Abstract
Purpose: The tumour microenvironment is frequently hypoxic, poorly perfused, and exhibits abnormally high interstitial fluid pressure. These factors can significantly reduce efficacy of chemo and radiation therapies. The present study aims to determine whether mild systemic heating alters these parameters and improves response to radiation in human head and neck tumour xenografts in SCID mice.
Materials and methods: SCID mice were injected with FaDu cells (a human head and neck carcinoma cell line), or implanted with a resected patient head and neck squamous cell carcinoma grown as a xenograft, followed by mild systemic heating. Body temperature during heating was maintained at 39.5 ± 0.5 °C for 4 h. Interstitial fluid pressure (IFP), hypoxia and relative tumour perfusion in the tumours were measured at 2 and 24 h post-heating. Tumour vessel perfusion was measured 24 h post-heating, coinciding with the first dose of fractionated radiotherapy.
Results: Heating tumour-bearing mice resulted in significant decrease in intratumoural IFP, increased the number of perfused tumour blood vessels as well as relative tumour perfusion in both tumour models. Intratumoural hypoxia was also reduced in tumours of mice that received heat treatment. Mice bearing FaDu tumours heated 24 h prior to five daily radiation treatments exhibited significantly enhanced tumour response compared to tumours in control mice.
Conclusions: Mild systemic heating can significantly alter the tumour microenvironment of human head and neck tumour xenograft models, decreasing IFP and hypoxia while increasing microvascular perfusion. Collectively, these effects could be responsible for the improved response to radiotherapy.
Introduction
Squamous cell cancers of the head and neck account for approximately 3% of all cancers in the USA and are the sixth most common cancer worldwide [Citation1,Citation2]. Treatment options include surgery, radiation and chemotherapy. Patients with advanced disease are at high risk for relapse, and of the 600 000 cases of squamous cell carcinoma of the head and neck worldwide, the 5-year survival rate is only 40–50% [Citation2]. New treatment strategies are needed to improve overall survival for these patients. Several studies have demonstrated that poor oxygenation [Citation3] and elevated tumour interstitial fluid pressure (IFP) [Citation4,Citation5] negatively impact the efficacy of therapy. Elevated IFP develops in tumours due to the formation of irregular and leaky vasculature arising from rapid and uncontrolled neo-angiogenesis, [Citation6,Citation7] and due to the absence of a well-developed lymphatic system [Citation8]. Increased oxygen consumption by rapidly dividing cancer cells [Citation9], heterogeneous distribution of vessels [Citation10], and inadequate vascular perfusion in tumours lead to the development of hypoxic regions within tumours [Citation11].
We have previously shown in non-head and neck tumour models that mild systemic heating of mice bearing murine tumours at 39.5 °C for 4–6 h improves blood flow, decreases both IFP and hypoxia, while improving the efficacy of radiation therapy [Citation12]. We hypothesise that systemic heating activates strong normal thermoregulatory responses, resulting in significant changes in flow patterns of normal blood vessels which are receiving neurovascular signals to cause both constriction and dilation of various vascular beds to help dissipate heat to the surface of the body. Tumour blood vessels, although abnormal and not capable of receiving all, or even some of these neurovascular signals, could still exhibit changes in their capacity due to upstream and downstream functional changes. However, there is no previous information on whether squamous cell carcinomas, such as most head and neck cancers, will be similarly affected by mild systemic heating. Therefore, the current pilot study was performed to obtain some proof-of-principle information that the above approach could be extended to treat human head and neck tumour xenografts grown subcutaneously in SCID mice. If successful, these data could help support the implementation of new clinical trials in patients receiving radiotherapy for head and neck cancer. The human head and neck tumour cell line FaDu and a xenograft derived from a surgical specimen of a patient’s head and neck tumour were used in this study.
Materials and methods
Tumour models
FaDu, a cell line derived from a human pharyngeal squamous cell carcinoma, was obtained from ATCC. 5 × 105 cells were subcutaneously implanted on the right side of the back in 6–8-week-old female SCID mice. When used for IFP and relative tumour perfusion measurements, tumours were grown to approximately 400–500 mm3. Larger tumours were used for IFP and relative perfusion measurements in order to have enough surface area for relative tumour perfusion measurements by laser Doppler flowmetry (LDF) and enough volume to reliably measure the IFP. Smaller sized tumours (average size 200 mm3) were used for experiments on the combined effect of heat and radiation in order to allow enough time to see differences in growth before tumours reached the maximum size limit as per the approved Institutional Animal Care and Use Committee protocol. The typical time for the tumours to reach ∼150–200 mm3 was 21 days from injection. Surgical specimens from resected patient head and neck tumour were grown as xenografts in SCID mice to obtain donor tumours which were then cut into 2–3 mm3 pieces and implanted subcutaneously in the flank of 6–8-week-old female SCID mice. The tumours grew to about 400–500 mm3 in an average of 30 days from implantation and were used for IFP, relative perfusion measurements. Tumour volumes were determined from measurements made using calipers along the short (w) and the long (l) axis and by using the formula, volume = (w2 × l)/2.
Mild systemic heating
Systemic heating was conducted as previously described [Citation12,Citation13]. Temperature transponders (Bio Medic Data Systems, Seaford, DE, USA) were implanted subcutaneously in the scruff of the neck in mice at least 48 h prior to whole body heating. Mice were given intraperitoneal injection (i.p.) of 1 mL of saline 30 min prior to heat treatment to prevent dehydration. Mice were then either placed in a cabinet at 22 °C where their body temperature remained at 37 °C or in preheated cages in an incubator (model BE500, Memmert, Schwabach, Germany) set at 38.5 °C, and their body temperatures were raised to 39.5 °C for duration of 4 h. Typically, the body temperature of the mice increased to 39.5 °C within 15 min of being placed in the 37.5 °C incubator. Body temperature was monitored over this 4-h period every 30–45 min using a temperature reader (Bio Medic Data Systems) and incubator temperature was adjusted accordingly to keep the mice body temperature at 39.5 °C. Duration of heating for all studies was 4 h at 39.5 °C.
Tumour interstitial fluid pressure
Tumour IFP was measured using a micro pressure transducer (Model SPR 524, Millar Instruments, Houston, TX) as described previously [Citation12]. Briefly, the transducer was coupled to a wing-tipped 23½ gauge needle catheter filled with sterile saline. The analogue output from the transducer was amplified (Millar Pressure Control Unit, model PCU-2000) and the amplified signal was fed to an analogue to digital converter (model DT9816, Data Translation, Marlboro, MA), the digital signal output was then fed through a USB adapter to a computer. The data was collected at 1 kHz and averaged over 1 s using a program developed and compiled in the laboratory using Measure Foundry (Version 4.07, Data Translation). After the needle was inserted in the tumour, the pressure was allowed to equilibrate and multiple measurements were taken at different depths inside the tumour and the readings were then averaged. The instrument was calibrated from 0 to 30 cm of water using a water manometer.
Tumour perfusion
LDF was performed using an OxyFlo (Oxford Optronix, Abingdon, UK) fitted with a surface fibre-optic probe. Fur from the skin above the tumour was removed using a clipper and mineral oil was applied on the shaved skin for better optical coupling. The probe was placed at five to six different locations over the tumour surface and five measurements taken at each location over a period of 2–5 min. Relative microvascular perfusion is the product of mean red blood cell velocity and the mean red blood cell concentration in the tissue volume under illumination from the probe and is displayed by Oxford Optronics instruments in relative units called blood perfusion units (BPU). The measurements over the whole tumour were then averaged and expressed as BPU. The probe was calibrated every day before use using a mobility standard obtained from the manufacturer.
Near infrared fluorescent liposomes
The number of perfused blood vessels in the tumours was determined using 100 nm sized liposomes containing a near infrared fluorescent (NIRF) dye-labelled lipid. The liposomes were prepared using high pressure extrusion of a mixed lipid dispersion containing distearoyl phosphatidylcholine (DSPC), cholesterol and PEG-5000 distearoyl phosphatidylethanolamine (PEG 5000-DSPE) in the ratio 1:0.5:0.05 (by mole) and a NIRF membrane dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indodicarbocyanine-5,5′-disulphonic acid (DiIC18(5)-DS) (Invitrogen, Carlsbad, CA) added to the lipid mixture at 0.1% of the total lipid molar concentration. The NIRF liposomes were prepared in HEPES buffered saline (pH 7.40). The excitation and emission maxima of the liposomes were determined to be 650 and 670 nm respectively using a QuantaMaster™ 30 phosphorescence/fluorescence spectrofluorometer system (PTI, Edison, NJ).
Tumour vascular perfusion using NIRF liposomes
The number of perfused blood vessels in the head and neck tumour xenografts was enumerated using NIRF liposomes. The control and heated tumour-bearing mice were injected with 200 μL of the liposomes via the tail vein 2 h after heating. The mice were euthanised 24 h later and the tumours excised and frozen in optimal cutting temperature (OCT) compound from Tissue-Tek®. The frozen tumours were sectioned using a cryomicrotome (Shandon Cryotome, Thermo Scientific, Waltham, MA) at a thickness of 8 µm. The sections from the patient head and neck tumour xenografts were subsequently viewed using a Nikon Eclipse Ti (Tokyo, Japan) inverted fluorescence microscope fitted with a Cy5 filter cube and fluorescence images recorded using a digital camera (model RTke SPOT, Sterling Heights, MI). The micrographs were recorded at both 4× and 20× for image processing. Sections of FaDu tumour xenografts were viewed using an Axioskop 2 MOT fluorescence microscope (Zeiss, Jena, Germany) with an extended IR range digital camera (SPOT Xplorer) and the micrographs were recorded at 5× and 10× magnification.
The number of perfused blood vessels in each field of view (FOV) in a tumour section was determined by using US National Institutes of Health (NIH) ImageJ image processing software. The perfused vessels in the micrographs at 20× (patient tumour) and at 10× (FaDu tumours) were identified by setting an intensity threshold of 200–255 for the 8-bit monochrome images. Particle analysis was then performed for enumerating the highly fluorescent spots with areas with minimum and maximum areas of 5 and 5000 pixels respectively.
Tumour hypoxia using hypoxyprobe immunohistochemistry
Pimonidazole HCl (Hypoxyprobe-1™, Burlington, MA) at 60 mg/kg was given by i.p. injection, in control unheated and in heated mice 2 h post-heating. The mice were sacrificed 1 h later; the tumours were excised, fixed in 4% formalin and embedded in paraffin for sectioning. The hypoxic areas in the tumour sections were identified by immunohistochemistry (IHC) of Hypoxyprobe-1 adducts of proteins and peptides formed at partial pressures of O2 < 10 mm Hg and the sections were counter-stained with haematoxylin and eosin (H&E). The sections were viewed by bright field microscopy and micrographs recorded at 10× using a Zeiss microscope (as above). The number of stained pixels in a field of view (FOV) at 10× was determined by image processing using ImageJ. The bright field images were first processed by splitting the RGB micrographs and the blue image was then converted to binary by setting the threshold from 0 to 149. Noise reduction was performed by two erosion cycles followed by two dilate cycles on the binary image. The total number of black pixels was then counted using the histogram function and averaged for all micrographs for each tumour from unheated control and heated mice. Tumours used to determine hypoxia were 400–500 mm3, though extensive hypoxia was found even in smaller ∼150–200 mm3-sized tumours (data not shown).
Radiation therapy
Mice given radiation treatment were anaesthetised with isofluorane (induction at 3–5%; maintenance at 1–2%), and shielded with 2-mm thick lead plates with an aperture to limit radiation exposure to only the tumour. Radiation treatments were administered using an RT 250 Orthovoltage X-ray unit (Philips, Best, the Netherlands) at 75 kV with a 1 × 2 cm cone. Radiation was given for 5 days in 2 Gy fractions for a total of 10 Gy. Treatment times were calculated using current dosimetry data provided by radiation physicists at Roswell Park Cancer Institute, and depth dose curves for 75 kV energy level.
Combined heat and radiation therapy
Heating and five-fractionated radiation treatment: Twenty FaDu mice with tumours that were approximately 200 mm3 were grouped (five per group) such that each group had similar average tumour size: group 1 = unheated control; group 2 = heat only; group 3 = radiation only, and group 4 = heat plus radiation. Groups 2 and 4 were heated to a body temperature of 39.5 °C for 4 h (day 24). Groups 3 and 4 received local tumour radiation at 2 Gy per day for five consecutive days (days 25–29), a total of 10 Gy of radiation. Tumour volumes were measured between 1–3-day intervals. Statistical significance was determined using ANOVA.
Results
Mild systemic heating of mice bearing human head and neck tumour xenografts results in reduced tumour IFP and increased relative tumour perfusion
Interstitial fluid pressure in FaDu tumours in SCID mice was significantly reduced from 17.75 cm/H2O in unheated control mice to 14.88 cmH2O (P < 0.01) at 2 h and to 14.19 cmH2O (P < 0.01) at 24 h after mild heat treatment of tumour-bearing animals (body temperature was maintained at approximately 39.5 °C for 4 h) (). Heating the animals also resulted in a significant increase in relative tumour microvascular perfusion as measured in blood perfusion units (BPU). This elevation was detected at 2 h post-heating (370 BPU, p < 0.001) and was still evident at 24 h post-heating (322 BPU, p < 0.001) as compared to unheated control (124 BPU) ().
Figure 1. Interstitial fluid pressure decreases and perfusion measured by LDF increases following systemic heating (n = 5). (A) Plot of IFP measured in FaDu tumours 2 h and 24 h after systemic heating to 39.5 °C for 4 h. A significant reduction in IFP was observed in the tumours in heated mice as compared to those in unheated control animals (ANOVA p < 0.01**). (B) Plot of LDF measurements on FaDu in blood perfusion units (BPU) in tumours showing significant increase in relative tumour perfusion in heated mice at 2 h and 24 h post-heating in comparison to those in unheated animals (ANOVA p < 0.001***). (C) Plot of tumour interstitial fluid pressures (IFP) in xenografts of a resected patient head and neck tumour (no. 19705) grown subcutaneously in SCID mice at 2 h and 24 h after systemic heating to 39.5 °C for 4 h. IFPs in tumours post-heating show significant decrease (ANOVA p < 0.01**). (D) Plot of relative tumour perfusion measurements using LDF on mice bearing the same patient tumour xenografts show small increases in relative tumour perfusion in heated mice at both 2 h and 24 h post-heating. The increases were not statistically significant.
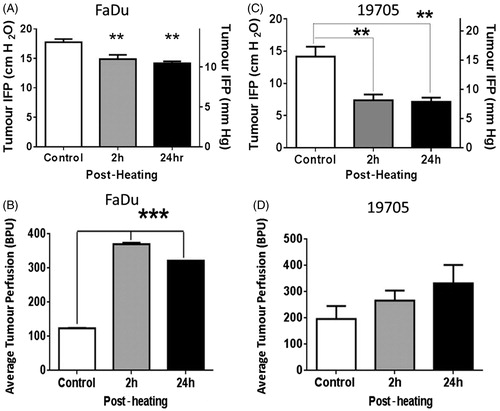
Interstitial fluid pressures (IFP) in the xenograft tumours grown from the resected patient head and neck squamous cell carcinoma (no. 19705) were also reduced in mice tested 2 h after heating (body temperature of 39.5 °C for 4 h) (p < 0.01). Unheated control mice had an IFP of 14.17 cmH2O while heated mice had an IFP of 7.39 cmH2O, this reduction in IFP was evident in mice measured 24 h post-heating (7.13 cmH2O) (). IFP measurement at the 2 - and 24-h time points were made on different groups comprised of five mice each. The mice were euthanised immediately after IFP measurement. Further, relative perfusion in the patient tumour xenograft model showed an upward trend, as measured at both 2 h and 24 h post-heating (increasing from an average of 197 BPU in controls to 266 BPU at 2 h and 331 BPU at 24 h BPU post-heating). However, despite the trend towards increased relative perfusion following heating, there was no significant difference in the patient xenograft model. It is likely that the ability to accurately measure changes in relative tumour perfusion may depend upon the individual tumour architecture and degree of vascularisation. H&E stained sections of the patient tumour xenografts show that tumours consisted of a thin layer of viable cells while the majority of the tumour is filled with dense extracellular collagen matrix (data not shown). The presence of dense extracellular matrix filling most of the tumour volume and extensive necrosis in this tumour could make the relative tumour perfusion measurements unreliable. Tumours that are well vascularised, e.g. FaDu (above) and CT26 [Citation12] show increases in relative tumour perfusion following heating tumour-bearing mice.
Heating tumour-bearing mice increases the number of perfused tumour blood vessels
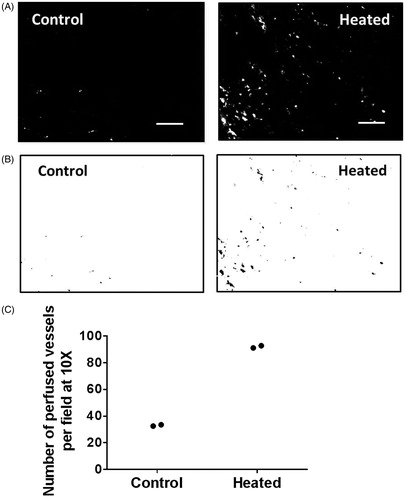
Perfused tumour blood vessels in FaDu and resected patient tumour xenografts were examined using fluorescence microscopy of frozen tumour sections. NIRF liposomes are useful for looking at perfused blood vessels because the fluorescent dye is covalently linked to the lipid head group and is thus contained within the liposomes and does not spread quickly into the stroma, as it happens when small dye molecules are used. The lighter grey areas indicate extravasated fluorescent liposomes. Few vessels were seen to contain fluorescent liposomes in sections from FaDu tumours in unheated control mice (, left panel) indicating their relatively poor perfusion. In contrast, many perfused blood vessels are evident in tumour sections from heated mice (, right panel). The highly perfused vessels were identified by applying a threshold intensity to the same fluorescence micrographs, and the results are shown in (control tumour, left panel and heated tumour right panel). The number of perfused vessels in tumours from control and heated mice (n = 2 for both groups) were enumerated by particle analysis on micrographs of six fields per section, and the results are plotted in . There was an increase in the number of perfused blood vessels in tumours from heated mice as compared to those from unheated mice (average of 92 in heated mice versus an average of 33 in unheated control mice, n = 2 per group). This is only preliminary data and further study is warranted.
Figure 2. Tumour vascular perfusion is increased following systemic heating of mice bearing FaDu tumours (n = 2). Fluorescent liposomes were injected 2 h post-heating when body temperature has returned to homeostatic level. (A) Representative fluorescence micrographs (10×) showing perfusion of FaDu tumour vessel in unheated control and in heated mice, and (B) the corresponding binary images. (C) Plot of the number of perfused vessels in the tumours determined using NIH ImageJ particle analysis routine shows an increase in the number of perfused vessels in tumour from mice that were heated (average 92 in two tumours) and unheated control (average 33 in two tumours). Scale bar = 100 µm.
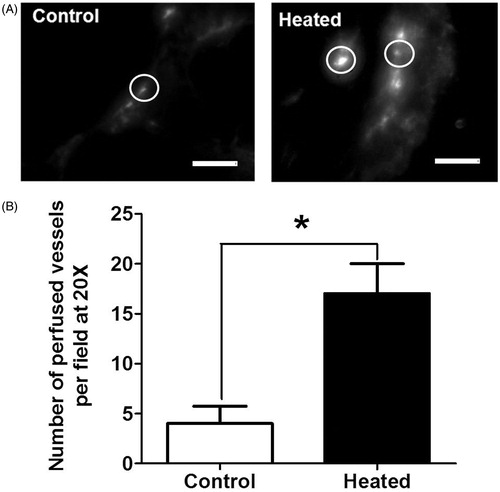
An increase in the number of perfused tumour vessels was also observed after heating the patient tumour-derived xenografts in SCID mice (n = 3 per group). compares representative fluorescence micrographs from a tumour from an unheated and a heated mouse. There are a greater number of perfused vessels (circled) in the heated tumours and they have higher intensity as compared to the perfused blood vessels in tumours from unheated mice. Circles highlight representative vessels, where the brightest intensity is the vessel and the grey is the extravasated liposomes (). A higher magnification was used for patient tumour-derived xenografts because these tumours have an interior of sloughed keratinised debris with only a rim of viable tissue making blood vessels difficult to visualise at lower magnification. Analyses of fluorescence micrographs, as shown earlier for FaDu tumours, show a fourfold increase in the number of functioning vessels (from 4 to 17, p < 0.05) in tumours from heated mice ().
Figure 3. The number of perfused vessels in tumours is increased following the heating of patient tumour xenograft tumour-bearing mice (n = 3) after systemic heat treatment. (A) Representative fluorescence micrographs (20×) showing perfused blood vessels in patient tumour (no. 19705) xenografts in unheated control (left) and in heated mice (right). Circles highlight representative vessels; the brightest intensity is the vessel and the grey is extravasated liposomes. (B) Plot of the number of perfused vessels determined using NIH ImageJ on the micrographs above. The numbers of perfused vessels show statistically significant increase. Scale bar = 100 µm (Student t-test *p < 0.05).
Mild systemic heating reduces hypoxia in FaDu tumours
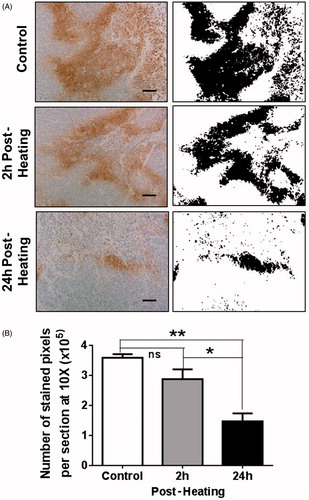
Hypoxic regions in tumours were detected using immunohistochemistry for pimonidazole adducts of protein and peptides using Hypoxyprobe-1. (panels on the left) shows representative micrographs of IHC-stained areas in tumour sections from control unheated mice and tumours from heated animals at 2 h and 24 h post-heating. Extensive hypoxia (brown stain) is seen in control tumour sections (top left) and slightly less staining is seen at 2 h post-heating (centre left) and staining is largely reduced at 24 h post-heating (bottom left). Binary images of the stained regions obtained using NIH ImageJ are shown in the panels on the right and the total area stained with IHC was analysed using the imaging software and plotted in . The average numbers of stained pixels per field at 10× were 3.59 × 105 in control unheated tumours, and 2.87 × 105 and 1.47 × 105 in heated tumours at 2 h and 24 h post-heating respectively, i.e. post-heating, the hypoxic areas in tumours (2 h and 24 h) were reduced to approximately 80% and 40% of that in control unheated tumours. The reduction in hypoxia was not significant at 2 h post-heating but at 24 h post-heating there was statistically significant decrease as compared to the control (ANOVA p < 0.01; n = 3).
Figure 4. Mild systemic heating improves tumour oxygenation. (A) Hypoxic areas in tumour sections were identified using immunohistochemistry (IHC) to label pimonidazole hydrochloride (Hypoxyprobe-1) adducts of proteins and peptides that are formed at low oxygen tension. Panels on the left are micrographs (10×) from tumour sections from a control unheated mouse and from heated mice 2 h and 24 h post-heating. Panels on the right are binary images of the micrographs on the left; the binary threshold was done using NIH ImageJ. The micrographs show extensive hypoxic areas in the control tumour and in the tumour 2 h post-heating. The hypoxic area is much reduced at 24 h post-heating. (B) A plot of the number of IHC stained sections from unheated control mice and those from mice at 2 h and 24 h post-heating. The pixel counts were obtained using NIH ImageJ. There was no significant decrease in the number of hypoxic pixels at 2 h post-heating but at 24 h post-heating there was significant decrease in hypoxia in the tumours. Scale bar = 100 µm (n = 3 per group, ANOVA *p < 0.05; **p < 0.01).
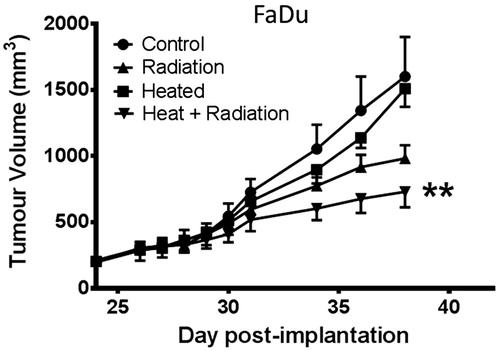
Systemic heating enhances FaDu tumour response to radiation therapy
The effect of systemic heating on the response of FaDu tumours to fractionated radiotherapy is shown in . The study was started 24 days after tumour implantation when the tumours reached approximately 200 mm3 in volume. Four groups of mice (n = 5) were used; the control (group 1) did not receive any treatment while the other three groups received systemic heating only (group 2), fractionated radiation only (group 3) and systemic heating followed by the first of five 2 Gy per day radiation treatments 24 h later (group 4). The groups that received heating or heating and radiation were heated on day 24; the groups that received either radiation alone or combined heat and radiation received the five daily treatments on days 25–29. The growth rates of the control and heated only groups were very similar. The group that received the combined heating and radiation treatments showed the best anti-tumour response. This was also the only group that had average tumour volumes that were significantly different from the average tumour volumes in the control group (ANOVA p < 0.01).
Figure 5. Radiation efficacy is improved by heat treatment prior to radiation therapy. Plot of growth of FaDu tumour xenografts in four groups (n = 5). Group 1 unheated control, group 2 heated (day 24), group 3 local radiation at 2 Gy on days 25–29, group 4 heating on day 24 followed by 2 Gy local radiation on days 25–29 starting 24 h post-heating. Tumour growth in only group 4, heating and radiation showed significant tumour response to therapy as compared to the control group (ANOVA **p < 0.01).
Discussion
High IFP and impaired tumour perfusion and blood flow can each significantly limit the delivery of drugs and oxygen [Citation4,Citation14,Citation15], resulting in tumour resistance to treatment. Reduction of both tumour hypoxia and IFP is thus a high priority in devising effective tumour treatment protocols. Our previous work [Citation12] demonstrated that mild systemic heating can reduce IFP and improve blood flow and the efficacy of radiotherapy. Although this is a limited study, here we show that mild systemic heating reduces tumour IFP () and hypoxia () and increases relative tumour perfusion and the number of perfused vessels (, and ) in head and neck tumour xenografts which are likely responsible for increasing the efficacy of radiation ().
Systemic heating of tumour-bearing mice increased the number of perfused blood vessels in FaDu and in patient-derived tumour xenografts following heating ( and ). This increase in the number of perfused tumour vessels would likely be expected to deliver more oxygen into the tumour interior and, we predicted, that the degree of hypoxia would therefore be decreased in heated tumours. We found that hypoxia in FaDu tumours, measured by Hypoxyprobe IHC () was indeed decreased following heating, especially at 24 h post-heating (, p < 0.1). This decrease in hypoxia at 24 h post-heating is the basis for the timing of the combined heat and radiation treatment schedule.
Local heating of superficial tumours has been reported to increase tumour blood flow and oxygenation [Citation16–18]; however, temperatures used in those studies were higher (40–42 °C) as compared to 39.5 °C used for systemic heating in our earlier reports [Citation12,Citation13] and in the current study. It is, however, possible that the increase in tumour temperature during systemic heating could also have influenced tumour blood flow and oxygenation.
Other treatments have been shown to reduce IFP in tumours such as anti-angiogenic therapies; however, there are added risks to the use of these drugs [Citation19–26] that could be avoided by the use of mild systemic or regional heating. For example, the anti-VEGF agent bevacizumab reduces tumour blood flow and volume, but increases tumour cell invasion, and does not reduce hypoxia [Citation24,Citation26,Citation27]. Targeting epidermal growth factor receptor using erlotinib had only modest clinical activity as a single agent; however, erlotinib treatment resulted in reduced tumour expression of HIF1α and vascular endothelial growth factor (VEGF) in human head and neck tumour cells SQ20B [Citation28]. While we do not currently have human data on the impact of mild heating on tumour blood flow in tumours of cancer patients, these earlier studies suggest regulation of tumour vasculature to improve blood flow but not vascular shut down may be an important key to improved tumour response.
In our previous study we showed that mild systemic heating can improve tumour vascular function. Tumour vessels which were previously collapsed, or otherwise non-perfused, appear to become perfused following an elevation of core body temperature. Our work also shows that this treatment is associated with decreased IFP and hypoxia [Citation12], as shown in and . That mild heating itself has no growth stimulatory effect on tumours is demonstrated by the fact that tumour growth in control and heated mice is identical [Citation12,Citation13] (). This and a lack of any other observable toxicity from mild systemic heating suggest its use as a promising adjuvant to current clinical therapies which are often toxic.
We postulate that tumour perfusion, measured by both relative microvascular perfusion and the number of perfused vessels is increased as a result of a normal physiological thermoregulatory response that is generated to help dissipate heat by increasing blood flow to the surface of the body [Citation12,Citation29]. This increased blood flow through normal blood vessels may then indirectly increase the perfusion of tumour vessels as the blood makes its way from the heated core to the body’s surface. Therefore, the use of head and neck models of subcutaneous tumours is likely to be effective in modelling clinical head and neck disease, as these tumours are often located superficially in patients. The decrease in tumour IFP is possibly due to improved drainage of tumour interstitial fluid at the low pressure end of tumour capillaries, but we currently have no evidence to confirm this supposition. The increase in the number of functioning blood vessels following heating would then enable better fluid drainage from tumour interstitium. It is also possible that heating improves lymphatic drainage, but this may not be as likely since functional intratumoural lymphatics are typically absent [Citation8,Citation30]. In normal tissue excess fluid is drained at the low pressure end of the capillaries and is regulated by oncotic pressure in the interstitium [Citation14] and lymphatics, which provides an ‘overflow’ mechanism due to protein leakage [Citation31]. A similar fluid dynamic would occur in tumours if adequate functioning tumour vessels were present. Systemic heating induced an increase in the number of perfused tumour blood vessels ( and ) and relative tumour perfusion (), and may allow for a period of vascular normalisation that can increase drainage of interstitial fluids from tumours via capillaries and reduce IFP.
Based upon previous clinical studies [Citation32,Citation33] and our experience with murine tumours [Citation12], one would predict that if mice bearing FaDu tumours are heated prior to radiation there would be an enhanced tumour response to radiotherapy. shows that there is an enhancement in efficacy of radiotherapy on FaDu tumours by combining heating followed by fractionated radiation treatment starting 24 h post-heating.
While patient-derived xenograft tumours would also be expected to have improved efficacy of radiotherapy in combination with systemic heating, these studies could not be performed here due to the difficulty of standardising growth in these xenografts. This is likely caused by the fact that the interior of those tumours, like their human counterparts, contain sloughed keratinised debris with only a rim of viable tissue, making accurate volume measurement and assessment of response to therapy difficult.
The present study provides further rationale for using heat as an adjuvant in the clinical setting of cancer treatment, while providing further questions to be studied. Moving forward to expand this type of analysis to multiple cancer models, including those for which there are limited treatment options, is an exciting possibility. Future studies aimed at expanding our current knowledge and further elucidating the mechanisms for the reduction of IFP and hypoxia following mild systemic heating are in progress.
Conclusions
Using two different human head and neck tumour xenograft models, we show here that when the body temperature of tumour-bearing mice is increased for 4 h to approximately 39.5 °C, there is significant increase in relative tumour perfusion lasting at least 24 h post-heating. At the same time, there is a reduction in IFP in these tumours. Based upon our previous reports on the effect of mild heating in syngeneic murine tumours [Citation12,Citation13] and the current study, we suggest that the thermoregulatory consequences of a mild elevation in body temperature are a useful adjuvant to cancer therapies due to its ability to increase tumour vascular perfusion and oxygenation while lowering IFP. These studies therefore provide pre-clinical support for new clinical trials using regional or systemic mild heating to elevate core temperature prior to radiation and/or chemotherapy, and also immunotherapies, since this treatment should increase the access to immune cells via increased vascular perfusion.
Acknowledgements
The authors thank Bonnie Hylander for her advice on the manuscript and data, and also Jeanne Prendergast for her help in managing the lab and many aspects of this research.
Declaration of interest
The authors report no potential conflicts of interest. This work was made possible by support from the US National Institutes of Health (grants CA135368, CA94045, and CA071599) and the Dr. med h.c Erwin Braun Foundation. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300
- Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9–22
- Gray L, Conger AD, Ebert M, Hornsey S, Scott O. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Brit J Radiol 1953;26:638–48
- Jain RK. Transport of molecules in the tumor interstitium: A review. Cancer Res 1987;47:3039–51
- Jain RK. Determinants of tumor blood flow: A review. Cancer Res. 1988;48:2641–58
- Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res 1996;56:4264–6
- Folkman J. Tumor angiogenesis: Therapeutic implications. New Engl J Med 1971;285:1182–6
- Butler TP, Grantham FH, Gullino PM. Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res 1975;35:3084–8
- Bensaad K, Harris AL. Hypoxia and metabolism in cancer. Adv Exp Med Biol 2014;772:1–39.
- Kuszyk BS, Corl FM, Franano FN, Bluemke DA, Hofmann LV, Fortman BJ, et al. Tumor transport physiology: Implications for imaging and imaging-guided therapy. Am J Roentgenol 2001;177:747–53
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004;14:198–206
- Sen A, Capitano M, Spernyak JA, Schueckler J, Thomas S, Singh A, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia, and enhances efficacy of radiotherapy in murine tumor models. Cancer Res 2011;71:3872–80
- Xu Y, Choi J, Hylander B, Sen A, Evans SS, Kraybill WG, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia 2007;23:513–27
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – An obstacle in cancer therapy. Nature Reviews Cancer. 2004;4(10):806–13
- Jain RK. Barriers to drug delivery in solid tumors. Sci Am 1994;271:58–65
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984;44(Suppl10):S4721–30
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001;155:515–28
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21:761–7
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med 2001;7:987–9
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005;307(5706):58–62
- Jain RK. Taming vessels to treat cancer. Sci Am 2008;298:56–63
- Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res 2007;67:2729–3523
- Kłosowska-Wardȩga A, Hasumi Y, Burmakin M, Åhgren A, Stuhr L, Moen I, et al. Combined anti-angiogenic therapy targeting PDGF and VEGF receptors lowers the interstitial fluid pressure in a murine experimental carcinoma. PLoS ONE 2009;4(12). doi: 10.1371/journal.pone.0008149
- Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SAA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA 2011;108:3749–54
- Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Schultz L, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neuro-Oncol 2011;99:237–42
- Heijmen L, Ter Voert EG, Punt CJ, Heerschap A, Oyen WJ, Bussink J, et al. Monitoring hypoxia and vasculature during bevacizumab treatment in a murine colorectal cancer model. Contrast Media Mol Imaging 2014;9:237–45
- Thaker AA, Razjouyan F, Woods DL, Haemmerich D, Sekhar K, Wood BJ, et al. Combination therapy of radiofrequency ablation and bevacizumab monitored with power Doppler ultrasound in a murine model of hepatocellular carcinoma. Int J Hyperthermia 2012;28:766–75
- Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS ONE 2009;4(8). doi: 10.1371/journal.pone.0006539
- Gordon CJ. Temperature Regulation in Laboratory Rodents. New York: Cambridge University Press, 1993
- Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: A molecular and functional evaluation. Cancer Res 2000;60:4324–7
- Waterhouse J, Sawdon M, Kirkman E. Capillary dynamics and the interstitial fluid-lymphatic system. Anaesth Intens Care Med 2013;14:72–8
- Milosevic M, Fyles A, Hedley D, Pintilie M, Levin W, Manchul L, et al. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res 2001;61:6400–5
- Yeo SG, Kim JS, Cho MJ, Kim KH, Kim JS. Interstitial fluid pressure as a prognostic factor in cervical cancer following radiation therapy. Clin Cancer Res 2009;15:6201–7
