Abstract
Purpose: The aim of this study was to determine whether localised drug release using thermosensitive liposomal doxorubicin (TLD) and mild hyperthermia produced by a clinical magnetic resonance high intensity focused ultrasound (MR-HIFU) system improves anti-tumour efficacy over TLD alone in rabbit Vx2 tumours. Materials and methods: Rabbits bearing one Vx2 thigh tumour (n = 6 per group) were administered TLD (1.67 mg/kg) either with or without MR-HIFU mild hyperthermia (20 min, 42.0 °C). Tumour progression was measured using contrast-enhanced T1-weighted MR imaging. Toxicity was evaluated by changes in body weight, blood counts, and blood chemistry. Tumour volume, body weight, and blood data were acquired weekly for the first month and biweekly thereafter. Results: Rabbits treated with TLD plus MR-HIFU mild hyperthermia had target region temperatures with spatial-median, temporal-mean of 41.4° ± 0.6 °C; 10th and 90th percentile temperatures were 40.2 and 42.7 °C. All six rabbits that received TLD alone had rapid tumour progression and reached the tumour size end point (maximum dimension >6 cm) within 24 days. Four of six rabbits treated with TLD plus MR-HIFU mild hyperthermia survived to the study end point of 60 days; one reached tumour size end point, one had hyperthermia-related toxicity, all had at least a transient decrease in tumour volume. Weekly body weight, complete blood counts, and blood chemistry did not reveal additional evidence of drug or hyperthermia-related toxicity. Conclusions: Rabbit Vx2 tumours treated with a single infusion of TLD during MR-HIFU mild hyperthermia had reduced tumour growth vs. tumours treated with TLD alone. These findings are an important step toward clinical translation of localised drug delivery using MR-HIFU and TLD.
Introduction
Thermosensitive liposomal doxorubicin (TLD) with rapid release was developed to accommodate the temperatures actually achieved in tumours during clinical hyperthermia treatments (39–42 °C) [Citation1]. Several mechanisms may contribute to the efficacy of hyperthermia-mediated drug delivery observed in rodent models. Mild hyperthermia alone exerts an anti-tumour effect through protein denaturation leading to heat-induced cell kill or to immune system stimulation [Citation2]. It is also a potent modifier of blood flow [Citation3] and vascular permeability [Citation4], potentially increasing the delivery of drug carriers to the tumour vasculature, as well as their subsequent extravasation and accumulation in the tumour perivascular space [Citation5]. Doxorubicin cytotoxicity may be temperature-dependent, demonstrating enhanced cellular uptake [Citation6], cell kill [Citation6,Citation7], and in vivo tumour response [Citation8] with exposure to greater than 42 °C for 30 min or more. However, results from simulations [Citation9] and intravital microscopy studies [Citation10] suggest that the most important factors for the enhanced efficacy of mild hyperthermia with thermosensitive liposomes are rapid heat-triggered release and prolonged localised heating. Rapid release in a timescale of seconds allows the drug to be released and subsequently extravasated during transit through a tumour, while continued release during prolonged heating over many minutes maintains a high intravascular concentration of free doxorubicin. This combination provides a strong concentration gradient for bioavailable drug to extravasate into the tumour extracellular space, with high extracellular drug concentrations sustained long enough to allow cytotoxic quantities of drug to accumulate slowly within tumour cells.
Clinical translation of hyperthermia-mediated drug delivery requires the use of human-scale technologies for delivery of mild hyperthermia. Clinical studies with a commercial liposome formulation have used invasive radiofrequency ablation with drug release in the thermal margins intended to increase lesion size in liver tumours [Citation11], or superficial microwave and unfocused ultrasound hyperthermia in the range of 40–42 °C at depths of less than 3 cm for recurrent breast cancer at the chest wall [Citation12]. High intensity focused ultrasound (HIFU) is currently the only modality that provides non-invasive deep tissue heating with millimetre accuracy. Combining HIFU with quantitative volumetric temperature measurements obtained with magnetic resonance (MR) thermometry enables automatic control of temperatures within target volumes using ultrasound. This technology is approved in several jurisdictions for clinical use in the treatment of uterine fibroids [Citation13] and bone metastases [Citation14], with several other indications under clinical investigation [Citation15–17].
This combination of precise heating and temperature monitoring could also be used to achieve mild hyperthermia at the optimal temperature for drug release [Citation18]. Using MRI controlled focused ultrasound hyperthermia to trigger drug release from thermosensitive liposomes, locally enhanced drug deposition has been demonstrated in centimetre-sized regions of normal tissues [Citation19–21] and solid tumours [Citation22–24]. Recent studies have also demonstrated that hyperthermia-mediated doxorubicin release using HIFU improves intratumoural distributions of bioavailable drug and total drug levels vs. administration of free drug or TLD without heat [Citation22,Citation24,Citation25]. Also, TLD plus MR-HIFU mild hyperthermia in 4-mm diameter target regions has shown an anti-tumour effect in rats [Citation26]. However, the anti-tumour effect of hyperthermia-mediated local doxorubicin release in large animal tumour models using TLD and mild hyperthermia delivered by a clinical MR-HIFU system has not been demonstrated. The importance of evaluating the anti-tumour effect of hyperthermia-mediated drug delivery in a large animal model using clinical MR-HIFU parameters arises from the fact that mild hyperthermia in humans will need to cover regions greater than 1 cm in diameter. At this size, the targeted volume is much larger than the natural HIFU focal point. Dynamic steering of the focus is required to cover the target volume, and variations in tissue properties make it difficult to achieve spatial and temporal temperature uniformity. Variations in tissue temperature may interact with intratumoural fluctuations in perfusion and drug deposition to influence anti-tumour effect. In rodents, this volume of heating becomes a substantial fraction of the total body volume. This might not be a good representation of localised mild hyperthermia in a human, and may result in exaggerated systemic levels of released drug that could affect toxicity and anti-tumour effect. Large animal models enable the use of more realistic mild hyperthermia parameters and provide a better representation of localised heating and drug delivery, for evaluating the performance of these clinical systems prior to human studies.
The rabbit Vx2 tumour is a widely used large animal model of solid tumours for energy-based therapies, including HIFU [Citation27–29]. A tumour of epithelial origin in rabbits, the Vx2 grows rapidly in a number of anatomical sites, and is propagated serially through implantation of tumour fragments or direct injection of cell suspension [Citation30]. In the past, this tumour line has been used in comparative studies of doxorubicin delivery through venous or arterial injection [Citation31], and the rabbit has often been used as a sensitive model of doxorubicin cardiac toxicity [Citation32,Citation33]. Larger animals such as pigs are also suitable for evaluating mild hyperthermia using a clinical MR-HIFU, but lack well-established and characterised tumour models.
Localised drug release using MR-HIFU mild hyperthermia achieves 10–20-fold increased DOX deposition in Vx2 tumours, with high intracellular DOX uptake throughout the tumour [Citation22,Citation24]. Building on these results, this study addresses the question of whether localised release of doxorubicin from thermosensitive liposomes using mild hyperthermia produced with a clinical MR-HIFU system improves anti-tumour efficacy in the rabbit Vx2 tumour model.
Materials and methods
Animals and treatment groups
Study design
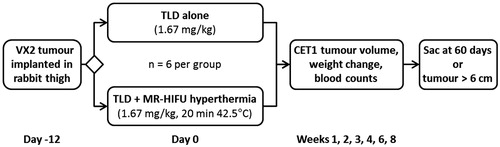
Rabbits bearing a Vx2 tumour in one thigh were administered TLD either with or without 20 min of mild hyperthermia to a target temperature of 42.0 °C using MR-HIFU hyperthermia (). On each treatment day, four rabbits bearing Vx2 tumours prepared from the same cell suspension were randomised equally into the two groups prior to imaging. A total of 12 rabbits were included in the study, six receiving drug alone, and six receiving drug plus heat. Tumour progression was then monitored using serial contrast-enhanced T1-weighted MR imaging, and toxicity was evaluated based on weight change and serial blood counts. MR imaging, body weight measurement, and blood collection were performed weekly for the first month and biweekly thereafter. End points for this survival study were maximum tumour dimension of greater than 6 cm, weight loss of greater than 20%, or survival for 60 days following treatment. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC).
Figure 1. Study design. Rabbits bearing Vx2 thigh tumours were randomised into groups that received either 1.67 mg/kg of TLD alone, or 1.67 mg/kg of TLD plus mild hyperthermia using a clinical MR-HIFU system. Tumour volume was followed on contrast enhanced T1-weighted MRI; body weight and blood counts were monitored for toxicity. Sac, sacrificed; TLD, thermosensitive liposomal doxorubicin.
Vx2 tumour preparation
Vx2 tumour cells were prepared from frozen samples as described previously [Citation22]. Tumour cell fragments stored in Hank’s bovine serum solution (HBSS) in 2-mL cryogenic tubes at −80 °C were thawed in a 37 °C water bath for 1 min, then diced in a glass dish and pressed through a 70-µm strainer into a 50-mL conical tube with 2 mL of HBSS. A small aliquot of the resulting cell suspension was mixed with 0.4% trypan blue and the concentration of viable cells was counted using a haemocytometer. The suspension was then diluted with HBSS to prepare an injection of 3–4 million cells in a volume of 0.4 mL, which was inoculated into the left posterior thigh of each rabbit in the study. Body weight at the time of tumour implantation was 3.0–3.5 kg.
Animal preparation for treatment
Animals were treated at 11–12 days after tumour inoculation. This time period was chosen because it typically resulted in a tumour of 1–2 cm in diameter as measured on T1-weighted contrast-enhanced images. This size of tumour was within the treatable range using the clinical MR-HIFU system with this animal model. On treatment day, rabbits assigned to both treatment groups were anaesthetised by intramuscular injection of ketamine (50 mg/kg/h) and xylazine (10 mg/kg/h), and had one ear vein and one ear artery cannulated. The tumour-bearing leg was depilated to enable transmission of ultrasound into the thigh, and then the rabbit was placed in the lateral decubitus position on a custom-made animal adaptor attached to a clinical MR-HIFU system. The rectal and skin temperatures of the animal were monitored using fibre-optic temperature sensors (Neoptix, Quebec City, Canada). Following treatment, rabbits were returned to the animal facility for recovery, where they were administered intramuscular analgesics and subcutaneous fluids. Between imaging time points, rabbits were monitored daily by vivarium staff for general well-being and were provided with analgesia and dietary enrichment as needed.
Administration of thermosensitive liposomal doxorubicin
Thermosensitive liposomal doxorubicin was provided by the manufacturer (Thermodox®, Celsion, Lawrenceville, NJ). TLD was diluted for infusion in an equal volume of 5% dextrose. An infusion of 1.67 mg/kg of doxorubicin followed by a 5% dextrose flush of 3 mL was administered intravenously into the ear vein over a period of 5 min using an MR-compatible power injector (Medrad Spectris Solaris EP, Bayer Healthcare, Whippany, NJ). In rabbits receiving hyperthermia, infusion was initiated during focused ultrasound heating, once the average target temperature reached the mild hyperthermia range (at least 40 °C). In rabbits receiving drug alone, infusion was performed with the rabbit positioned on the animal adaptor for the MR-HIFU system, but without ultrasound heating and temperature mapping.
Mild hyperthermia system
Mild hyperthermia algorithm and treatment strategy
Mild hyperthermia was produced within the rabbit thigh using a clinical MR-HIFU system (Sonalleve V1, Philips Medical Systems, Vantaa, Finland) attached to a 3-T MRI scanner (Achieva, Philips Healthcare, Best, Netherlands). The MR-HIFU system uses a 256-element phased array therapeutic ultrasound transducer built into an MRI patient bed [Citation34]. To deliver energy to regions larger than the 1 × 1 × 7-mm3 natural focus, the ultrasound focus is steered electronically along the boundary of circular trajectories with 4-, 8-, 12- and 16-mm diameters. In this study, a sonication frequency of 1.2 MHz was used.
For mild hyperthermia in animals, a modified version of the binary feedback control algorithm developed by Partanen et al. [Citation35] was used, with the goal of maintaining a mean temperature of 42.0 °C in the tumour for 20 min. The input for feedback control was the mean temperature measured in voxels within the target diameter on slices defined across the ultrasound focus. During the initial heat-up phase, the interior circles were heated at the maximum prescribed power (typically 12 or 16 W) until they reached 39 °C. Next, the outer circle was heated at the same power until voxels in this circle reached the lower limit of the target temperature band. In these experiments the temperature band was defined with a lower limit of 42.0 °C, and upper limit of 42.5 °C. In the maintenance phase, zero power was applied until the mean temperature of the pixels along the boundary of any circle dropped below 42.0 °C, at which point the under-heated circle was sonicated at five eighths of the prescribed power. When sonicating, power was shut off for an image acquisition period if the mean temperature in any circle exceeded the upper limit of the target temperature band (42.5 °C), or if the maximum temperature in the target region exceeded a safety threshold of 44.0 °C.
Mild hyperthermia treatment cells of 12 mm in diameter were used to cover the extent of the identified tumour. In cases where the tumour shape was elongated perpendicular to the beam path (2/6 rabbits), two treatment cells were defined and sonicated in succession, allowing for tissue cooling between the two mild hyperthermia sonications. Drug infusion occurred during the first sonication. In cases where subject motion disrupted temperature mapping during mild hyperthermia (1/6 rabbits), hyperthermia was stopped, the tissue was allowed to cool, and a second mild hyperthermia sonication was started to reach the full prescribed treatment time. The infusion of TLD was not stopped, and allowed to continue for the full 5 min.
Animal adaptor and imaging coil
A customised experimental set-up was developed in order to use the clinical focused ultrasound ablation system for mild hyperthermia in rabbits. This set-up is illustrated in and described below.
Figure 2. Experimental set-up for mild hyperthermia in rabbit Vx2 tumours using a clinical MR-HIFU system. Left: Axial survey image of a rabbit on top of a water-filled animal adaptor. A waterproofed receive-only imaging coil fits around the lower leg. The bottom film of the animal adaptor is coupled to the window of the clinical HIFU system by a gel pad; the HIFU transducer is in the oil bath below. Overlays indicate the relative size of the ultrasound beam path (dashed) and treatment cell (shaded). Right: Rendering of the animal adaptor designed for the clinical HIFU system. The detachable lid (A) is a polyimide film glued to an acrylic ring. The cylindrical water bath (B) is a 3D-printed shell that holds a volume of degassed water, which is heated by water pumped through a coiled channel printed into the walls of the cylinder. Polyimide film (C) forms the base.
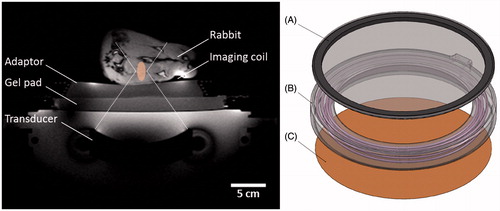
In clinical focused ultrasound therapy, a series of short ablative sonications is delivered with acoustic coupling to the patient’s skin provided by a gel pad. Longer exposure durations in targets close to the skin could cause accumulation of dissipated heat in the gel pad and reduce heat dissipation from the skin. Mild hyperthermia in rabbits requires the ability to freely dissipate heat from the body into the ultrasound coupling medium. It also requires that the temperature at the skin surface matches the animal’s body temperature, to ensure that the reference temperature added to MR temperature difference maps is representative of the actual tissue temperature throughout the targeted limb at the start of the sonication. Finally, the treatment depth in a rabbit is much smaller than for a human, so the animal should be placed higher up off the HIFU table to allow effective targeting.
For this study, an animal adaptor was designed to provide acoustic coupling to the rabbit thigh through a bath of warm degassed water. The adaptor is essentially a cylindrical water bath designed to fit on top of the acoustic window of the clinical system, supporting the subject at the depth of the ultrasound focus, allowing for convective heat dissipation from the skin, and maintaining normal body temperature within the thigh (). This water bath was 3D-printed in a material that approximates the properties of polycarbonate using stereolithography (Viper SLA System, ClearVue material, 3D Systems, Valencia, CA). The temperature of the water in the reservoir was regulated by flowing warm water through channels integrated into the wall of the adaptor. The tube’s inlet and outlet were connected by a luer-lock connector to the modified hoses of a standard water blanket heating pump (T/Pump Model TP-500, Gaymar, Orchard Park, NY) located outside the MR room. The temperature set point of the heating pump was manually adjusted to achieve matching fibre optic temperature measurements in the rectum of the animal and at the skin surface away from the target zone. The permanent base and removable lid of the water bath were made with a 25 -µm polyimide film window which provided coupling to a gel pad below (Aquaflex, Parker Laboratories, Fairfield, NJ), and to the rabbit above.
In cases where tumour position forced the beam path to propagate out through the skin of the inner thigh rather than into the pelvis, heating of the opposite leg was avoided by allowing the beam to propagate into a sealed bag of degassed water placed between the legs of the animal. To prevent excessive heating at skin interfaces between the legs, the inner thighs were carefully depilated and coupled to the water bag with ultrasound gel.
To achieve high SNR for MR thermometry in the targeted rabbit thigh, a custom-made single-loop receive-only surface coil was placed under the rabbit’s leg on top of the adaptor. To prevent water from damaging the coil it was sealed with epoxy into a 3D-printed clamshell case.
Imaging parameters
The use of a single channel imaging coil allowed for the optimisation of imaging parameters for treatment planning and MR thermometry (). Treatment planning to determine tumour location and prescribe a heated volume was based primarily on transverse T1-weighted images acquired before and 1 min after intravenous injection of 0.2 mL/kg gadodiamide MRI contrast agent (Omniscan, GE Healthcare). In some cases, T2-weighted images acquired in a sagittal orientation to the rabbit were also useful in confirming the tumour location. The same post-contrast T1-weighted imaging scan was used to monitor tumour response on subsequent imaging days. The potential for susceptibility-related effects of dynamic gadolinium levels in tissue during MR temperature mapping was mitigated because of the time required to plan and prepare for sonication. A minimum of 24.8 min (60.2 ± 32.7 min) elapsed between administration of MRI contrast agent and the first MR-HIFU hyperthermia sonication, which is more than three times the reported gadodiamide distribution half-life in rabbits (7.2 ± 1.7 min), and comparable to the elimination half-life (38 ± 3.0 min) [Citation36]. At this point the plasma concentration is expected to be lower than 1 mM, which is unlikely to cause significant error in MR temperature measurements [Citation37].
Table 1. MR imaging parameters used in this study.
During sonication, tissue temperatures were continuously monitored in a dynamic scan using temperature maps calculated from multi-shot gradient-echo EPI images. Six 5-mm thick slices covering a 16-cm field of view were acquired every 10 s, with 1 mm in-plane spatial resolution. Three slices were automatically placed perpendicular to the beam centred on the ultrasound focus (sagittal to the rabbit), two orthogonal slices were automatically placed along the beam path centred on the focus (transverse and coronal to the rabbit), and one image was manually placed to detect near field heating where the ultrasound beam entered the thigh. As the region of interest was primarily water-based muscle tissue, the water-selective fat suppression technique typically employed in uterine fibroid ablation was not used. This allowed for a slice thickness of less than 7 mm.
Temperature changes during heating were calculated and displayed using a proton resonance frequency shift coefficient of 0.0094 ppm/°C. The absolute temperature was determined by adding the temperature changes calculated from the proton resonance frequency shift method to the average of the measurement of body temperature obtained from the fibre-optic sensors placed in the rectum and skin. The drift correction algorithm used in the hyperthermia exposures was based on the proprietary drift correction technique used clinically for uterine fibroid ablation [Citation34]. Throughout the sonication a global temperature offset was applied to each incoming image based on a temporal fit of the apparent background temperature change in the three image planes automatically prescribed across the ultrasound focus over the previous five dynamics. This apparent background temperature change was the spatial average of all pixels of the image excluding unreliable regions with temporal temperature standard deviation greater than 3 °C and a region centred on the target volume where heating was expected to occur.
Assessment of tumour growth
Treatment efficacy was evaluated based on tumour progression on late-phase contrast-enhanced T1-weighted images acquired weekly for the first month and biweekly thereafter. Image acquisition was initiated 1 min after injection of contrast material. Tumour volume was calculated using customised software written in MATLAB (Mathworks, Natick, MA). Transverse image slices used for contouring were displayed with fixed window and level settings, and an orthogonal reconstruction of the 3D dataset was displayed in a separate window to guide the user to the region of interest. Volume was calculated by manually contouring the enhancing tumour region on each transverse slice, multiplying the contour area by the slice thickness, and summing over all slices. Datasets from different treatment groups, rabbits, and time points were displayed to the investigator without identifying information and in random order to reduce bias.
Assessment of treatment toxicity
Early doxorubicin-related toxicity was monitored by percentage change in body weight and changes in complete blood counts. Dermal toxicity related to liposome and drug accumulation in the skin, including alopecia and skin lesions, was monitored clinically. The major long-term toxicity of doxorubicin is degeneration of cardiac muscle, which was evaluated by elevated serum levels of creatine phosphokinase and lactate dehydrogenase, and later evaluated using histology. Systemic toxicity to the liver and kidney were evaluated based on specific blood chemistry assays. Samples of serum and whole blood were collected from the ear artery prior to treatment and again prior to each imaging time point, and processed by a commercial veterinary laboratory (ANTECH Diagnostics, Mississauga, Ontario, Canada).
Statistics
Descriptive summary statistics are reported as mean values with standard deviation. Tumour volumes at multiple time points were compared using a one-way ANOVA with Bonferroni post-tests between groups at each week. A two-sided t-test was used to compare the post-treatment minimum in relative body weight between the two groups. Changes in blood measurements were compared using one-way repeated-measures ANOVA with Bonferroni post-tests against the pretreatment values. For all tests, differences were considered to be statistically significant for p less than 0.05.
Analysis of temperature maps was performed offline in MATLAB. The temperature accuracy of the mild hyperthermia algorithm was evaluated based on the temporal mean of the spatial median (T50), the T90 (temperature that 90% of the target region exceeds) and the T10 (temperature that 10% of the target region exceeds) in the target region. The duration over which the temporal mean and standard deviation were calculated was based on the start time of the maintenance phase of the feedback algorithm and the end time of the sonication, identified from system logs. The coverage of the target was evaluated based on the dimensions of the region with temporal mean of T50 greater than 40 °C.
For animals in the TLD plus MR-HIFU mild hyperthermia group, a post-hoc subgroup analysis was performed to identify relationships between anti-tumour efficacy and experiment parameters. Rabbits were classified into two subgroups: those having a strong response (smaller tumour size at 60-day end point than at day zero), vs. those that either had a weak response (60-day survival but larger tumour size than at day zero), no response (reached tumour size end point before 60-day end point), or treatment-related toxicity (sacrificed due to toxicity before 60-day end point). Two-way ANOVA with Bonferroni post-test was performed to identify significant differences in body weight, initial tumour size, body temperature, and quality of mild hyperthermia (T50, T90, T10, temporal SD of T50, width and length of the T50 >40 or 41 °C contours, time >40° or 41 °C, and median CEM43).
Results
Mild hyperthermia
Treatment planning
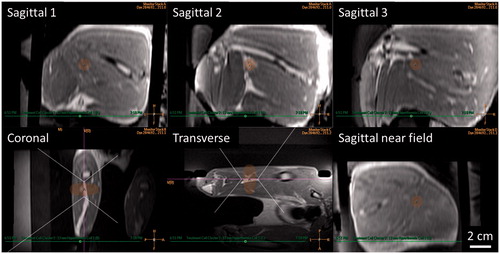
Vx2 tumours were accurately identified and targeted in the clinical treatment planning software using contrast-enhanced T1-weighted planning images (). At the time of treatment, average tumour volume was 0.90 ± 0.35 cm3, with maximum dimension of 1.68 ± 0.75 cm. Tumour delineation for treatment planning was greatly facilitated using multi-planar reconstruction in the treatment planning software. Orthogonal image reconstructions with transducer beam overlays were also helpful for defining transducer translation and rotation to achieve accurate tumour targeting while avoiding sonication through bone or air interfaces. Positioning of the rabbit on the animal adaptor placed the target region within the achievable targeting area of the clinical HIFU system. The large acoustic window of the adaptor and mechanical positioning range of the HIFU system allowed for adequate tumour targeting with minimal repositioning of the subject. However, the 12-mm diameter treatment cell had a length of approximately 20 mm, which is similar to the width of the rabbit thigh. To avoid skin heating, the treatment cell and the middle thermometry slice were centred within the thigh. For 2/6 rabbits, the tumour was slightly off-centre, and was thus covered by both the middle and bottom image slices. In 4/6 rabbits, the tumour was located near the femur, and temperature fluctuations in noisy pixels at the bone interface within 10 mm of the treatment cell intermittently exceeded the control algorithm’s maximum temperature threshold of 44 °C. This forced the controller to switch off power intermittently, resulting in lower median temperatures in the target region.
Figure 3. Mild hyperthermia treatment planning in a rabbit Vx2 thigh tumour using a clinical MR-HIFU system. Three-dimensional reconstruction of contrast-enhanced T1-weighted images acquired for mild hyperthermia treatment planning. Ultrasound beam and treatment cell overlays used to guide translation and rotation of transducer to avoid heating skin, bone, and sensitive structures. For figure clarity, semi-transparent ultrasound beam overlays shown by the treatment planning software are replaced here with dotted outlines. Treatment cell overlays are shaded.
Quality of temperature mapping
Temperature mapping was initiated when optical temperature measurements in the rectum and at the interface between the skin and water bath matched to within 1 °C. The observed baseline temperature was 34.2 ± 1.1 °C in the six rabbits receiving mild hyperthermia treatment. The temperature noise in the thermal maps had a spatial standard deviation of 0.8 ± 0.3 °C. An average background magnetic field drift of 0.55 ± 0.14 °C/min was measured and corrected. During long sonications, large drift artefacts developed at tissue interfaces away from the target region, but did not affect heating.
Accuracy of temperature control
The performance of the mild hyperthermia algorithm was assessed based on MR temperature measurements. shows an example of temperature maps acquired during MR-HIFU mild hyperthermia in a rabbit Vx2 tumour, as displayed by the treatment planning system. In the image slice centred on the tumour, a temporal mean, spatial median temperature of 41.0 °C was achieved over 18.5 min in the 12-mm diameter target region, with T90 and T10 of 39.8 and 42.0 °C. Temperatures sufficient for drug release (at least 40 °C) were reached within the target volume after 120 s.
Figure 4. MRI-controlled mild hyperthermia of a Vx2 tumour in a rabbit thigh using a clinical MR-HIFU system. Top: MR temperature maps acquired after 15 min of heating. The slice labelled Sagittal 2 is centred at the focus on the tumour. HIFU beam path is outlined in white, imaging slice locations are indicated in pink. High temperature measurements in the water outside of the rabbit thigh are the result of imaging artefacts. Bottom: Plot of median temperatures vs. time in three sagittal slices across the treatment cell. The range between T10 and T90 temperatures in the middle slice is shaded. Vertical lines indicate the start and end of thermosensitive liposomal doxorubicin (TLD) infusion, and end of sonication.
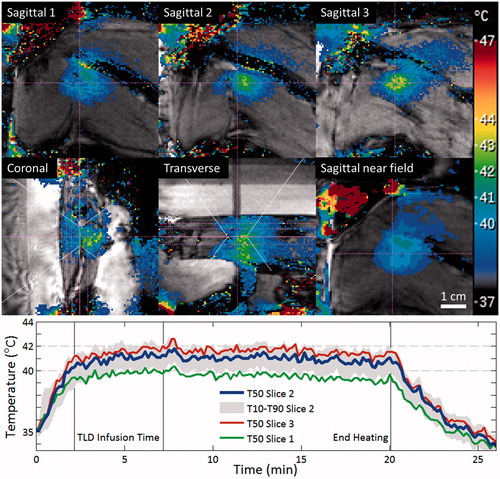
summarises a total of nine mild hyperthermia sonications in six rabbits. In two rabbits, two sonications were used to cover elongated tumours. In one rabbit where thermometry was disrupted by subject motion and sonication was aborted by the user, the tissue was allowed to return to resting temperature and then a second sonication was performed in the same location to reach the prescribed treatment time. For each sonication, temperature accuracy and temporal uniformity were evaluated based on the temporal average and standard deviation of the spatial-median temperature within the target diameter on the central perpendicular thermometry map (labelled Sagittal 2 in ). The duration over which the temporal mean and standard deviation were calculated was based on the start time of the maintenance phase of the feedback algorithm and the end time of the sonication; this duration was 18.4 ± 0.4 min for sonications that completed successfully, and ranged from 3.3–15.9 min for sonications that ended early due to motion. Spatial uniformity was evaluated based on the temporal average of the spatial T10 and T90 on the central thermometry map. Target coverage was evaluated based on the length and width of the region that had an average temperature of at least 40 °C. The mild hyperthermia algorithm provided adequate temperature control for drug release, with T50 temperatures above 40 °C in all cases, reaching that level with a rise time of 98 ± 35 s and varying temporally with a standard deviation of 0.4 °C over the treatment duration. However, T50 was consistently lower than the desired 42.0 °C and varied with a standard deviation of 0.6 °C between sonications. An average T90 to T10 temperature range of 40.2 to 42.7 °C indicated that the spatial uniformity of heating was sufficient to ensure drug release in the entire target region. During mild hyperthermia sonications, the region reaching optimal temperatures for maximal rate of drug release (greater than 41 °C) measured 14.1 × 20.7 mm, closely matching the target volume. The region reaching minimally adequate temperatures for drug release (greater than 40 °C) measured 18.5 × 28.7 mm, widened by thermal diffusion from the 12 × 30-mm region affected during short ablation exposures with the same sonication trajectory. Temperatures were maintained in the mild hyperthermia range of greater than 40 °C for an average of 17.3 min, with median thermal dose significantly exceeding the expected 5 CEM43 in only one rabbit. In cases of multiple sonications, the delay from the end of the first sonication to the start of the next ranged from 13 to 31 min.
Table 2. MR-HIFU mild hyperthermia of rabbit Vx2 tumours.
Tumour response
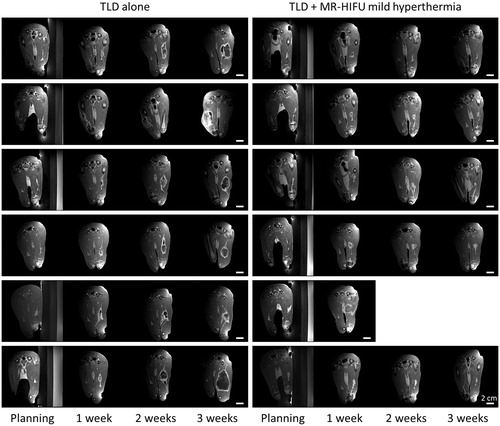
compares follow-up contrast-enhanced T1-weighted images of all rabbits. There is a marked decrease in tumour progression for the rabbits treated with TLD plus mild hyperthermia (right) compared to the rabbits treated with TLD alone (left).
Figure 5. Rabbit Vx2 tumour response after hyperthermia-mediated drug delivery using thermosensitive liposomal doxorubicin and MR-HIFU. Contrast-enhanced T1-weighted images from treatment planning and 1, 2, and 3 weeks post-treatment. Axial images through the 3D volume are shown at the location of the maximal tumour extent. Left: Rabbits treated with TLD alone. Right: TLD plus MR-HIFU mild hyperthermia. Scale bar = 2 cm. TLD, thermosensitive liposomal doxorubicin.
Tumour volume vs. time and overall survival are summarised in . All rabbits that received TLD alone had rapid monotonic tumour progression and reached the tumour size end point (maximum dimensions >6 cm) within 24 days of treatment. All rabbits treated with TLD plus mild hyperthermia had at least a transient decrease in tumour volume. Four of six rabbits treated with TLD plus mild hyperthermia survived to 60 days, and only one reached the tumour size end point. Tumour volume by week is summarised in . Significant differences were not observed in initial tumour volume or at 7 days after treatment, but the TLD plus mild hyperthermia group had a significantly smaller tumour volume starting at two weeks after treatment. No rabbits in the TLD alone group survived past 4 weeks.
Figure 6. Effect of TLD with or without MR-HIFU mild hyperthermia in rabbit Vx2 tumours. (A) Tumour volume calculated from 3D contrast-enhanced T1-weighted MR images. Inset shows the volume at the time of treatment. (B) Overall survival. All rabbits in the TLD group reached the end point of maximum tumour dimension exceeding 6 cm. One rabbit in the TLD + MR-HIFU group experienced severe toxicity and was sacrificed at 7 days, one reached tumour size end point at 53 days, the other four survived to the end of the study. TLD, thermosensitive liposomal doxorubicin.
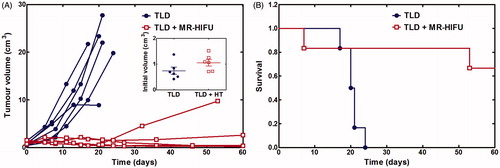
Table 3. Tumour volume grouped by week.
Upon necropsy, secondary tumours were discovered in 3/6 rabbits treated with TLD alone, and 1/6 for TLD plus mild hyperthermia. In the TLD group these arose in the thigh adjacent to the original tumour (two rabbits) and in the liver (one rabbit). In the TLD plus mild hyperthermia group, one rabbit had a large abdominal mass.
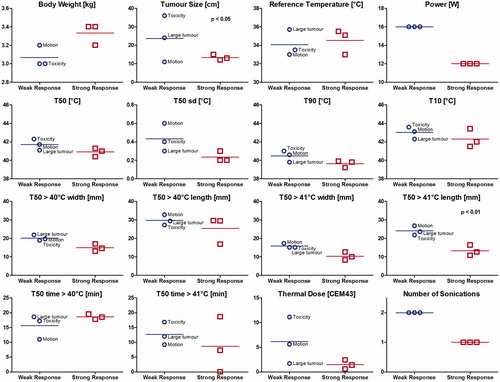
For animals treated with TLD plus MR-HIFU mild hyperthermia, presents a subgroup analysis comparing rabbits with tumour size at day 60 smaller than at day zero (n = 3), against the three rabbits that either had initial tumour regression but relapsed requiring sacrifice before day 60 (n = 1), had larger tumour size at day 60 than at day zero (n = 1), or that were sacrificed due to acute toxicity (n = 1). More data is required for this post-hoc subgroup analysis to produce significant results; however, rabbits that required two sonications either because of large tumour size or motion during hyperthermia were less likely to have a lasting positive treatment outcome. The weak response rabbit that had a larger tumour at day 60 than at day 0 had the shortest duration above 40 °C of all rabbits, with two sonications at the same target, the first interrupted by motion. The rabbit that reached tumour size end point at day 53 had two sonications with good heating, but a large initial tumour size. The rabbit that was sacrificed early due to thermally induced toxicity had the largest initial tumour size and received two sonications that each had a thermal dose above 10 CEM43. Use of higher power may be related to the larger temporal standard deviation of the median temperature and higher thermal dose in the weak responders.
Figure 7. Subgroup analysis among rabbits treated with thermosensitive liposomal doxorubicin plus MR-HIFU mild hyperthermia. Strong response: rabbits with smaller tumour volume at the 60-day study end point than at day 0. Weak response: rabbits with tumour relapse (tumour size end point before day 60, or larger tumour size at day 60 than at day 0) or acute toxicity (sacrificed prior to day 60). The weak response rabbit labelled ‘motion’ had short duration of heating above 40 °C, the rabbit labelled ‘large tumour’ had a large initial tumour size, and the rabbit labelled ‘toxicity’ was sacrificed early due to acute treatment-related toxicity attributable to high thermal dose.
Toxicity
To evaluate the acute toxicity of mild hyperthermia in addition to TLD, rabbits were monitored for skin sores and difficulty walking. One rabbit in the mild hyperthermia group was sacrificed at the first post-treatment time point due to behavioural indications of distress and difficulty walking which resulted in a 14% weight loss in one week. This animal had received two sonications to cover a large tumour, achieving a median thermal dose greater than 5 CEM43; contrast enhanced images at 7 days indicated necrosis of the surrounding muscle (). No other rabbits demonstrated signs of damage attributable to HIFU. In the first four rabbits from both groups, skin sores related to depilation were observed. These lesions resolved after treatment with topical antibiotics and zinc oxide cream, and were avoided in later experiments by minimising the depilated area. Sores did not match the reported appearance of cutaneous toxicity related to liposomal doxorubicin in dogs [Citation38]. In all rabbits, minor bruising was observed at sites of intramuscular anaesthesia injection in the paraspinal muscles. Decreased appetite occurred in all animals, and was resolved with dietary enrichment of fresh fruits and vegetables. No signs of diarrhoea, vomiting, or urine discoloration were observed.
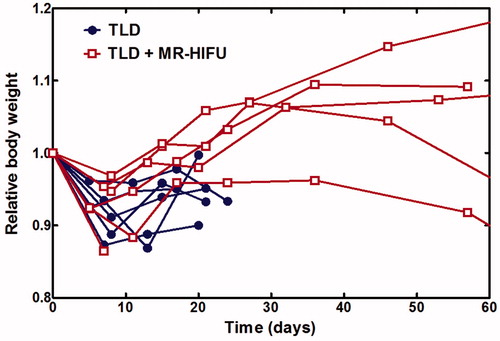
Overall systemic toxicity was compared based on changes in body weight following treatment. There was no significant difference in initial body weight between rabbits treated with TLD alone or with TLD plus heat (3.26 ± 0.14 vs. 3.18 ± 0.19 kg, p = 0.428). plots relative body weight over time. Rabbits in both groups experienced an early weight decrease followed by recovery, with disease progression causing a second weight decrease in some animals. Comparing the first decrease in body weight, there was no significant difference for rabbits receiving TLD alone vs. TLD plus mild hyperthermia (minimum relative body weight 0.90 ± 0.03 vs. 0.92 ± 0.04, p = 0.4027), suggesting that tumour burden and anaesthesia had larger effects than drug or mild hyperthermia toxicity.
Figure 8. Relative body weight over time. All rabbits experienced an initial decrease in body weight and subsequent recovery. TLD, thermosensitive liposomal doxorubicin.
Systemic toxicity was also compared based on complete blood counts and blood chemistry assays. lists weekly measurements for both groups taken immediately before treatment to 3 weeks post-treatment; for the TLD plus MR-HIFU group, data at 8 weeks is included. For each parameter, the results of one-way repeated-measures ANOVA with Bonferroni post-tests against the pretreatment values are shown. Use of one-way repeated-measures ANOVA precluded testing across groups, but enabled within-group comparisons at all time points for the TLD plus mild hyperthermia group.
Table 4. Rabbit haematology and chemistry follow-up. For each measurement, mean and standard error are shown, as well as results of one-way repeated-measures ANOVA, with Bonferroni post-tests against pretreatment value (week 0) for that group. Values outside of normal range are highlighted in bold.
Myelosuppression is a common dose-limiting toxicity for doxorubicin, often including leucopenia, neutropenia, thrombocytopenia, or anaemia. For rabbits receiving TLD alone, white blood cell counts were increased from baseline at all time points; for TLD plus mild hyperthermia this increase was delayed and followed a transient decrease in neutrophils. The TLD plus MR-HIFU group also had increased basophils at week 8, but remained within the normal range. A small decrease in red blood cell counts occurred at week 1 for both treatments but was not significant. Increasing platelet counts for the TLD alone group may have been related to disease progression.
Hepatic, renal, and cardiac toxicity were evaluated based on changes in blood chemistry. Before treatment, both groups had globulin and total protein levels at the low end of the normal range. For the TLD only group, decreased albumin at weeks 1 and 2 may have been related to decreased appetite. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and creatinine, which were monitored for increases related to liver, muscle, or cardiac damage, did not have a significant increase across either group. Likewise, stable levels of blood urea nitrogen (BUN) indicated that renal failure did not occur. In the TLD only group, cholesterol increased following treatment, but remained within the normal range. Consistent glucose elevation across all rabbits was likely related to the use of ketamine anaesthesia, but could also be a result of stress or decreased food intake. At the last follow-up, one rabbit in the TLD alone group had elevated ALT, AST, and creatinine with stable cholesterol, BUN, and ALP, suggesting toxicity to skeletal or cardiac muscle. Necropsy revealed pale streaking of cardiac muscle consistent with myocardial degeneration, consistent with doxorubicin toxicity.
Previously, creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) were identified as potential markers for early doxorubicin-related cardiac toxicity in rabbits [Citation32]. In our study, neither parameter demonstrated significant differences during follow-up. A transient increase in CPK, indicative of damage to skeletal or cardiac muscle, was observed in weeks 1 and 2 for the TLD plus mild hyperthermia group, but was not statistically significant. This measurement was likely confounded by a number of factors including stress, the use of ketamine anaesthesia, and damage induced by intramuscular injection of anaesthesia. LDH, which is also sensitive to muscle damage, gradually increased beyond the normal range for both groups, but this change was not statistically significant.
Discussion
This study represents in vivo validation of the anti-tumour efficacy of localised hyperthermia-mediated doxorubicin release in a large animal tumour model using a clinical MR-HIFU system and commercially developed thermosensitive liposomal doxorubicin. Rabbit Vx2 thigh tumours treated with a single intravenous infusion of TLD plus localised mild hyperthermia had a significant reduction in tumour growth with respect to tumours treated with TLD alone. These findings support the results of previous small animal studies, and constitute an important step towards the clinical translation of mild hyperthermia and thermosensitive liposomes for localised drug delivery.
Dose determination for hyperthermia-mediated drug delivery
Previous studies have demonstrated that hyperthermia-mediated release from TLD achieves locally increased doxorubicin deposition in heated vs. unheated tissues [Citation22–24,Citation39]. This increased therapeutic index can be utilized for either increased local tumour control or decreased systemic toxicity. The doxorubicin dose for this study was selected after reviewing the literature for preclinical efficacy and toxicity of doxorubicin, liposomal doxorubicin, and thermosensitive liposomal doxorubicin. As described below, the TLD dose used in this study was chosen slightly below the reported minimally effective dose in rabbit Vx2 tumours, thereby avoiding systemic toxicity and relying on enhanced accumulation in tumours to achieve anti-tumour effect. It was also comparable to biologically equivalent doses used in rodent studies of tumour regression using mild hyperthermia and thermosensitive liposomal doxorubicin.
Doxorubicin anti-tumour efficacy and toxicity in rabbits
Swistel et al. [Citation31] reported that single intravenous doses of 1 mg/kg free doxorubicin in rabbits with Vx2 thigh tumours had no effect on tumour growth compared to saline-treated controls, while 2 mg/kg achieved a tumour growth plateau at 4 weeks; 3 mg/kg was still well tolerated and achieved complete response in 6/12 rabbits. At 4 mg/kg, rabbits experienced severe toxicity including alopecia, limb wasting, muscle atrophy, and weight loss.
In a toxicity study, Jaenke et al. [Citation32] treated rabbits with a free doxorubicin dose of 1.2 mg/kg per week for 3–11 weeks. Reversible bone marrow suppression was identified after 3 weeks of treatment. Cumulative doses of greater than 10 mg/kg resulted in progressive degeneration of cardiac muscle at 3 and 6 months. Affected animals had sustained elevation of creatine phosphokinase (an indicator of muscle and myocardial damage) and lactate dehydrogenase (elevated during tissue breakdown, but may also be related to tumour growth). Congestive heart failure was observed in 2/6 rabbits in the maximum cumulative dose group (24 mg/kg).
Using conventional liposomal doxorubicin, which circulates for several days and slowly accumulates in the tumour, incidence of doxorubicin-related cardiotoxicity was reduced even after cumulative doses of 21 mg/kg (1 mg/kg every 5 days) [Citation33]. With free doxorubicin, severe cardiomyopathy was observed in 10 of 15 rabbits at 14 mg/kg; congestive heart failure caused the death of five animals. With liposomal drug, minor cardiomyopathy was detected on histology for only three of 15 animals at 14 mg/kg, and one of 10 at 21 mg/kg. However, liposomal doxorubicin accumulation in the skin caused significant dermal toxicity. After 5 weeks (cumulative dose of 7 mg/kg), open sores in the paws, as well as redness and painful crusting of the skin under the limbs forced treatment to be postponed for 14 days to allow recovery; however, skin lesions led to early sacrifice in six of 25 rabbits. Reversible, but dose-limiting dermal toxicity has also been documented in humans for the same liposome formulation [Citation40].
In our study, a TLD dose of 1.67 mg/kg was used. This is comparable to the 2 mg/kg minimum effective single dose of free doxorubicin in rabbit Vx2 thigh tumours, but achieved tumour response similar to that observed at higher doses. Our single doxorubicin dose provides a much lower cumulative drug or liposome exposure than what has been shown to cause dermal or cardiac toxicity, and only one case of possible drug-related cardiotoxicity was observed.
Efficacy of hyperthermia-mediated doxorubicin release in small animal tumour models
Converting the dose used in this study to other species using normalised body surface area [Citation41], our rabbit dose can be compared to thermosensitive liposomal doxorubicin doses used in previous mouse and rat studies of tumour regression. A dose of 1.67 mg/kg in rabbits is equivalent to 6.67 mg/kg in mice and 3.34 mg/kg in rats. In mice, 5 mg/kg led to tumour regression in human squamous cell carcinoma xenografts [Citation42], JC murine mammary carcinoma [Citation43], and EMT-6 tumours [Citation39]. At 6-7 mg/kg, TLD plus hyperthermia achieved varying degrees of improved survival over TLD or heat alone in several other mouse tumour lines [Citation44]. A similar systemic dose of TLD in mice with bilateral SKOV-3 tumours demonstrated that heating of only one tumour had an anti-tumour effect on both the heated and non-heated tumours [Citation45]. In rats, 5 mg/kg showed improved survival in a fibrosarcoma model [Citation46]. Recently, de Smet et al. described tumour growth delay in rat R1 rhabdomyosarcoma for 2 mg/kg of TLD with MR-HIFU mild hyperthermia [Citation26]. In most studies, doxorubicin without heat, heat alone, and TLD alone were all similar to untreated control.
It should be noted that in our study, baseline body temperature was in most cases well below the typical physiological levels in rabbits or humans. This is an important point, as the release rate for similar liposome formulations increases with temperature in the range of 30–37 °C [Citation47]. The expected impact of lower systemic temperature would be reduced systemic exposure to doxorubicin, resulting in reduced efficacy for TLD alone and improved localisation for TLD plus mild hyperthermia, with lower systemic toxicity for both groups. Additionally, systemic temperatures below the normal physiological range imply low temperatures in the target thighs for animals in the TLD alone group that received no localised heating. This could have resulted in vasoconstriction and reduced liposome accumulation in the tumour.
Use of clinical HIFU system and Vx2 model
Preclinical studies of tumour response with thermosensitive liposomal doxorubicin have been limited to small animal models. Most have used water bath heating techniques [Citation39,Citation42,Citation44–46], with one study using pulsed ultrasound heating without temperature monitoring [Citation43], and recent reports using MRI-controlled heating in rats [Citation23,Citation25,Citation48]. Our study provides efficacy data for hyperthermia-mediated drug delivery with thermosensitive liposomes in a non-rodent tumour model. A clinical MR-HIFU system was adapted for used in rabbits, achieving mild hyperthermic temperatures in a region of rabbit thigh that included the extent of implanted Vx2 tumours. The relative size of the rabbit with respect to the target region and the HIFU system allowed us to use an accurately localised, clinically relevant mild hyperthermia technique to investigate the efficacy of thermally mediated drug delivery. It also served to demonstrate some of the technical challenges of using a clinical system for preclinical studies, and of using a HIFU ablation system for mild hyperthermia.
During mild hyperthermia sonications in rabbit thigh, temperature was maintained within the effective range of 40–43 °C, but was consistently lower than the set point of 42.0 °C. A focal region within the tumour experienced temperatures greater than 41 °C where optimal drug deposition at the maximal release rate for TLD could be expected. The region that experienced temperatures greater than 40 °C, where substantial drug release still occurs but at a slower rate, extended beyond the target zone in both the axial and transverse directions. The clinical transducer was designed to heat large regions deep within the human body, operating at low frequency (1.2–1.4 MHz) with a long focal depth (12 cm). Scanning the focus around a 12-mm diameter treatment cell delivered energy to a region 20 mm in length, with temperatures above 40 °C spreading to 29 mm. This typically spanned the entire rabbit thigh, making it difficult to localise heating along the beam. In , transverse temperature maps showed temperature in the mild hyperthermia range not only in the tumour, but also at the skin where the beam entered and exited the thigh, and in the muscle between the tumour and the inner thigh. To improve localisation of heating in shallow targets in animal models, higher frequency transducers could be used to provide increased ultrasound absorption in tissue [Citation19]. Alternatively, localisation of drug delivery could be improved by combining hyperthermia-triggered release with localised cell permeabilisation using ultrasound-mediated microbubble cavitation, which is less dependent on the thermal properties of the tissue [Citation49].
This work also highlights several challenges of using clinical MR-HIFU ablation systems for mild hyperthermia. One aspect is the increased sensitivity of small temperature elevations in mild hyperthermia exposures to fluctuations in temperature measurements. In this study, long repetition times were employed to achieve smoothly varying MR temperature measurements with high signal to noise. However, in several cases low median target temperatures occurred as a result of intermittent fluctuations in temperature readings near the bone interface. Future implementations of the feedback algorithm should investigate spatial and temporal imaging filters to make the system more robust to oscillating temperature readings. Another difference between ablation and mild hyperthermia is the duration of MR thermometry acquisition. During ablation, temperature maps are acquired over a series of short 1-2 min sonications during which background field drift is typically a minor concern. During mild hyperthermia sonications with over 20 min of continuous thermometry, spatially varying magnetic field drift causes measurable temperature mapping errors not corrected for by the standard zero-order drift correction technique. These could be observed in , with false temperature readings in regions outside the region of expected heating, and with the final temperature cooling below the baseline. Further investigations are warranted in the development of improved drift correction techniques for mild hyperthermia. Finally, the clinical application of MR-HIFU mild hyperthermia will require coverage of larger volumes than typically targeted in individual ablation sonications. In this study, two mild hyperthermia sonications were used for complete heating of elongated tumours, with 13 to 31 min between sonications. TLD was infused at the beginning of the first sonication. This variability in the duration and timing of mild hyperthermia is only expected to have a major effect if heating occurred when systemic concentrations of TLD were low. Based on the reported plasma half-life for TLD in rabbits of approximately 1 to 2 h [Citation22,Citation24], the second sonications in this study were performed within the mild hyperthermia window. In humans the initial t1/2 is approximately 1 to 1.5 h [Citation11], and larger target volumes will need to be considered. Strategies for large volume heating using combined electronic and mechanical beam steering [Citation50] and improved drift correction based on spatial fitting of magnitude-weighted temperature maps are currently being developed for clinical use.
Future directions
This study demonstrated high anti-tumour efficacy using thermosensitive liposomal doxorubicin and mild hyperthermia with a single dose of doxorubicin lower than those previously shown to have tumour control in rabbits. Future preclinical studies should seek to examine the effect of doxorubicin dose on the balance between anti-tumour efficacy and dose-limiting toxicity, as well as the dependence of outcomes on heating duration and temperature. Studies should also compare commercial TLD with other thermosensitive liposome formulations, as well as free and conventional liposomal doxorubicin. Subsequent investigations should also consider that the anti-tumour effects observed in this study may involve contributions from ultrasound pressure-mediated enhancements in drug delivery, or from hyperthermia-induced anti-tumour immune response.
The potential to improve local control with a low doxorubicin dose promised by this approach may find particular utility in the treatment of paediatric solid tumours. For sarcoma and neuroblastoma, doxorubicin is commonly administered as an adjuvant to local treatment, but is associated with a high rate of cumulative cardiac toxicity [Citation51]. In these patients, hyperthermia-mediated doxorubicin delivery could be used to reduce systemic toxicity without sacrificing anti-tumour effect by decreasing the injected dose or the number of doses, or to safely enhance local tumour control.
Acknowledgements
The authors would like to acknowledge the excellent animal care support provided by Alexandra Garces, Shawna Rideout, Carrie Purcell, and Loa Croft. Mohammad Kazem designed and manufactured the water bath and the case for the imaging coil.
Declaration of interest
Thermosensitive liposomal doxorubicin was provided by Celsion Corporation. This work was funded by the Ontario Institute for Cancer Research. Robert Staruch performed these experiments while a student at Sunnybrook Research Institute, supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Alexander Graham Bell Canada Graduate Scholarship. He is now a paid employee of Philips, the manufacturer of the clinical MR-HIFU system used in this study. The other authors report no additional conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Needham D, Dewhirst M. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 2001;53:285–305
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33–56
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21:761–7
- Kong G, Braun R, Dewhirst M. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000;60:4440–5
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001;61:3027–32
- Hahn G, Braun J, Har-Kedar I. Thermochemotherapy: Synergism between hyperthermia (42-43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci 1975;72:937–40
- Herman T. Temperature dependence of adriamycin, cis-diamminedichloroplatinum, bleomycin, and 1, 3-bis (2-chloroethyl)-1-nitrosourea cytotoxicity in vitro. Cancer Res 1983;43:517–20
- Overgaard J. Combined adriamycin and hyperthermia treatment of a murine mammary carcinoma in vivo. Cancer Res 1976;36:3077–81
- Gasselhuber A, Dreher MR, Rattay F, Wood BJ, Haemmerich D. Comparison of conventional chemotherapy, stealth liposomes and temperature-sensitive liposomes in a mathematical model. PLoS One 2012;7:e47453
- Manzoor A, Lindner L, Landon C, Park J, Simnick A, Dreher M, et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res 2012;72:5566–75
- Wood B, Poon R, Locklin J, Dreher MR, Ng K, Eugeni M, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol 2012;23:248–55
- Zagar TM, Vujaskovic Z, Formenti S, Rugo H, Muggia F, O’Connor B, et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int J Hyperthermia 2014;30:285–94
- McDannold N, Tempany CM, Fennessy FM, So MJ, Stewart EA, Jolesz FA, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology 2006;240:263–72
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging-guided focused ultrasound. Radiology 2008;249:355–63
- Chopra R, Colquhoun A, Burtnyk M, N’djin WA, Kobelevskiy I, Boyes A, et al. MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: Initial feasibility in humans. Radiology 2012;265:303–13
- Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. Lancet Neurol 2013;12:462–8
- Furusawa H, Namba K, Thomsen S, Akiyama F, Bendet A, Tanaka C, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: Reliability and effectiveness. J Am Coll Surg 2006;203:54–63
- Hijnen N, Langereis S, Grüll H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Deliv Rev 2014;72:65–81
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2011;27:156–71
- Staruch R, Chopra R, Hynynen K. Hyperthermia in bone generated with MR imaging-controlled focused ultrasound: Control strategies and drug delivery. Radiology 2012;263:117–27
- Gasselhuber A, Dreher MR, Partanen A, Yarmolenko PS, Woods D, Wood BJ, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: Computational modelling and preliminary in vivo validation. Int J Hyperthermia 2012;28:337–48
- Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2012;28:776–87
- Smet De M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J Control Release 2011;150:102–10
- Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 2012;158:487–94
- De Smet M, Hijnen NM, Langereis S, Elevelt A, Heijman E, Dubois L, et al. Magnetic resonance guided high-intensity focused ultrasound mediated hyperthermia improves the intratumoral distribution of temperature-sensitive liposomal doxorubicin. Invest Radiol 2013;48:395–405
- De Smet M. MR-HIFU mediated local drug delivery using temperature-sensitive liposomes. Eindhoven University of Technology, PhD Thesis, 2013
- Hazle JD, Jason Stafford R, Price RE. Magnetic resonance imaging-guided focused ultrasound thermal therapy in experimental animal models: Correlation of ablation volumes with pathology in rabbit muscle and VX2 tumors. J Magn Reson Imaging 2002;15:185–94
- McDannold N, King R, Jolesz F, Hynynen K. Usefulness of MR imaging-derived thermometry and dosimetry in determining the threshold for tissue damage induced by thermal surgery in rabbits. Radiology 2000;216:517–23
- Wijlemans JW, Deckers R, van den Bosch MA, Seinstra BA, van Stralen M, van Diest PJ, et al. Evolution of the ablation region after magnetic resonance-guided high-intensity focused ultrasound ablation in a Vx2 tumor model. Invest Radiol 2013;48:2–7
- Rous P, Kidd J, Smith W. Experiments on the cause of the rabbit carcinomas derived from virus-induced papillomas. J Exp Med 1952;96:159–74
- Swistel A, Bading J, Raaf J. Intraarterial versus intravenous adriamycin in the rabbit Vx2 tumor system. Cancer 1984;53:1397–404
- Jaenke RS. Delayed and progressive myocardial lesions after adriamycin administration in the rabbit. Cancer Res 1976;36:2958–66
- Working PK, Newman MS, Sullivan T, Yarrington J. Reduction of the cardiotoxicity of doxorubicin in rabbits and dogs by encapsulation in long-circulating, pegylated liposomes. J Pharmacol Exp Ther 1999;289:1128–33
- Köhler M, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009;36:3521–35
- Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012;28:320–36
- Harpur ES, Worah D, Hals P-A, Holtz E, Furuhama K, Nomura H. Preclinical safety assessment and pharmacokinetics of gadodiamide injection, a new MRI contrast agent. Invest Radiol 1993;28:S28–43
- Hijnen NM, Elevelt A, Pikkemaat J, Bos C, Bartels LW, Grüll H. The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. J Ther Ultrasound 2013;1:8. doi:10.1186/2050-5736-1-8. http://www.jtultrasound.com/content/1/1/8
- Vail DM, Kravis LD, Cooley AJ, Chun R, MacEwen EG. Preclinical trial of doxorubicin entrapped in sterically stabilized liposomes in dogs with spontaneously arising malignant tumors. Cancer Chemother Pharmacol 1997;39:410–16
- Tagami T, Ernsting MJ, Li S. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release 2011;152:303–9. doi: 10.1016/j.jconrel.2011.02.009
- Alvarez Secord A, Jones EL, Hahn CA, Petros WP, Yu D, Havrilesky LJ, et al. Phase I/II trial of intravenous Doxil and whole abdomen hyperthermia in patients with refractory ovarian cancer. Int J Hyperthermia 2005;21:333–47
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008:659–61
- Kong G, Anyarambhatla G, Petros W, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000;60:6950–7
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007;13:2722–7
- Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia 2010;26:485–98
- Viglianti BL, Dewhirst MW, Boruta RJ, Park J-Y, Landon C, Fontanella AN, et al. Systemic anti-tumour effects of local thermally sensitive liposome therapy. Int J Hyperthermia 2014;6736:1–8
- Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, et al. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. J Natl Cancer Inst 2007;99:53–63
- Mills J, Needham D. The materials engineering of temperature-sensitive liposomes. Methods Enzymol 2004;387:82–113
- Hijnen NM, Heijman E, Köhler MO, Ylihautala M, Ehnholm GJ, Simonetti AW, et al. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. Int J Hyperthermia 2012;28:141–55
- Yudina A, Moonen C. Ultrasound-induced cell permeabilisation and hyperthermia: Strategies for local delivery of compounds with intracellular mode of action. Int J Hyperthermia 2012;28:311–19
- Yarmolenko PS, Gallardo ENC, Partanen A, Ranjan A, Burke C, Bartels LW, et al. Large volume, conformal hyperthermia with magnetic resonance-guided high intensity focused ultrasound. Soc Therm Med 2012. Society for Thermal Medicine 2012 Annual Meeting, April 13–16, 2012. Portland, OR. Abstract number 0019.https://stm.conference-services.net/programme.asp?conferenceID=2837&action=prog_list&session=19599
- Huh WW, Jaffe N, Durand J-B, Munsell MF, Herzog CE. Comparison of doxorubicin cardiotoxicity in pediatric sarcoma patients when given with dexrazoxane versus as continuous infusion. Pediatr Hematol Oncol 2010;27:546–57
