Abstract
Purpose: The purpose of this study is to evaluate the fluctuations of coagulation parameters during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) and confirm beyond doubt that epidural anaesthesia is safe with this type of operations. Materials and methods: This is a prospective clinical study of consecutive patients who had cytoreductive surgery and HIPEC. An epidural catheter was inserted into all patients. Peripheral venous blood samples in specific time points of the procedure were tested for complete blood count, prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalised ratio (INR), fibrinogen, D-dimer, and expression of the GpIIb/IIIa platelet receptor. Results: A total of 51 consecutive patients were included in this study. The initial mean (SD) platelet count decreased significantly to a mean of 250.6 (105.4) 109/L (p < 0.001). Fibrinogen levels decreased to 295.9 (127.4) mg/dL (p = 0.009). D-dimer levels increased to 5.3 (3.1) mg/dL (p < 0.001). APTT increased from 30.8 (5.8) s to 35.1 (4.6). The mean INR increased significantly to 1.5 (0.5) (p < 0.001). The total number of GpIIb/IIIa platelet receptors showed no significant variation throughout the measurements and was 72603.2 before HIPEC, 80772.4 during, and 77432.1 after. All the parameters examined, despite significant fluctuations remained in levels that would permit perioperative epidural analgesia. No related complications were recorded. Conclusion: Our results support the belief that epidural analgesia is a safe option in cytoreductive surgery and HIPEC despite certain intraoperative fluctuations in coagulation parameters. It is of major importance to regulate any abnormalities observed during surgery. There are no available data regarding the occurrence of coagulopathy in the post-operative period.
Introduction
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been used in selected patients with primary or secondary peritoneal surface malignancy. Encouraging results show a survival benefit in well-selected patients with acceptable morbidity and mortality rates [Citation1–3]. Historically, the standard treatment of patients with peritoneal carcinomatosis was tumour debulking followed by adjuvant chemotherapy that left little hope for cure. This aggressive approach that combines peritonectomy procedures and complex organ resections aims to eradicate all macroscopic disease [Citation4]. The perfusion of the abdominal cavity with heated chemotherapeutic agents targets all microscopic residual disease. Perioperative intraperitoneal chemotherapy after maximal cytoreductive surgery has been shown to be effective in the treatment of pseudomyxoma peritonei [Citation1], diffuse malignant peritoneal mesothelioma [Citation2], gastrointestinal cancer with peritoneal carcinomatosis [Citation5], and peritoneal sarcomatosis [Citation6]. This treatment has recently been used as an alternative for recurrent and persistent ovarian cancer and also as upfront treatment in FIGO stage III ovarian cancer [Citation7–9]. As a result of the promising findings of cohort studies, more than 60 clinical trials on HIPEC are currently registered with ClinicalTrials.gov [Citation10].
The perioperative management of patients for cytoreduction and HIPEC is a challenge for surgeons, anaesthesiologists and intensivists [Citation11]. Standard anaesthesiology protocols implemented in major abdominal surgery may need adjustment as the patient is subjected to major surgery with significant fluid and blood loss concomitantly with chemotherapy and serious fluctuations in core temperature. The combination of the above has an impact on most physiological parameters including haemodynamic status, renal function, respiratory efficiency and coagulation [Citation12,Citation13]. The severity of this impact has not been studied extensively, and more data are needed for the standardization of the perioperative care of our patients. During cytoreductive surgery and HIPEC the maintenance of an effective clotting mechanism is of major importance. Coagulopathy is part of a chain of events that may lead to considerable intraoperative blood loss. This will put at risk the quality of surgery, increase the need for transfusions and endanger the patient’s post-operative course. Significant coagulopathy also affects the management of the patient by the anaesthesiologist and the use of epidural analgesia in particular [Citation14]. Epidural anaesthesia and analgesia is not considered safe in cases of severe coagulopathy as it may lead to complications such as epidural haematomas and abscesses. Although epidural analgesia is a broadly used technique and is regarded as the standard analgesic technique for major abdominal surgery [Citation15], in the case of cytoreductive surgery there is no international consensus regarding the management of perioperative pain [Citation16].
The purpose of this study is to evaluate the fluctuations of coagulation parameters during extensive surgery and HIPEC. The findings of the study may be used as confirmation of the safety of epidural analgesia during the surgical treatment of the patients.
Methods
This is a prospective observational study of 51 consecutive patients with peritoneal surface malignancy treated with cytoreductive surgery and HIPEC in the Department of Surgery of Didimoticho General Hospital between May 2011 and April 2012. The hospital is a designated national referral centre for cases of peritoneal carcinomatosis. The study was approved by the ethical committee of the hospital and all patients gave written informed consent. Inclusion criteria were age above 16 years and Karnofsky performance status scale >50%. Exclusion criteria were severe cardiovascular or respiratory disease, whole blood count <4000/mm3, platelet count <150,000/mm3, renal or hepatic failure, pregnancy, multiple partial intestinal obstruction or extensive involvement of the surface of the small bowel as well as distant and non-resectable metastases.
Anaesthesia and operative procedure
An epidural catheter was introduced at the levels of T12-L1 before the induction of anaesthesia. Anaesthesia was induced with intravenous (i.v.) infusion of propofol 2.5 mg/kg, rocuronium 1 mg/kg and fentanyl 150 mcg/kg. Maintenance of anaesthesia was achieved with sevoflurane and additional i.v. rocuronium according to patient needs. After the induction of anaesthesia patients received 5 mg/mL solution of ropivacaine bolus via the epidural catheter in doses of 2 mL by neurotome from the point of entry of the epidural catheter up to the level of T6. After the settlement of the epidural, analgesia patients received a continuous epidural infusion of 2 mg/mL solution of ropivacaine with a flow of 8 mL/h until the end of the operation.
Peritonectomy procedures included pelvic peritonectomy, greater omentectomy with or without splenectomy, lesser omentectomy with resection of the omental bursa, cholecystectomy, right and left subdiaphragmatic peritonectomy, and parietal peritonectomy. All patients underwent surgery with the intention to perform complete cytoreduction. Resections of other organs, small and/or large bowel, and the stomach were performed when needed. The extent of peritoneal dissemination was assessed by the peritoneal cancer index (PCI) and the completeness of cytoreduction with the cytoreduction score (CC) as previously described [Citation15].
Intraoperative monitoring included heart rate (ΗR), invasive systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), central venous pressure (CVP), cardiac output (CO), stroke volume (SV), and systemic vascular resistance (SVR). Patients were given intravenous fluids beginning 12 h prior to surgery and according to individual needs. During surgery patients were liberally given crystalloids with close monitoring of the urine output. Transfusions were kept to a minimum and depending on intraoperative losses were aimed to keep haemoglobin levels above 9 mg/dL. Fresh frozen plasma was given in an effort to stabilise prothrombin time (PT)/international normalised ratio (INR) below 1.2. During the 90 min of HIPEC patients were given additional crystalloids to establish a minimal urine output of 400 mL/15 min.
After the completion of cytoreduction and before intestinal reconstructions (when needed) HIPEC was performed using the open Coliseum technique for 90 min [Citation14]. In brief, the abdominal cavity was filled with 3–5 L of normal saline and the chemotherapeutic agents were circulated in the abdominal cavity after reaching the temperature of 42 °C. The choice of chemotherapy regimens depended on the origin of the malignancy and the courses of chemotherapy the patient had previously received. Chemotherapy-naïve and platinum-sensitive ovarian cancer patients were given cisplatin (50 mg/m2) in combination with doxorubicin (15 mg/m2) for 90 min. Platinum-resistant patients received melphalan (70 mg/m2) or gemcitabine (1000 mg/m2) for 60 min. Colon cancer patients received mitomycin C (10 mg/m2). Pancreatic cancer patients received gemcitabine (1000 mg/m2) for 60 min. Gastric cancer patients received mitomycin C (10 mg/m2) and cisplatin (50 mg/m2).
Heated chemotherapy was performed using the SunChip system (Gamidatech, Eaubonne, France). The heat exchanger kept the fluid at >43 °C so that the intraperitoneal fluid was maintained at approximately 42 °C.
Blood sampling and analysis
Peripheral venous blood samples were obtained just after the induction of anaesthesia (time point 1), 2 h after the beginning of cytoreduction (time point 2), at the beginning of HIPEC (time point 3), in the middle of HIPEC at 45 min (time point 4), at the end of HIPEC at 90 min (time point 5) and just before the awakening of the patients (time point 6).
The parameters examined were platelet count, fibrinogen levels, D-dimer levels, prothrombin time, activated partial thromboplastin time, international normalised ratio, all of which were measured in all time points, and also expression of the GpIIb/IIIa platelet receptor which was calculated only at time points 2, 4 and 6.
Platelet count was determined by haematology analyser Sysmex XE-2100 (Sysmex America, Inc. in Lincolnshire, IL). D-dimer concentrations were determined by use of a quantitative enzyme-linked immunosorbent assay based on the ‘sandwich’ type immunoenzymatic method, with final measurements achieved through fluorescence detection. Fibrinogen levels were determined with an ACL-9000 (Diamond Diagnostics, Holliston, MA) coagulation analyser, using a PT-fibrinogen high sensitivity reagent, which is a high-sensitivity calcium thromboplastin that allows the simultaneous determination of PT and fibrinogen levels. aPTT was measured using an ACL-9000 coagulation analyser and INR was calculated by the formula INR = (PT patient/PT normal)ISI.
The expression of the platelet membrane receptor GpIIb/IIIa was studied with flow cytometry (Epics XL-MCL system, Beckman-Coulter) using a specialised kit (ADIAflo Platelet occupancy, American Diagnostics Corporations, Hauppauge, NY). The total number of GpIIb/IIIa platelet receptors was estimated by using the recorder fluorescence intensity, as previously described with the use of monoclonal antibodies [Citation18,Citation19]. A standard curve was initially constructed as described in the kit and the number of receptors per platelet was extrapolated by the standard curve.
Statistical analysis
Quantitative data were expressed as mean (SD), median or range. Univariate analysis was performed with the chi-square test and the Fisher exact test where applicable for categorical variables and the Mann-Whitney U test for numerical variables. Differences between time points were analysed by Wilcoxon test. All statistical analyses were performed using the statistical package SPSS statistics 18.0 (SPSS Inc, Chicago, IL). Significance was tested at the 5% level of statistical significance (p < 0.05).
Clinical trial registration
As this was an observational clinical study without intervention in humans, it was not registered in an international clinical trial registry.
Results
A total of 19 men and 32 women who underwent cytoreductive surgery and HIPEC were included in this study. The characteristics of the patients are listed in . The duration of surgery reached a median of 310 min (range 245–510). In total, six patients received a median of three (range 2–8) fresh frozen plasma transfusions while five patients were transfused with a median of two (range 1–6) red blood cells. No serious intraoperative complications occurred during HIPEC. There was no significant bleeding during surgery or in the immediate post-operative period. There was no re-operation for bleeding and no recorded incident of epidural haematoma or abscess.
Table 1. Demographics of 51 patients undergoing cytoreductive surgery and HIPEC.
The mean (SD) platelet count just after the induction of anaesthesia was 324.9 (128.1) × 109/L. It decreased significantly to a mean of 250.6 (105.4) 109/L (p < 0.001) in time point 2 and then it stabilised at these levels.
Fibrinogen levels were 431.8 (220.3) mg/dL in the first measurement and did not present any significant change until halfway through HIPEC (time point 4), when they decreased to 295.9 (127.4) mg/dL (p = 0.009). Then, in the last two measurements, fibrinogen levels presented a non-significant decline ().
Figure 1. Fibrinogen levels were seen decreasing in different time points during cytoreductive surgery (A) as relevant D-Dimer levels were increasing (B).
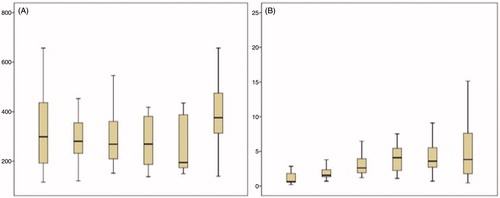
D-dimer levels initially presented a non-significant decline from 3.0 (2.2) mg/dL in time point 1, to 1.8 (1.0) mg/dL in time point 2. Then, they increased to 2.7 (1.7) mg/dL (non-significant change) in time point 3. They continued to increase to 4.0 (2.1) mg/dL in time point 4 (p < 0.001) and to 4.9 (3.3) mg/dL in time point 5 (p < 0.001). Thereafter, D-dimer levels presented a non-significant increase to 5.3 (3.1) mg/dL in time point 6 ().
PT presented a steady increase from 11.7 (1.1) s in time point 1 to 13.2 (1.3) s (p < 0.001) in time point 2, 13.9 (1.6) s (p = 0.01) in time point 3, 15.4 (2.3) s (p < 0.001) in time point 4, and 16.1 (2.4) s (p = 0.002) in time point 5. Then, it presented a non-significant decline to 15.7 (2.8) s.
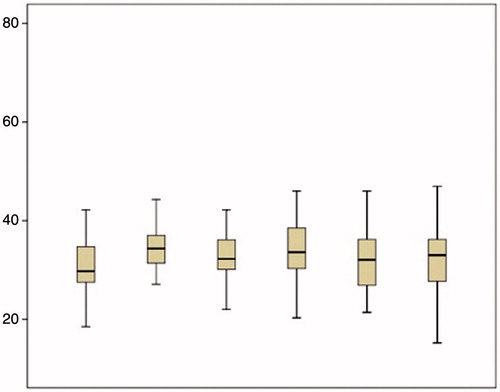
Activated PTT increased from 30.8 (5.8) s in the first measurement to 35.1 (4.6) s in time point 2 (p < 0.001) and then did not present any significant change ().
The mean INR was 0.9 (0.1) in the first measurement and increased significantly to 1.1 (0.2) in the second measurement (p < 0.001), to 1.2 (0.3) in the third measurement (p = 0.01), to 1.4 (0.4) in the fourth measurement (p < 0.001) and to 1.5 (0.5) in the fifth measurement. Then it stabilised at these levels (). Changes in platelets count, fibrinogen levels, D-dimer levels, PT, aPTT and INR are also depicted in .
Figure 3. INR levels are seen within normal limits at different time points during cytoreductive surgery and HIPEC.
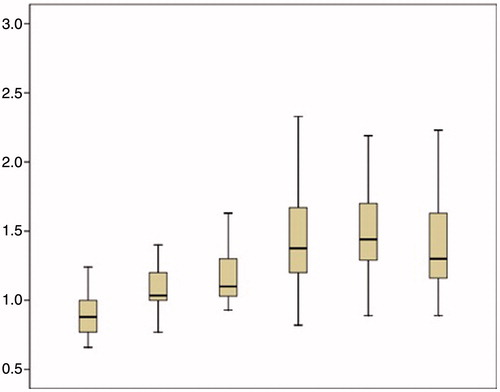
Table 2. Changes in coagulation parameters studied before, during and after HIPEC‡.
The total number of GpIIb/IIIa platelet receptors showed no significant variation throughout the measurements and was 72603.2 (25517.6) before HIPEC, 80772.4 (35183.2) in the middle of HIPEC and 77432.1 (25517.7) after HIPEC.
Discussion
Epidural analgesia, is an important component of perioperative pain management in major abdominal surgery. It is associated with reduced intraoperative opioid administration, earlier recovery of bowel function, fewer side effects and lower post-operative visual analogue scores (VAS) on pain [Citation14,Citation15]. Patients who are candidates for cytoreduction and HIPEC often suffer from chronic pain, which is associated with poor quality of life. Extensive use of analgesics together with epidural catheterisation may help these patients reduce the need for opioids [Citation20].
The main factors that could prevent the use of epidural analgesia are profound haemodynamic instability and coagulopathy. In large series, epidural analgesia has been proved a remarkably safe procedure with an epidural abscess rate of less than 1:1350 (versus 1:1368) and an epidural haematoma rate of less than 1:4100 [Citation21–23].
Spinal haematomas occur in the epidural space mostly without an obvious source of bleeding (arterial or venous). Most spinal haematomas are delayed and become symptomatic days after catheter placement, suggesting the bleeding is not arterial. It is also interesting that the amount of blood required for the neurological symptoms to develop varies and can be much less than what would be injected in a blood patch. The widespread prophylactic use of low molecular weight heparins (LMWH) may be responsible for a rise in the incidence of epidural haematomas but the actual risk is not clear, while the push for more thromboprophylaxis is global [Citation24–26]. In general, epidural catheters should be avoided in patients with profound coagulopathy. An important issue with the placement of epidural catheters and intraoperative coagulopathy is that the actual efficacy of clotting cannot be determined with standard point of care tests, while there are no firm cut-off points for the measured parameters that can be used for clinical decision-making. In our study, effort was taken to evaluate both the intrinsic, extrinsic and common pathways as well as the function of platelets during extensive cytoreductive surgery and HIPEC.
Cytoreductive surgery and HIPEC stimulate various physiological changes, affecting mainly oxygen consumption and haemodynamic parameters [Citation27]. Extensive surgery and HIPEC may cause significant fluid, blood and protein losses along with systemic hyperthermia, which leads to increased metabolic rate [Citation28,Citation29]. Although the effects of HIPEC on haemodynamics and fluid balance have been previously described, the effect on coagulation remains insufficiently understood. This makes the choice of epidural analgesia debatable in cytoreductive operations [Citation9,Citation15,Citation30,Citation31]. A question for further study is whether the known haemodynamic effects of epidural analgesia are intensified during HIPEC while disorders of haemostasis will increase the risk of epidural haematoma.
Intraoperative coagulopathy is a known complication of extensive surgery and HIPEC [Citation30,Citation31] and is probably caused by a combination of factors that include the high fluid volume required for resuscitation, the direct effect of intraperitoneal chemotherapy, hepatic toxicity due to antineoplastic agents and direct hepatic trauma [Citation30]. In our series of patients, statistically significant alterations were recorded in all parameters studied except total number of GpIIb/IIIa platelet receptors which showed no significant variation throughout the procedure. GpIIb/IIIa platelet receptor is an integrin complex found on platelets that acts as a receptor for fibrinogen and leads to platelet activation [Citation32]. Therefore it plays an important role in platelet aggregation and adhesion to endothelial surfaces, making agents that block the function of the GPIIb-IIIa complex a powerful new generation of antithrombotic drugs [Citation19]. The stable number of GpIIb/IIIa platelet receptors in our series of patients shows a lack of impact on them.
Fibrinogen levels showed a significant decline during HIPEC while D-dimer levels gradually increased as a result of fibrinogen degradation. PT and INR were gradually increased during HIPEC, while aPTT remained stable. Despite these changes, all parameters studied remained within a range that is generally considered to be safe for epidural analgesia [Citation21,Citation33,Citation34]. The absence of clinically evident complications connected to the epidural catheter such as haematoma, meningitis and epidural abscess cannot be assessed statistically as the total number of patients is low in relation to the rarity of the events. What this study shows is that while anaesthesiologists were reasonably concerned as to the safety of epidural analgesia in HIPEC, present data and experience support its safe use.
Similar results have been reported by Cooksley et al. counting platelets and INR before and after surgery [Citation27]. Schmidt et al., in a series of 78 consecutive patients, reported increase of the INR, reduced number of platelets 205 × 109/L versus 244.3 × 109/L (which was the lowest mean count recorded in our series) and a significantly prolonged aPTT (45.2 versus 37 in our patients) [Citation28]. However, even in patients with elevated aPTT, Schmidt et al. did not record serious complications from epidural catheter, and their patients who received epidural analgesia presented significantly shorter duration of ventilation and post-operative need of intravenous opioids.
This study confirms the safety of epidural analgesia but does not provide data on possible coagulopathy during the immediate post-operative period. For this to be adequately studied, further tests should be scheduled that would cover the duration of the indwelling of the catheter.
Conclusion
The effects of cytoreductive surgery and HIPEC on the clotting mechanism are not sufficiently understood so far. This raises concerns among anaesthesiologists regarding the use of epidural analgesia. In our series of patients the total number of GpIIb/IIIa platelet receptors showed no significant variation throughout the procedure, while the alterations of the other coagulation parameters remained within a range that is considered safe for epidural analgesia. Further studies are needed to assess the possible impact of various peritonectomy procedures, HIPEC techniques and antineoplastic agents on blood clotting.
Declaration of interest
A.A.T. performed all surgical operations, helped design the study, and approved the final manuscript. This was an observational clinical study that was approved by the Didimoticho General Hospital Review Board. The authors alone are responsible for the content and writing of the paper.
References
- Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449–56
- Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J Clin Oncol 2009;27:6237–42
- Yarema RR, Ohorchak MA, Zubarev GP, Mylyan YP, Oliynyk YY, Zubarev MG, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int J Hyperthermia 2014;30:159–65
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42
- Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Cancer 2010;116:5608–18
- Rossi RC, Deraco M, De Simone M, Mocellin S, Pilati P, Foletto M, et al. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis. Cancer 2004;100:1943–50
- Tentes AA, Kakolyris S, Kyziridis D, Karamveri C. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy in the treatment of advanced epithelial ovarian cancer. J Oncol 2012;2012:358341
- Munoz-Casares FC, Rufian S, Rubio-Carlos MJ, Díaz CJ, Díaz R, Casado A, et al. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin Transl Oncol 2009;11:753–9
- Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, et al., FROGHI Group. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435–43
- Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia 2015;15:1–6
- Webb CA, Weyker PD, Moitra VK, Raker RK. An overview of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for the anesthesiologist. Anesth Analg 2013;116:924–31
- Schmidt C, Moritz S, Rath S, Grossmann E, Wiesenack C, Piso P, et al. Perioperative management of patients with cytoreductive surgery for peritoneal carcinomatosis. J Surg Oncol 2009;100:297–301
- Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open ‘coliseum technique’. Ann Surg Oncol 2000;7:296–300
- Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: A population-based cohort study. Lancet 2008;372(9638):562–9
- Rodgers A, Walker N, Shug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from an overview of randomized trials. BMJ 2000;321:1–12
- Desgranges FP, Steghens A, Rosay H, Méeus P, Stoian A, Daunizeau AL, et al. Epidural analgesia for surgical treatment of peritoneal carcinomatosis: A risky technique? Ann Fr Anesth Reanim 2012;31:53–9
- Sugarbaker PH (ed). Management of Peritoneal Surface Malignancy Using Inytraperitoneal Chemotherapy and Cytoreductive Surgery. A Manual for Physicians and Nurses, 3rd ed. Grand Rapids, Michigan: Ludann, 1998
- Hezard N, Metz D, Nazeyrollas P, Nguyen P, Simon G, Daliphardt S, et al. Free and total platelet glycoprotein IIb/IIIa measurement in whole blood by quantitative flow cytometry during and after infusion of c7E3 Fab in patients undergoing PTCA. Thromb Haemost 1999;81:869–73
- Quinn M, Deering A, Stewart M, Cox D, Foley B, Fitzgerald D. Quantifying GPIIb/IIIa receptor binding using 2 monoclonal antibodies: Discriminating abciximab and small molecular weight antagonists. Circulation 1999;99:2231–8
- McQuellon RP, Loggie BW, Lehman AB, Russell GB, Fleming RA, Shen P, et al. Long-term survivorship and quality of lift after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol 2003;10:155–62
- Cameron CM, Scott DA, McDonald WM, Davies MJ. A review of neuraxial epidural morbidity: Experience of more than 8,000 cases at a single teaching hospital. Anesthesiology 2007;106:997–1002
- Christie IW, McCabe S. Major complications of epidural analgesia after surgery: Results of a six-year survey. Anaesthesia 2007;62:335–41
- Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal–epidural anesthesia. Anesth Analg 1994;79:1165–77
- Gogarten W, Vandermeulen E, Van Aken H, Kozek S, Llau JV, Samama CM, European Society of Anaesthesiology. Regional anaesthesia and antithrombotic agents: Recommendations of the European Society of Anaesthesiology. Eur J Anaesthesiol 2010;27:999–1015
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al., American College of Chest Physicians. Prevention of VTE in Nonorthopedic Surgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl2):e227S–77
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133: S381–453
- Cooksley TJ1, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J Surg Oncol 2011;9:169–73
- Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008;63:389–95
- Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: A multi-institutional experience. Ann Surg Oncol 2012;19:4244–51
- De la Chapelle A, Pérus O, Soubielle J, Raucoules-Aimé M, Bernard JL, Bereder JM. High potential for epidural analgesia neuraxial block-associated hypotension in conjunction with heated intraoperative intraperitoneal chemotherapy. Reg Anesth Pain Med 2005;30:313–14
- Desgranges FP, Steghens A, Mithieux F, Rosay H. Potential risks of thoracic epidural analgesia in hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2010;101:442
- Diz-Kücükkaya R, López JA. Inherited disorders of platelets: Membrane glycoprotein disorders. Hematol Oncol Clin North Am 2013;27:613–27
- Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantitation of 7E3 binding to human platelets. Blood 1996;88:907–14
- Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence- Based Guidelines (Third Edition). Reg Anesth Pain Med 2010;35:64–101