Abstract
Purpose: The aim of this study was to assess the safety and effectiveness of contrast-enhanced ultrasound (CEUS) on ultrasound guided high intensity focused ultrasound (USgHIFU) ablation of uterine fibroids. Methods: Thirty-three patients (37 fibroids) were randomly assigned to two groups: group A (17 patients, 20 fibroids) in which CEUS was used before, during and after HIFU treatment, and group B (16 patients, 17 fibroids) in which CEUS was not administered at all. Follow-up including contrast-enhanced magnetic resonance imaging (MRI) and a clinical questionnaire was performed, and technical success, ablation efficacy, volume reduction and complications were assessed. Results: Technical success was 100% in both groups. CEUS revealed residual enhancement in 40% of the patients in group A and the treatment was continued until the completion of ablation. MRI at 1 month after treatment revealed significant difference in the relative fibroid volume reduction rate between the two groups: 16.1% in group A versus 4.8%, in group B (p = 0.01). There was no statistically significant relative volume reduction rate for the results at 3, 6 and 12 months and no significant changes in the quality of life results or the complication rate. Conclusions: CEUS was safe and effective in enhancing US guidance during HIFU ablation of uterine fibroids. Moreover, the use of CEUS during HIFU sonication increased the ablation efficacy, leading to a more relevant fibroid volume reduction at 1 and 3 months. This gap disappeared after 6 months, when there were no differences between the two groups of patients at MRI. However, in our experience, USgHIFU represented a very effective method for the treatment of uterine fibroids, and the use of CEUS during HIFU procedure reduced the treatment time and treatment repetitions for incomplete fibroid ablation.
Introduction
Uterine fibroids are rather common benign tumours, affecting 20–40% of women aged 35 and above and may cause substantial symptoms affecting quality of life. In 10–20% of cases uterine fibroids may lead to severe bleeding, pelvic pain, and bulk-related symptoms [Citation1].
While hysterectomy has been the historical approach to symptomatic fibroids, currently many women prefer less invasive methods that may preserve the uterus, particularly during reproductive age. Therefore, more recently, emphasis has been placed on the development of less invasive treatments [Citation2,Citation3]. Among these, high intensity focused ultrasound (HIFU) ablation has shown promising results offering a minimally invasive solution for this common problem [Citation4,Citation5]. In the majority of the series reported, HIFU treatment of fibroids is performed under magnetic resonance guidance (MRgHIFU) which offers high quality images during fibroid ablation and can control heat delivery, thanks to some dedicated thermosensitive sequences; the main drawbacks of MRgHIFU are the lack of real-time image guidance during treatment and the significant length of the procedure reported [Citation6].
Contrast-enhanced ultrasound (CEUS) is an established diagnostic tool for the assessment of the post-ablation outcome in many organs [Citation7]. It is easy to use when compared to other cross-sectional imaging modalities and it may be performed in any intra-procedural or intra-operative setting [Citation8]. CEUS has already been shown to be useful for evaluating the therapeutic effect of percutaneous HIFU ablation for uterine fibroids when performed 1 week after treatment [Citation9]. In addition, CEUS appeared to enhance the effect of HIFU in an experimental study with rabbits [Citation10]. There is no study at the moment that evaluates the double effect of CEUS during HIFU treatment both as a real time diagnostic tool for the ablation guidance and also as a tool enhancing the ablative effect of the focused ultrasound beam. The purpose of this study is to assess the safety and the clinical results of the use of CEUS during ultrasound USgHIFU of uterine fibroids in women of reproductive age.
Materials and methods
Study design
It is an institutional review board approved randomised prospective study, requiring 12 months of accrual. The informed consent for participation in this study, explaining the risks of the procedure, the available alternatives (hysterectomy, myomectomy, percutaneous arterial embolisation) and the randomisation of the use of CEUS, were obtained from all patients before the enrolment. Every single patient was also discussed within a multidisciplinary board (MDB) together with the gynaecologists of our institution, and common consensus for HIFU treatment was obtained.
The inclusion criteria for this study were the following: women (1) above the age of eighteen (2) not planning to become pregnant within the following 2 months after the treatment, (3) with one or more symptomatic uterine fibroids (unusual vaginal bleeding, menstrual or pelvic pain) of any size, detected with both the two imaging modalities (US and MRI), (4) scoring above 20 on a validated symptom severity scale [Citation11] and (5) unresponsive to medical therapy (including progestin, oral contraceptives, and anti-inflammatory drugs). In addition, all patients needed to be able to understand and sign the consent form for participation in the study.
The exclusion criteria were: (1) pregnancy (defined by a positive pregnancy test), (2) breastfeeding, (3) presence of gynaecological malignancy in the past 5 years, (4) previous pelvic surgery (that led to the presence of numerous surgical clips and/or severe scarring), (5) pelvic inflammatory disease, (6) immunologic disorder and (7) abnormal coagulation status. Finally, active menstruation was a relative contraindication and the procedure was postponed if present.
Patients
Thirty-six consecutive women were initially recruited for this study. Three patients were excluded by the MDB: two of them due to the presence of a subserosal, pedunculated fibroid and one due to a previous pelvic surgery. In the first two cases there was a high risk for the fibroids to fall off into the peritoneal cavity after treatment. In the last, some artefacts were noted in the anterior abdominal wall due to residual suture material that would not allow the homogeneous ultrasound wave propagation.
Thirty-three patients with 37 fibroids were included in the study. Menorrhagia and/or metrorrhagia were the most frequent symptoms reported, occurring in 25/33 patients (75%). Dysmenorrhoea (n = 20/33, 60%), pelvic heaviness (n = 12/33, 36.3%) and urinary discomfort (n = 8/33, 24.2%) were other reported symptoms. In 11/33 (33.3%) two or more symptoms were present.
The patients were randomly divided into two groups: group A (17 patients, 20 fibroids) and group B (16 patients, 17 fibroids). Randomisation was performed using sealed envelopes. Thirty-four envelopes, 17 with the word ‘CEUS’ and 17 with the words ‘No CEUS’, were placed into a sealed box. Envelopes were extracted immediately before the procedure. There were 17 ‘CEUS’ envelopes extracted and 16 ‘No CEUS’. The last residual envelope was opened and ‘No CEUS’ was confirmed. In the patients assigned to group A, up to three vials of 2.4 mL of sulphur hexafluoride in the form of microbubbles (SonoVue, Bracco, Milan, Italy) were intravenously injected before, during and after USgHIFU ablation, whereas patients of group B did not receive any microbubble US contrast agent injection before, during or after the HIFU procedure (no placebo was administered).
The baseline characteristics of the two groups and the mean volume of the fibroids before treatment are shown in . The mean age of patients of group A was 43.1 years (range from 29 to 51, SD 5.3) and the mean age of patients of group B was 42 years (range from 32 to 52, SD 5.4). The mean fibroid volume for group A was 419.24 cm3 (range 47.3–1865, SD 409) and 189.58 cm3 (range 34.6–709.5, SD 190) for group B, with statistically significant difference between the two groups (p < 0.05).
Table 1. Characteristics of the 33 patients who underwent USgHIFU, and mean volumes before treatment.
Procedure preparation
A contrast-enhanced MRI scan was obtained in all cases 1 week prior to the procedure and the target fibroid volume was calculated from the T2 weighted images. The severity of the symptoms was assessed using the Uterine Fibroids Symptom and Quality of Life questionnaire (UFS-QOL) () [Citation11]. Pre-treatment US ‘simulation’ was also performed a couple of days before the treatment, with the ultrasound scanner (MyLab70 Gold US imaging device, Esaote, Genoa, Italy), which is part of the USgHIFU device, in order to evaluate the detection of the target fibroids and to assess the technical feasibility (acoustic path and bowel loops interference). All patients were given adequate bowel preparation the day before the treatment and instructed to fast for at least 8 h. The overlying skin was carefully shaved in order to avoid any potential interference from body hair, which may cause US wave reflection and the risk of skin damage. The procedure was performed under deep sedation with intravenous midazolam (1–10 mg) and fentanyl (25–100 μg) administered by an anaesthetist. Prior to placing the patient in the prone position, a Foley catheter was inserted in all patients in order to expand the bladder with distilled water. This is useful for the anterior and cranial dislocation of the uterus when needed. A purified water balloon was always placed between the HIFU transducer and the patient in order to compress and push the bowel loops away from the HIFU track and avoid the unpredictable presence of air bubbles in the US beam track, and at the same time to reduce the thickness of the subcutaneous fat layer. Patients were carefully positioned prone on the HIFU table, ensuring that the skin overlying the target lesion was in contact with the water balloon and the degassed water contained ino the water tank, where the HIFU transducer was placed.
Table 2. Clinical parameters included in the UFS-QOL [Citation11].
The target lesion was first detected with US using B-mode and colour Doppler imaging. A preliminary planning scan was then performed in order to define the suitable safe ablation track and was stored as a baseline cine loop. The diagnostic US machine settings, such as the focal zone and time-gain compensation, were optimised for the US guidance of HIFU treatment. The entire fibroid volume was then divided into 5-mm interspersed axial US slices, and recorded by the software of the HIFU equipment, as the treatment plan.
In group A, CEUS was first performed with a half dose of 2.4 mL (i.e. 1.2 mL) of sulphur hexafluoride microbubbles (SonoVue) injected via an antecubital vein, using a 20-gauge cannula as a rapid bolus followed by a 5-mL saline flush. Harmonic microbubble-specific imaging mode with low acoustic US pressure (3.5 MHz transducer; mechanical index <0.2; 12–13 frame rate/s) was performed as baseline study. The fibroids were continuously scanned after contrast medium injection and axial scanning acquisition of the whole treatment plan was achieved and recorded with cine-loop sequences. All group A patients were scanned with CEUS, immediately before the ablation and all CEUS examinations were monitored up to 5 min after contrast medium injection and recorded on the optical disk system of the diagnostic US machine. Group B patients were evaluated only with the pre-treatment B-mode US and colour Doppler images, acquired and recorded for the treatment plan.
USgHIFU treatment
USgHIFU ablation was performed on the JC-HIFU-system (Chongqing Haifu, HIFU-Tech, Chongqing, China). Ablative acoustic energy was produced by a transducer with a diameter of 20 cm, focal length of 15 cm, which operates at a frequency of 0.8 MHz. A MyLab70 US imaging device was used as the real-time imaging unit for HIFU guidance. The built-in 1.0–8.0-MHz diagnostic probe is located in the centre of the HIFU transducer.
Target regions, included within the recorded US scans of the treatment plan, were ablated with US energy delivered by the HIFU beam, guided by real time US imaging. Sonications (i.e. activations of the transducer for energy delivery) at every single focal spot position within the target were repeated until a tissue change was achieved on US imaging. Every sonication was 3 s long and was repeated at least three times with an interval of 3 to 4 s. The energy of every sonication was set to 250 W at the beginning and then increased up to 400 W, if needed, according to the tissue changes in echogenicity. This process was repeated on a slice-by-slice basis, in order to achieve complete ablation of the whole fibroid volume. During the ablation the real-time US scans obtained immediately before and after individual energy exposures (i.e. HIFU sonications) were compared in order to define the change in the echogenicity of the treated region, which is indicative of the extent of ablation (). The expected post-sonication appearance is that of an ill-defined hyperechoic area. This change in echogenicity may be achieved faster when the sonications are accompanied by tissue enhancement at the level of the HIFU focal spot after the intravenous injection of microbubble ultrasound contrast medium, due to the increased tissue impedance.
Figure 1. Plain US images collected (A) before and (B) after HIFU treatment show the difference in fibroid ecogenicity. In (B) disomogeneous hyperchoic changes of treated fibroid are visible.
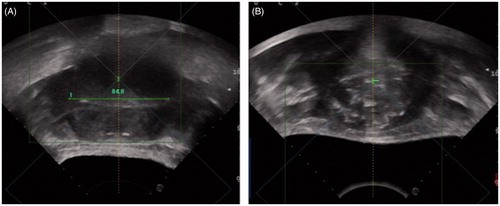
For this reason, for group A patients a second injection of a quarter of a dose (0.6 mL) of contrast medium was performed around 15 s before the first HIFU sonication. The target area at the focal spot level was monitored with US harmonic mode and the sonication was performed before the microbubbles washed-out. Further injections of the same small amount of contrast medium (0.6 mL) were then performed only when operators did not achieve enough echogenicity changes after 4 to 6 sonications at the same level. When the HIFU session was considered completed according to the echogenicity changes of the ablated target at the US greyscale, another 1.2 mL (half vial) of contrast medium was injected and the whole target volume was scanned with the US harmonic mode. Post-operative findings were then compared to the pre-operative CEUS cine loop. If post-operative evaluation detected no residual enhancement within the target volume, the HIFU session was concluded (). Conversely, if residual enhancement was still present it was interpreted as the presence of residual fibroid tissue (), and additional ablation sessions were performed immediately. No further CEUS was performed. No more than three vials (2.4 mL × 3 = 7.2 mL in total) were injected during each session in group A patients.
Figure 2. CEUS imaging before (A) and after (B) USgHIFU of 48 mm highly perfused uterine fibroma (*). In (B) CEUS shows a complete devascularisation of the treated fibroma. Arrow = left common femoral vein; arrowhead = bowel loop.
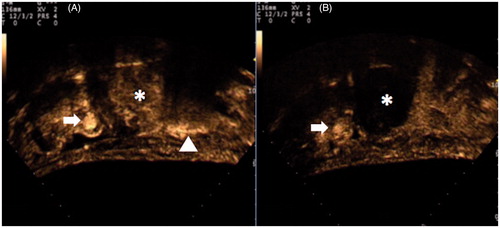
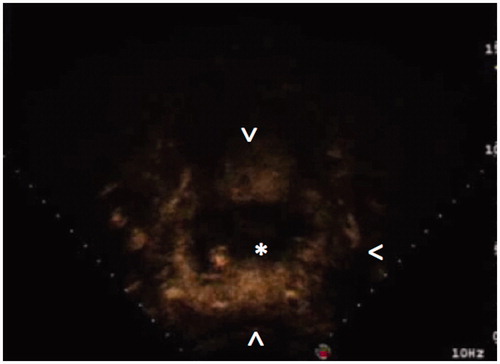
Figure 3. CEUS imaging during USgHIFU of a 75-mm highly perfused uterine fibroma shows a still highly perfused tissue (arrowheads) around the central, already treated, devascularised area (*).
In group B only the US evaluation of greyscale changes was used to assess the differences in the echogenicity of the treated region that were indicative of the extent of the ablated area.
Treatment length and amount of delivered energy were recorded in both the two groups.
Follow-up
All patients underwent clinical and imaging follow-up. At 1 month a clinical evaluation was performed with cell blood count and a new UFS-QOL questionnaire. Contrast-enhanced pelvic MRI, which is considered as the gold standard technique for assessing fibroid ablation, was compared to the CEUS scan performed at the end of the HIFU treatment. MRI was performed in all cases at 1, 3, 6 and 12 months, and fibroid mean volumes for groups A and B were calculated and compared.
Definitions and statistical analysis
Technical success was defined on the basis of the satisfactory change of the echogenicity (i.e. change of echogenicity of the whole extent of the target volume) and/or the absence of contrast enhancement at the end of the procedure. Differences between the two treatment groups (group A) and control (group B) in the distributions of volume at baseline were evaluated using a student’s t-test. At each follow-up point (1, 3, 6 and 12 months) the individual change of fibroid volume from baseline was calculated. The differences of the mean individual changes between the two groups were tested using a linear mix of repeated measures adjusting for the baseline value. In the regression analysis a cubic root transformation of the fibroid volume was used to reduce skew [Citation12]. All analyses were performed with SAS software version 9.2 (Cary, NC). All reported P-values were two-sided.
Results
Technical success was 100% in both the two groups and all the ablative sessions were carried out uneventfully.
In group A, at the end of the HIFU treatment CEUS revealed 8/20 (40%) fibroids with residual enhancement (even though a change in the greyscale echogenicity was noticed over the whole extent of the target fibroid), and further sonications were then performed at the level of the residual enhancing areas, until completion. In all group B patients the ablative session was stopped when the change in the greyscale echogenicity was noticed at the whole extension of the target fibroid. MRI at 1 month revealed 17/20 (85%) cases of group A with complete ablation of the fibroids without residual enhancement, whereas complete ablation was only assessed in 9/17 (52.9%) in group B patients (p < 0.05).
The average energy delivered was 398.842 J in group A patients and 699.896 J in group B patients (57% higher than in group A). Sonication time, which is the sum of the sonications used for the whole HIFU procedure, was 1485 s in group A and 2297 s in group B (64.4% longer than in group A).
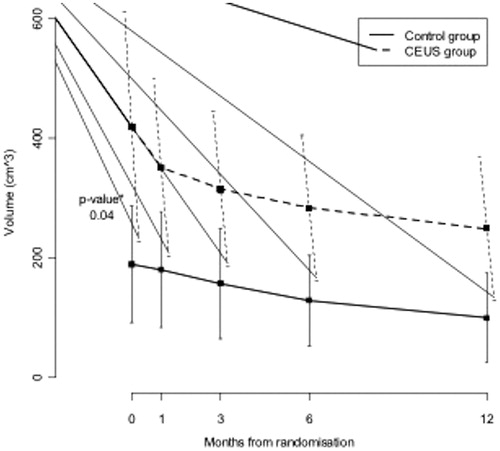
In the MRI scan 1 month post-procedure, the mean fibroid volume was 351.58 cm3 (SD 317) for group A and 180.47 cm3 (SD 189) for group B with relative volume shrinkage of 16.1% for group A and 4.8% for group B, and a statistically significant difference between the two groups (p < 0.05). A volume reduction in group A was also noticed on the scan 3 months post-procedure; however, it was not statistically significant (24.8% versus 17%, p = NS). No significant relative volume reduction rate was detected on MRI at 6 and 12 months (32.3% versus 31.9%, p = NS and 40.5% versus 47.2%, p = NS, respectively) (, ).
Table 3. Fibroid volume observed before (time 0) and after HIFU ablation (1, 3, 6 and 12 months): mean and standard deviation by randomisation group.
UFS-QOL questionnaire mean change scores were all greater than or equal to 16 points, but no differences were noted between groups A and B after treatment. In four cases (one of group A and three of group B) there was significant persistence of symptoms and a new HIFU treatment was performed in each case after a mean time of 4.3 months.
Complications
During the HIFU ablation most patients experienced minor abdominal pain, but as soon as the procedure stopped the pain was significantly reduced and patients were pain free within 1 h post-procedure.
Hospital stay was free of complications and all patients from both groups were discharged on the day of the treatment. In two cases (one from group A and one from group B) skin erythema was noticed at the end of the HIFU session, which disappeared within 1 week. There were no significant post-procedural complications.
Discussion
Uterine fibroids are very common benign smooth-muscle tumours, which may cause significant symptoms affecting the quality of life of women. The historical treatment for fibroids was hysterectomy, however social and cultural changes, along with technological improvements in medicine, have increased the desire for women to retain their uterus, particularly if they are affected by this problem at a reproductive age [Citation13,Citation14].
Even less invasive surgical solutions such as myomectomy are linked to a high morbidity (incontinence, vaginal vault prolapse, and risk for premature ovarian failure), long recovery times and high costs, and therefore alternative and less invasive therapies for the treatment of symptomatic fibroids [Citation15], such as uterine artery embolisation (UAE) [Citation16,Citation17], cryomyolysis [Citation17], and recently even cryoablation [Citation18] and radiofrequency thermal ablation (RFA) [Citation19] have been developed.
HIFU ablation is a ‘needle free’ ablative treatment modality. HIFU technology provides a potential therapeutic method for the precise ablation of entire tumours of different sizes and shapes without damaging overlying and/or surrounding vital structures. Previous clinical experiences regarding solid tumours have already been published with very promising results [Citation20]. During focused ultrasound (HIFU) therapy, the high-intensity US beam is focused selectively within the targeted tumour tissue, and while the beam travels through adjacent tissues, the temperatures within the focal spot rise rapidly, resulting in the thermal ablation of target tissue. This technology may provide a valid alternative to surgery or other invasive treatments for women with symptomatic uterine fibroids who wish to preserve their fertility, and avoid potential complications such as necrosis or injury of surrounding organs.
For these purposes several studies on HIFU have been performed reporting an improvement in patient’s symptoms and a reduction of fibroid size over time. After early feasibility studies [Citation21,Citation22] published in 2003, Taran et al. [Citation23] in 2009 described the treatment of 109 patients with HIFU, concluding that MR guided focused ultrasound (MRgFUS) treatment of uterine leiomyomas leads to a clinical improvement with fewer significant clinical complications and adverse events, compared to hysterectomy at 6-month follow-up. However, so far, despite the fact that a considerable amount of published data is available regarding MRgFUS for uterine fibroids, there are limited papers on the use of USgHIFU.
Zhou et al. [Citation9] studied 64 patients who underwent HIFU treatment of fibroids aiming for symptomatic relief. The authors used CEUS for evaluating the local results within 1 week after HIFU ablation and revealed that 11 fibroids required new HIFU treatment, according to the residual enhancement assessed with enhanced US imaging. The authors concluded that CEUS is potentially useful for the evaluation of early therapeutic effect of HIFU. However, in this study CEUS was not performed in the same setting and all the cases that needed further HIFU treatment, required a new treatment session. In a study by Meng et al. [Citation24] the authors compared USgHIFU and radiofrequency ablation (RFA) in the treatment of uterine fibroids; again the authors, from the same group of the previous quoted publication [Citation24], used CEUS for evaluating the treatment efficacy, but not as an intra-procedural evaluation tool. In our series, all patients in group A were evaluated with CEUS during the HIFU session and further sonications were performed in the same setting, if needed, according to the enhanced US imaging. In our study, CEUS performed during HIFU treatment allowed for the early detection of residual unablated (i.e. still enhancing) regions in eight fibroids that were then completely treated within the same session. However, in 3/20 (15%) fibroids of group A, post-treatment MRI revealed still viable tissue that was missed with intra-procedural CEUS. This problem was presumably due to poor fibroid US detection, due to the inherent inhomogeneity of thickness of skin and fat, in the beam pathway, which became hyperechoic during HIFU treatment, due to the energy absorption, refraction, and reflection.
Another advantage of the intra-procedural administration of CEUS is the enhancement of the HIFU effect. HIFU appears more efficient after intravenous microbubble injection as highlighted in the work of He et al. [Citation10]. This study focused on HIFU ablation of rabbit hepatic VX2 tumour. One group received SonoVue intravenous microbubbles injection and a second group (control group) only saline, during HIFU tumour ablation. Histopathology assessment showed local residual viable tumour tissue in 10% of the SonoVue group and 47% in the control group. Our results are comparable and show that the intravenous injection of US contrast medium during HIFU ablation induced a significantly faster shrinkage of uterine fibroids than without the use of CEUS 1 month after treatment. The difference in fibroid baseline volume between the two groups of treated patients (419.24 cm3 in group A versus 189.58 cm3 in group B) is probably the reason why the difference in tumour shrinkage was not statistically significant at 3 and 6 months, and showed no difference when assessed at 1 year using MRI.
However, the sonication time and the delivered energy of HIFU ablations were much lower in group A patients than in group B, and this represents a practical advantage coming from the use of intravenous US contrast medium during the treatment, which may allow for an overall shorter treatment time. The need for a lower amount to achieve effective ablation might even reduce the risk of HIFU-induced complications (such as skin burns), which are clearly related to the amount of energy employed during the sonication process.
We did not notice any clear disadvantage in using US contrast medium during the HIFU session, but a possible limitation could be the increased cost of the procedure.
Our study is the first blind, randomised comparison of USgHIFU with and without intra-procedural CEUS, to our knowledge. Due to the blind randomisation a significantly larger fibroid volume was noticed in group A and this may be considered as another a limitation of the study. Patients were randomly assigned to the two groups immediately before treatment, and it was not possible to predict the distribution of the fibroid sizes in the two groups. We presume that this factor may have affected the results, and the better results of group A would have been even more marked if the volume of fibroids of the two groups had been of more similar size. Another limitation may be the fact that three patients in group B benefited from a second ablation. This may partly explain the greatest change in volume in group B at 12 months compared with group A, in which only one patient underwent a second ablation.
Conclusions
In our experience USgHIFU with intra-procedural CEUS is safe and allows for early detection of residual fibroid tissue after ablation, reducing the risk of further treatments.
The use of CEUS during HIFU offers a more consistent ablative effect that, in the case of treatment for uterine fibroids, is manifest as faster volume shrinkage. In the long term there is no a clear benefit, however due to its safety profile, the shorter sonication time, the lower amount of HIFU energy and the short-term clinical benefits, we may conclude that intra-procedural CEUS is a useful tool for USgHIFU.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ankem K. Information-seeking behaviour of women in their path to an innovative alternate treatment for symptomatic uterine fibroids. J Med Libr Assoc 2007;95:164–172
- Apgar BS, Kaufman AH, George-Nwogu U, Kittendorf A. Treatment of menorrhagia. Am Fam Phys 2007;75:1813–19
- Evans P, Brunsell S. Uterine fibroid tumors: Diagnosis and treatment. Am Fam Phys 2007;75:1053–8
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med Biol 2004;30:245–60
- Rabinovici J, Inbar Y, Revel A, Zalel Y, Gomori JM, Itzchak Y, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol 2007;30:771–7
- Hesley GK, Gorny KR, Henrichsen TL, Woodrum DA, Brown DL. A clinical review of focused ultrasound ablation with magnetic resonance guidance: An option for treating uterine fibroids. Ultrasound Q 2008;24(2):131–9
- Gallotti A, D'Onofrio M, Ruzzenente A, Martone E, De Robertis R, Guglielmi A, Pozzi Mucelli R. Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med 2009;114:1094–105
- Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol 2009;31:15–24
- Zhou XD, Ren XL, Zhang J, He GB, Zheng MJ, Tian X, et al. Therapeutic response assessment of high intensity focused ultrasound therapy for uterine fibroid: Utility of contrast-enhanced ultrasonography. Eur J Radiol 2007;62:289–94
- He W, Wang W, Zhou P, Wang YX, Zhou P, Li RZ, et al. Enhanced ablation of high intensity focused ultrasound with microbubbles: An experimental study on rabbit hepatic VX2 tumors. Cardiovasc Intervent Radiol 2011;34:1050–57
- Spies JB, Coyne K, Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS–QOL, A new disease specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002; 9:290–300
- Bland JM, Altman DG. Transformations, means, and confidence intervals. Br Med J 1996;312:1079
- Hickey M, Farquhar CM. Update on treatment of menstrual disorders. Med J Aust 2003;178:625–9
- Evans P. Uterine fibroid tumors: Diagnosis and treatment. Am Fam Physician 2007;75:1452–8
- Siskin G. New treatments for uterine fibroids. Tech Vasc Interv Radiol 2006;9:12–18R
- Lumsden MA. Embolization versus myomectomy versus hysterectomy. Hum Reprod 2002;17:253–9
- Bratby MJ, Hussain FF, Walker WJ. Outcomes after unilateral uterine artery embolization: A retrospective review. Cardiovasc Interv Radiol 2008;31:254–9
- Cowan BD, Sewell PE, Howard JC, Arriola RM, Robinette LG. Interventional magnetic resonance imaging cryoablation of uterine fibroids tumors: Preliminary observation. Am J Obstet Gynecol 2002;186:1183–7
- Recaldini C, Carrafiello G, Laganà D, Cuffari S, Bergamini V, Ghezzi F, Fugazzola C. Percutaneous sonographically ablation of medium-sized fibroids: Feasibility study. Am J Roentgenol 2007;189:1303–6
- Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. Am J Roentgenol 2010;195:W245–52
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003;226:897–905
- Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a non-invasive thermoablative technique. Am J Obstet Gynecol 2003;189:48–54
- Taran FA, Tempany CM, Regan L, Inbar Y, Revel A, Stewart EA, MRgFUS Group. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol 2009;34:572–8
- Meng X, He G, Zhang J, Han Z, Yu M, Zhang M, et al. Comparative study of fibroid ablation rates using radio frequency or high-intensity focused ultrasound. Cardiovasc Intervent Radiol 2010;33:794–9