moving during sonication. If the resulting treatment cells are conformed to the tumour contour there is no need to make multiple layers to cover the whole tumour, thus contributing to increased treatment speed.
As the duration (i.e. the ablation zone size) per sonication increases, the cooling interval between sonications has to be elongated in order to prevent accumulation of heat and subsequent adverse effects on organs in the near field. In spite of improved treatment speeds, the rate of near-field thermal injury in volumetric MR-HIFU has been reported to be higher than that by MR-HIFU system using a point-by-point sonication method [Citation39]. This problem may be because the optimal cooling interval of the volumetric MR-HIFU therapy has not been well-established due to difficulties caused by individual variability in heat absorption and tolerance. One possible solution may be active cooling of the skin surface by circulating chilled water, as is being tested in one clinical trial (NTR 4089; Nederland Trial Register). This may be beneficial for further accelerating treatment speed as well as attenuating the effect of individual variability.
Organ-dedicated designs of MR-HIFU systems
Other than the extracorporeal MR-HIFU systems for general purposes, special designs of MR-HIFU systems dedicated to specific organs are under investigation or have been commercialised recently. As for the brain, the dedicated MR-HIFU system (ExAblate Neuro) with a helmet-shaped, ‘numerous-elemented’ transducer is under clinical investigation for the treatment of diseases arising from the deep brain area such as essential tremor, Parkinsonism, and neuropathic pain [Citation40,Citation41]. Prostate-dedicated MR-HIFU systems adopt either a transrectal (ExAblate OR) or transurethral approach (Philips Healthcare) using a spoon-shaped multi-elemented transducer with a water bag for acoustic coupling and cooling, or a stick-shaped, mechanically rotating, multi-elemented transducer, respectively [Citation42,Citation43]. For the treatment of bone pathologies such as metastatic bone tumours, osteoid osteoma, and facet joint osteoarthritis (mainly for pain palliation), a general extracorporeal MR-HIFU system can be utilised with a patient positioned with the target lesion in contact with the HIFU window on the table. The conformal bone system (ExAblate OR) dedicated to bone treatment allows a more comfortable patient position because manual positioning of the transducer is feasible [Citation44–46]. For breast cancer, a general extracorporeal MR-HIFU system can also be used. However, the chest wall, the lung and the heart may be at risk, especially if the tumour is deeply located [Citation47,Citation48]. To minimise this risk, a MR-HIFU system with a breast-dedicated platform (Philips Healthcare) has adopted the strategy of lateral sonications by using a recently developed cup-shaped transducer design [Citation49].
Future applications of MR-HIFU therapy
Organ applicability
MR-HIFU up to now has focused on ablation therapy with the intention of inducing tumour necrosis or achieving pain control. Based on current research and development, it is predicted that ablation therapy by MR-HIFU will be extended to other organs such as the liver, the pancreas, and the kidney that are currently barely accessible. The difficulties are mainly due to respiratory motion and acoustic shadowing by the ribs, the lower lung and the bowel loops. As for respiratory motion, the aforementioned strategies for MR thermometry and motion tracking techniques will contribute to overcoming this limitation. With respect to acoustic shadowing by the bowel loops or the lower lung, artificial ascites and/or artificial pleural effusions may be helpful as in US-guided radiofrequency ablation of the liver [Citation50]. For shadowing by the rib cage, selective activation of only the elements corresponding to the intercostal spaces appears to be the most promising solution [Citation51–53].
Mild hyperthermia
Mild hyperthermia by MR-HIFU represents another promising field of clinical application for the treatment of cancers. MR-HIFU is able to induce temperature elevations to 42–43 °C by using lower-energy sonication. Homogeneous heating covering a large area was reported feasible by the clinically available MR-HIFU system using volumetric sonication with closed-loop feedback control [Citation54,Citation55].
Mild hyperthermia by volumetric MR-HIFU could be useful in combination with lysolipid thermally sensitive liposomal doxorubicin or other anticancer drugs (LTSL) for targeted drug delivery. LTSL is a drug capable of releasing encapsulated doxorubicin when exposed to mild hyperthermia. When LTSL is combined with volumetric MR-HIFU-induced mild hyperthermia, it is not hard to anticipate that anticancer drugs could be delivered to the targeted tissue very efficiently at lower systemic drug concentrations under straightforward temperature monitoring [Citation56].
It has long been established that cancer tissue exposed to mild hyperthermia for a prolonged period becomes more susceptible to chemo- or radiotherapy than to physiological conditions (i.e. chemo- or radio-sensitisation) [Citation57,Citation58]. However, this concept has not been popularised clinically because of deterioration of the patient’s condition when systemically exposed to heat for a long time, as well as poor heating efficiency of the devices used. However, MR-HIFU is an ideal tool to realise this therapy principle because it is able to non-invasively induce localised, mild hyperthermia to the cancer tissue within a very short duration (i.e. only several minutes) under accurate temperature monitoring conditions. Heating localised only to the target tumour is expected to have little effect on systemic body temperature. If these therapy principles become clinically available, the efficacy of anti-cancer therapies for neoplasms arising from ultrasound-accessible organs may be greatly improved, thus may provide a breakthrough in the field of medical and radiation oncology.
Comparison of MR-HIFU with US-HIFU
The biggest merit of MR-HIFU as compared to US-HIFU is the feasibility of thermometry, which contributes to enhanced therapeutic efficacy by ensuring temperature rise of the target as well as enabling closed-loop feedback control. Furthermore, MR thermometry is beneficial for research because it makes quantitative analysis feasible. Superior targeting capability is another advantage of MR. MR imaging has better tissue contrast and a larger field of view than US. In addition, image quality shows no deterioration as HIFU sonications are repeated, which is one of the drawbacks of US-HIFU. MR-HIFU also confers a safety advantage over US-HIFU with respect to MRI being able to anatomically and thermometrically visualise the surrounding sensitive organs. Manipulation of the internal organs such as the bowel loops is more intuitive under MR image guidance than US guidance.
The biggest disadvantage of MR-HIFU as compared to US-HIFU is its higher purchase and maintenance costs. In terms of imaging, MR-HIFU is more susceptible to motion and has lower temporal resolution. However, the temporal resolution of current clinical MR-HIFU systems is not very problematic clinically. For instance, the refresh rate of magnitude images and thermometric measurements is only 2–3 s. US propagation can be predicted better by US rather than MR imaging.
In terms of organ applicability, MR-HIFU is mandatory for the treatment of brain or bony lesions. MR-HIFU and US-HIFU may be superior for treatment of the uterus, prostate, and breast. For the moving organs such as the liver and the kidney, US-HIFU is superior to MR-HIFU at this time. However, with advances in MR technology, MR-HIFU will likely become competitive to US-HIFU in the near future. With regard to mild hyperthermia therapy, MR thermometry and volumetric sonication methods are essential.
Conclusion
MR imaging plays a profoundly important role in HIFU therapy not only for guiding and monitoring but also for pre- and post-therapeutic assessment. The biggest advantage of MR-HIFU over US-HIFU is the feasibility of thermometry and better targeting capability, which is beneficial for ensuring the safety and efficacy of ablation therapy. Moreover, recent technological advances such as MR thermometry for moving organs, MR-ARFI and volumetric mild hyperthermia techniques will contribute to expanding the territory of HIFU therapy to ablation of the moving organs, targeted drug delivery and chemo- or radio-sensitisation. In spite of these features and the technology’s potential, the largest obstacle to clinical implementation of MR-HIFU is its higher cost compared to US-HIFU, which impedes more widespread clinical adoption of MR-HIFU therapy. Therefore, the shifting balance of these advantages and disadvantages of MR-HIFU will determine how fast this therapeutic modality will advance in the future.
Declaration of interest
This work was supported by a Samsung Medical Centre grant (GFO1130071). The author alone is responsible for the content and writing of the paper.
References
- Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: An overview for radiologists. Korean J Radiol 2008;9:291–302
- Park MJ, Kim YS, Keserci B, Rhim H, Lim HK. Volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids: Treatment speed and factors influencing speed. Eur Radiol 2013;23:943–50
- Yoon SW, Lee C, Cha SH, Yu JS, Na YJ, Kim KA, et al. Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: A pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol 2008;18:2997–3006
- Kim YS, Kim JH, Rhim H, Lim HK, Keserci B, Bae DS, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: Initial clinical outcomes. Radiology 2012;263:600–9
- Kim YS, Kim BG, Rhim H, Bae DS, Lee JW, Kim TJ, et al. Uterine fibroids: Semiquantitative perfusion MR imaging parameters associated with the intraprocedural and immediate postprocedural treatment efficiencies of MR imaging-guided high-intensity focused ultrasound ablation. Radiology 2014:132719
- Park MJ, Kim YS, Rhim H, Lim HK. Technique to displace bowel loops in MRI-guided high-intensity focused ultrasound ablation of fibroids in the anteverted or anteflexed uterus. Am J Roentgenol 2013;201:W761–4
- Funaki K, Fukunishi H, Funaki T, Kawakami C. Mid-term outcome of magnetic resonance-guided focused ultrasound surgery for uterine myomas: From six to twelve months after volume reduction. J Minim Invasive Gynecol 2007;14:616–21
- Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 2007;196:184 e181–6
- Kim YS, Lim HK, Kim JH, Rhim H, Park BK, Keserci B, et al. Dynamic contrast-enhanced magnetic resonance imaging predicts immediate therapeutic response of magnetic resonance-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Invest Radiol 2011;46:639–47
- Rivens I, Shaw A, Civale J, Morris H. Treatment monitoring and thermometry for therapeutic focused ultrasound. Int J Hyperthermia 2007;23:121–39
- Quesson B, de Zwart JA, Moonen CT. Magnetic resonance temperature imaging for guidance of thermotherapy. J Magn Reson Imaging 2000;12:525–33
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90
- Peters RD, Hinks RS, Henkelman RM. Ex vivo tissue-type independence in proton-resonance frequency shift MR thermometry. Magn Reson Med 1998;40:454–9
- Baron P, Ries M, Deckers R, de Greef M, Tanttu J, Köhler M, et al. In vivo T2-based MR thermometry in adipose tissue layers for high-intensity focused ultrasound near-field monitoring. Magn Reson Med 2013;72:1057–64
- Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 2013;33:1555–68
- Meshorer A, Prionas SD, Fajardo LF, Meyer JL, Hahn GM, Martinez AA. The effects of hyperthermia on normal mesenchymal tissues. Application of a histologic grading system. Arch Pathol Lab Med 1983;107:328–34
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787–800
- Mougenot C, Quesson B, de Senneville BD, de Oliveira PL, Sprinkhuizen S, Palussiere J, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU). Magn Reson Med 2009;61:603–14
- McDannold N, Hynynen K. Quality assurance and system stability of a clinical MRI-guided focused ultrasound system: Four-year experience. Med Phys 2006;33:4307–13
- Kim YS, Park MJ, Keserci B, Nurmilaukas K, Köhler MO, Rhim H, et al. Uterine fibroids: Postsonication temperature decay rate enables prediction of therapeutic responses to MR imaging-guided high-intensity focused ultrasound ablation. Radiology 2014;270:589–600
- Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci 2006;5:167–71
- Fischer K, Gedroyc W, Jolesz FA. Focused ultrasound as a local therapy for liver cancer. Cancer J 2010;16:118–24
- Köhler MO, Denis de Senneville B, Quesson B, Moonen CT, Ries M. Spectrally selective pencil-beam navigator for motion compensation of MR-guided high-intensity focused ultrasound therapy of abdominal organs. Magn Reson Med 2011;66:102–11
- Holbrook AB, Santos JM, Kaye E, Rieke V, Pauly KB. Real-time MR thermometry for monitoring HIFU ablations of the liver. Magn Reson Med 2010;63:365–73
- Grissom WA, Lustig M, Holbrook AB, Rieke V, Pauly JM, Butts-Pauly K. Reweighted l1 referenceless PRF shift thermometry. Magn Reson Med 2010;64:1068–77
- de Senneville BD, Mougenot C, Moonen CT. Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound. Magn Reson Med 2007;57:319–30
- Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng 2004;6:229–48
- McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys 2008;35:3748–58
- Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med 2011;65:738–43
- Kaye EA, Pauly KB. Adapting MRI acoustic radiation force imaging for in vivo human brain focused ultrasound applications. Magn Reson Med 2013;69:724–33
- Holbrook AB, Ghanouni P, Santos JM, Medan Y, Butts Pauly K. In vivo MR acoustic radiation force imaging in the porcine liver. Med Phys 2011;38:5081–9
- Marsac L, Chauvet D, Larrat B, Pernot M, Robert B, Fink M, et al. MR-guided adaptive focusing of therapeutic ultrasound beams in the human head. Med Phys 2012;39:1141–9
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003;226:897–905
- Kim YS, Keserci B, Partanen A, Rhim H, Lim HK, Park MJ, et al. Volumetric MR-HIFU ablation of uterine fibroids: Role of treatment cell size in the improvement of energy efficiency. Eur J Radiol 2012;81:3652–9
- Gorny KR, Hangiandreou NJ, Hesley GK, Gostout BS, McGee KP, Felmlee JP. MR guided focused ultrasound: Technical acceptance measures for a clinical system. Phys Med Biol 2006;51:3155–73
- Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009;36:3521–35
- InSightec. Product/ExAblate OR. Available at: http://www.insightec.com/ExAblate-Operation-Room-Future.html (accessed 22 September 2012)
- Park MJ, Kim YS, Rhim H, Lim HK. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol 2014;25:231–9
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640–8
- Bauer R, Martin E, Haegele-Link S, Kaegi G, von Specht M, Werner B. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord 2014;20:S197–9
- Lindner U, Ghai S, Spensieri P, Hlasny E, Van Der Kwast TH, McCluskey SA, et al. Focal magnetic resonance guided focused ultrasound for prostate cancer: Initial North American experience. Can Urol Assoc J 2012;6:E283–6
- Siddiqui K, Chopra R, Vedula S, Sugar L, Haider M, Boyes A, et al. MRI-guided transurethral ultrasound therapy of the prostate gland using real-time thermal mapping: Initial studies. Urology 2010;76:1506–11
- Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicentre study. Ann Surg Oncol 2009;16:140–6
- Napoli A, Mastantuono M, Cavallo Marincola B, Anzidei M, Zaccagna F, Moreschini O, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013;267:514–21
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain – Case series of an innovative new technique. Eur Radiol 2012;22:2822–35
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused ultrasound surgery of breast cancer: Correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat 2003;82:93–101
- Furusawa H, Namba K, Nakahara H, Tanaka C, Yasuda Y, Hirabara E, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer 2007;14:55–8
- Merckel LG, Bartels LW, Köhler MO, van den Bongard HJ, Deckers R, Mali WP, et al. MR-guided high-intensity focused ultrasound ablation of breast cancer with a dedicated breast platform. Cardiovasc Intervent Radiol 2013;36:292–301
- Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: Initial experience. Am J Roentgenol 2008;190:91–8
- Aubry JF, Pernot M, Marquet F, Tanter M, Fink M. Transcostal high-intensity focused ultrasound: Ex vivo adaptive focusing feasibility study. Phys Med Biol 2008;53:2937–51
- Quesson B, Merle M, Köhler MO, Mougenot C, Roujol S, de Senneville BD, et al. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med Phys 2010;37:2533–40
- Salomir R, Petrusca L, Auboiroux V, Muller A, Vargas MI, Morel DR, et al. Magnetic resonance-guided shielding of prefocal acoustic obstacles in focused ultrasound therapy: Application to intercostal ablation in liver. Invest Radiol 2013;48:366–80
- Salgaonkar VA, Prakash P, Rieke V, Ozhinsky E, Plata J, Kurhanewicz J, et al. Model-based feasibility assessment and evaluation of prostate hyperthermia with a commercial MR-guided endorectal HIFU ablation array. Med Phys 2014;41:033301
- Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012;28:320–36
- de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J Control Release 2011;150:102–10
- Dahl O. Interaction of hyperthermia and chemotherapy. Recent Results Cancer Res 1988;107:157–69
- Bull JM. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res 1984;44:S4853–6
