Abstract
Purpose: To establish optimum conditions for anti-tumour therapy, we evaluated the efficacy of doxorubicin using liposomal doxorubicin and local hyperthermia to improve the anti-tumour efficacy over liposomal doxorubicin alone in rabbit VX2 tumours. Materials and methods: A VX2 tumour model was established in New Zealand white rabbits, which were randomly divided into five groups: 1) control, 2) free doxorubicin hydrochloride (Dox), 3) liposomal doxorubicin hydrochloride (L-Dox), 4) L-Dox plus 41 °C thermotherapy (L-Dox + 41 °C TT); and 5) L-Dox plus 43 °C thermotherapy (L-Dox + 43 °C TT). To achieve complete tumour remission, multiple high-dose administrations (5 mg/kg, once per week for a total of 3 weeks) were given. An ultrasound hyperthermia instrument was used to induce local hyperthermia and the systemic toxicity of Dox was evaluated by changes in weight, blood count and serum lactic dehydrogenase. The anti-tumour effect of Dox was evaluated by observing the gross tumour volume, weight and rabbit survival. Results: The white blood cell count following administration of Dox or L-Dox was lower than for control animals and those treated with L-Dox + 41 °C TT. There was no difference between the groups with regard to the red blood cell count. Compared with the control and Dox groups, tumour proliferation was significantly inhibited following administration of L-Dox, L-Dox + 41 °C TT and L-Dox + 43 °C TT, as evidenced by the difference in tumour volume, weight and survival time. Differences in tumour proliferation were also found between the L-Dox and thermotherapy groups. Conclusion: Local hyperthermia combined with L-Dox can significantly improve anti-tumour efficacy and reduce systemic toxicity.
Introduction
Since dose-limiting toxicity and poor local accumulation often result in a lack of adequate drug concentration at the tumour site, many drugs that have strong anti-tumour efficacy in vitro are not suitable for in vivo use. In the past few years, various studies have demonstrated improved site-specific drug delivery, including passive targeting and the active targeting of tumour or endothelial cells, as well as triggered release from drug delivery systems (DDSs), such as nanoparticles [Citation1]. Such systems have two stages: first, the drug carrier passes through permeable tumour vessels, and then the embedded drug is released into the intercellular space within the tumour [Citation2]. In theory, a carrier can accumulate selectively in the tumour region due to the increased permeability of new vessels and the retention effect [Citation3]. If the embedded drug is then released within the tumour tissue, the efficacy of the treatment may be increased and the side effects reduced, thereby achieving the aims of targeted drug delivery.
Doxorubicin (Dox) is an anthracycline antibiotic that is routinely administered in the treatment of, for example, breast, lung, stomach and thyroid cancers, as well as Hodgkin’s lymphoma; however, due to its severe side effects, its use is limited [Citation4]. To reduce such side effects, liposomal chemotherapy is recommended [Citation5]. Thus, PEGylated-liposomal Dox (Doxil®) has been developed as a nano-drug for clinical purposes and has been approved by the US Food and Drug Administration (FDA). It is indicated for advanced ovarian cancer, breast cancer, Kaposi’s sarcoma and multiple myeloma caused by AIDS [Citation6]. However, studies have found that use of liposomes does not significantly improve the efficacy of chemotherapy due to the slow, passive release of the embedded drug [Citation7–9]. Other studies have concluded that carrier size, limited flow of tissue fluid, and the compact nature of the tumour interstitial matrix limit the penetration of carriers into the extravascular extracellular space (EES) [Citation10].
Thermotherapy significantly improves the efficacy of chemoradiotherapy and reduces side effects [Citation11]. Common methods of heating include hot water [Citation12], ultrasound [Citation13] and microwaves [Citation14]. Kong et al. [Citation15] were the first to study the effect of thermotherapy on carrier extravasation, concluding that thermotherapy can improve the extravasation of liposomes. Li et al. [Citation16] studied thermotherapy in mouse B16 melanoma, human BLM melanoma, BFS-1 sarcoma and Lewis lung cancer models, demonstrating stable extravasation of thermosensitive liposomes during thermotherapy administered at 41 °C for 1 h. Meanwhile, we have developed an ultrasonic thermotherapy system that maintains a constant temperature throughout treatment by means of intelligent control. However, in-depth study focused on stable treatment is lacking and the effects of thermotherapy are not yet clear.
In the present study we used New Zealand white rabbit VX2 tumours as a model to study the anti-tumour effect of liposomal doxorubicin hydrochloride as an injection (Libaoduo®) combined with local hyperthermia. The PEGylated liposomal doxorubicin injection (L-Dox, Libaoduo®) is the first nano-drug to have been developed for clinical applications in China. Furthermore, compared with Doxil®, which gives similar results in animal and clinical trials, L-Dox has no statistically significant difference with regard to the half-life (t1/2), area under curve (AUC) and the peak concentration (Cmax). L-Dox has also been approved by the State Food and Drug Administration (SFDA) in China for clinical therapy. It can also protect the liposome from recognition by the mononuclear macrophage system (MPS), which consequently extends the circulatory lifetime of the drug. The particle size is 70∼130 nm and the encapsulation efficiency is greater than 90%. To achieve complete remission of tumours, a multiple high-dose administration strategy was implemented. Compared with traditional chemotherapy, local hyperthermia combined with L-Dox significantly inhibited the tumour growth rate, improved survival time and reduced systemic side effects.
Materials and methods
Reagents
L-Dox for injection (Libaoduo®, 20 mg/10 mL, Shanghai Fudan Zhangjiang Pharmaceutical, Shanghai, China), Dox for injection (25 mg, Sangon Biotech, Shanghai), ketamine (0.1 g/2 mL, Jiang Su Heng Rui Medicine, Lianyungang, China), Sumianxin (40 mg/2 mL, Jilin Huamu Animal Health Product, Changchun, China), saline (0.9%/500 mL, Hunan Kelun Pharmaceutical, Yeyang, China), anerdian (60 mL; Shanghai LiKang Disinfection Technology, Shanghai) and depilatory cream (30 g; Payven Cosmetics, Shenzhen, China) were used as received.
Animals
All animal-related procedures were approved by and conducted under the guidelines of the Shanghai Jiaotong University Animal Care and Use Committee. New Zealand white rabbits aged 2–3 months and weighing 2.0–2.3 kg were obtained from the Experimental Animal Department of Shanghai Ninth People’s Hospital School of Medicine, Shanghai Jiaotong University. They were housed in an ambient environment of 50–60% humidity, 20–22 °C with a 12 h/12 h light/dark cycle. The animals were given free access to food and water.
Instruments
A MyLab Twice ultrasound system (Esaote, Genova, Italy) was used for ultrasonography. The ultrasonic hyperthermia system was developed by the Biomedical Instrument Institute, School of Biomedical Instruments of Shanghai Jiao Tong University. This system had the following components: a controllable high frequency power supply, an ultrasound applicator, a water-cooling device, a multichannel temperature measurement circuit, a multichannel pulse width modulation generator circuit, an analogue-to-digital converter (ADC) card, a computer, and an electricity source.
The high frequency power supply was developed and purpose-built by the Biomedical Instrument Institute, School of Biomedical Engineering, Shanghai Jiao Tong University. The instrument had two driving frequencies: 1.1 MHz and 3.4 MHz. The diameter of the circular plane transducer was 40 mm, with a thickness of 2 mm. The instrument had only one transducer. The structure of the transducer was planar and the material was a piezoelectric ceramic. The cooling device made use of the Peltier effect, with semiconductor refrigeration on one face and heat produced on the other face. The model number of the ADC card was PCI-1710 (Advantech, Taipei, Taiwan). The card had five measure and control functions, including auto-scan/gain scan, single and variable analogue input, peripheral component interconnect (PCI) bus plug and play, first-in-first-out (FIFO) buffer and a programmable counter.
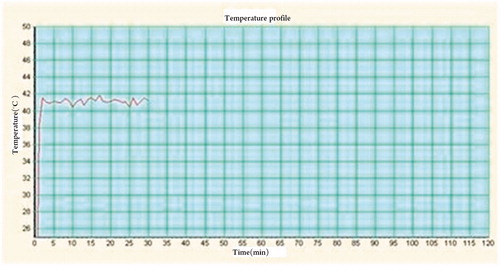
In order to ensure the temperature of the lesion area stabilized in the target temperature fluctuation range (±0.5 °C) during treatment (), the system used an integral algorithm to take the closed-loop proportional-integral-derivative (PID) controller for output energy, which had been calibrated by national medical device testing institutions that were designated by the Chinese SFDA.
Preparation of the VX2 tumour models in rabbits
Preparation of the VX2 tumour block
Ketamine (0.2 mL/kg) and sumianxin (0.2 mL/kg) were intramuscularly injected into rabbits with VX2 tumours for anaesthesia. Rabbits were then strapped to a platform in the supine position and the skin conventionally prepared at the tumour site. A transverse incision was made at the tumour site after anerdian disinfection, and the subcutaneous tissue and fascia were then successively cut open until both sides of the tumour were completely stripped and the fascia, fat and connective tissue removed and placed in saline. Euthanasia was performed immediately after removal of the tumour tissue.
The tumour tissue was cut into 1 mm3 cubes with ophthalmic scissors. Single cell suspensions were immediately prepared with a gentleMACS™ automated tissue processor (Cologne, Germany), followed by stirring with a glass rod. Samples of the suspension were taken for trypan blue staining and haemocytometry. The suspension was then adjusted to a concentration of 106 living cells/mL and placed in a series of 1.5-mL bottles that were immediately cooled in an ice box and transported to the animal operating room for inoculation.
Inoculation and monitoring
New Zealand white rabbits were anaesthetized by intramuscular injection with ketamine (0.2 mL/kg) and sumianxin (0.2 mL/kg). Both lower extremities were shaved and depilated, and approximately 1.5 mL of the prepared tumour cell suspension was injected into the superficial muscle of the right hind leg under ultrasound guidance.
Ultrasonography was used to monitor the growth of the tumours for 6 days after inoculation. Then, when the tumour size was greater than 1 cm in any dimension, analyses were undertaken once every 3 days.
Hyperthermia equipment preparation
The water in a Technotrans cooling system was replaced to ensure that almost no air content was present in the water of the circulation loop. The operating system’s software was used to input basic information regarding the rabbits. The thermotherapy instrument settings were as follows: treatment temperature 41 °C or 43 °C, water temperature 35 °C, treatment frequency 1.1 MHz, treatment voltage/acoustical power 30 V/22 W and voltage duty ratio 100%.
Treatment
Thirty VX2 tumour-bearing rabbits were randomly divided into five groups: 1) control, 2) free Dox, 3) L-Dox, 4) L-Dox plus 41 °C thermotherapy (L-Dox + 41 °C TT), and 5) L-Dox plus 43 °C thermotherapy (L-Dox + 43 °C TT).
The rabbits were anaesthetized by intramuscular injection with ketamine (0.2 mL/kg) and sumianxin (0.2 mL/kg) before treatment and an electric blanket was placed on the leg without the tumour to maintain a core body temperature of 34–37 °C after anaesthesia. The rabbits in groups two received Dox (5 mg/kg) and the rabbits in groups three, four ,five received L-Dox (5 mg/kg) by slow intravenous bolus injection into the ear vein; the duration of the injection was longer than 5 min, after which 1 mL saline was injected to wash out the ear vein. The body temperature, breathing and heart rate were monitored. Saline treatment was used for the control group at a dose of 5 mL/kg over 5 min.
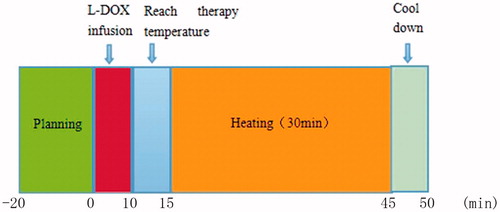
For the L-Dox + TT groups, thermotherapy was conducted immediately after the administration of L-Dox and the output power of the heating system was continuous. The treatment time was set at 40 min (allowing 5–10 min for the treatment temperature to be reached). The skin of the hind leg was depilated and disinfected with alcohol wipes. A thermal needle was then inserted into the central part of the tumour in the subcutaneous muscle layer and the heating probe was placed on the skin over the tumour after the application of a heat-conducting gel, followed by continuous heating for 30 min (). Thermal treatment was performed once per week for a total of 3 weeks.
Monitoring
Observations were made on day 7 after treatment (i.e. day 30), at which point ketamine (0.2 mL/kg) and sumianxin (0.1 mL/kg) were intramuscularly injected to anaesthetise the rabbits. Scalp acupuncture was used to puncture the ear vein to phlebotomise the animals and the volume of blood was 1 mL. The blood was placed in anticoagulant tubes and placed on ice prior to blood cell haemocytometry; three blood samples were taken for each group after 21 days of treatment.
Following tumour inoculation, observations were made until the maximum diameter of the tumour reached 30 mm, the survival time after inoculation reached 50 days, or weight loss was >20%. At the study end point, the rabbits were anaesthetised first and then euthanised by injection of 20–50 mL air through the ear vein.
Statistical analysis
SPSS 19.0 statistical software was used for analysis. Experimental data were expressed as the mean ± standard error of the mean. A Kolmogorov–Smirnov test was used to inspect the normality of data, while Levene’s test was used to inspect the homogeneity of variance. A Kruskal–Wallis test or one-way analysis of variance (ANOVA) was used for overall comparison among the multi-group mean, and the Mann–Whitney method or the Bonferroni method was used for comparison among groups; p < 0.05 was considered statistically significant.
Results
At 10 days after transplantation the mean tumour volume reached 607 mm3 and the average diameter was 12.5 ± 2.3 mm. During the study, one rabbit after one treatment and two rabbits after two treatments in the control group, and one rabbit after two treatments in the Dox group reached an end point (maximum tumour diameter > 30 mm) and were euthanised; meanwhile, one rabbit died of an anaesthesia overdose in the L-Dox + 41 °C TT group during the second treatment. The treatment cycle was completed for the remaining rabbits.
Systemic toxicity of L-Dox combined with local hyperthermia
We evaluated the systemic toxicity after multiple dosing with L-Dox over 21 days (n = 6 per group, n = 5 in the L-Dox + 41 °C TT group).
To assess the acute toxicity of L-Dox combined with hyperthermia, the appearance of skin ulcers or trouble walking after treatment was noted. These symptoms were not found in rabbits of any group as a result of L-Dox treatment. However, a skin burn was noted in one rabbit in the L-Dox + 43 °C TT group. This was due to an error in the Technotrans cooling and circulation system, which was modified to prevent reoccurrence.
On day 1 of inoculation and day 9, 16, 23, 30, 37, 44 and 50 after inoculation, the rabbits in each group were weighed and the weights recorded. If the previous weight ratio was close to 80% then the rabbit was weighed once a day until the end point of observation. The weight ratio was calculated using the following formula.
where MD0 is the weight on the day of inoculation and MD1 is the weight 1 day after inoculation. There was no difference in the weights between the groups before treatment and after the first week of treatment; however, during the second and third week of treatment, the diet, excretion, mental state and other general conditions of tumour-bearing rabbits slightly deteriorated across all groups. The weight loss rate of five of the six rabbits in the Dox group reached 20% within 7 days, while four of the six rabbits in the L-Dox group reached the end point of observation within 10 days and all of the rabbits in the L-Dox + 43 °C TT group reached the end point of observation within 15 days. Meanwhile, two of the five rabbits in the L-Dox + 41 °C TT group reached the end point of observation at 50 days ().
Table 1. Changes in the weight ratio of VX2 tumor-bearing rabbits with the course of treatment. The rate of weight loss of four rabbits in the Dox group reached 20% within 7 days after three treatments (i.e. 31 days), four rabbits in the L-Dox group reached the end point of observation within 10 days (i.e. 34 days), all rabbits in the L-Dox + 43 °C group reached the end point of observation within 15 days (i.e. 37 days) and two rabbits in the L-Dox + 41 °C group reached the study end point of observation of 50 days.
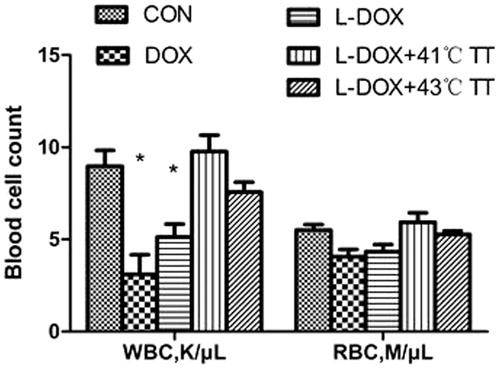
The white blood cell counts for the Dox and L-Dox groups were significantly lower than the control group and the L-Doc + 41 °C TT group, and there was no difference between the groups with regard to the red blood cell count ().
Effect of L-Dox combined with local hyperthermia on the average tumour volume
We evaluated the therapeutic effect of L-Dox combined with local hyperthermia on rabbits with VX2 tumours in the superficial layer of the unilateral thigh (n = 25). Rabbits received L-Dox treatment on day 10, 17 and 24 after inoculation at a dose of 5 mg/kg. Ultrasonic inspection and measurements were made on day 9, 16, 23, 30, 37, 44 and 50 after inoculation (i.e. the day before treatment, day 6, 13, 20, 27, 34 and 41 after treatment). If the maximum diameter of the tumour reached close to 30 mm, daily monitoring was performed until an end point of observation was reached. The tumour volume was measured from the long diameter (L) and the short diameter (S) in situ using the following formula: V = L × S2/2. The tumour proliferation rate was calculated using (VT1 – VT0)/VT0 × 100%, where VT1 represents the volume after administration and VT0 represents the volume prior to administration. From this data, tumour growth curves were prepared.
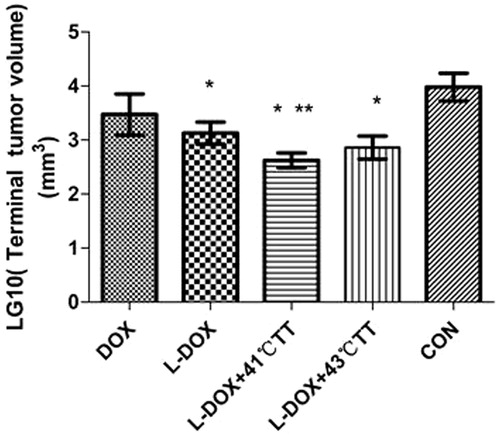
On day 9 after inoculation (i.e. before treatment), there was no difference in the tumour volume between the groups. However, over time, the tumour volume increased in those rabbits treated with Dox alone, as well as the control group. Meanwhile, the tumour volume remained stable in those rabbits treated with L-Dox and L-Dox + 43 °C TT after the third assessment (i.e. after two treatments), and also in those rabbits treated with L-Dox + 41 °C TT after the second assessment (i.e. after one treatment). The tumour volumes were compared at the end of the study period and it was found that the mean volume for those rabbits that received treatment was less than that of the control group and the differences between the Dox, L-Dox and L-Dox + TT groups were statistically significant (p < 0.05, ). In addition, there was a difference between the L-Dox + 41 °C TT and L-Dox groups (p < 0.05), while there was no difference between the L-Dox + 41 °C TT and L-Dox + 43 °C TT hyperthermia groups.
Figure 4. Effect of L-Dox combined with local hyperthermia on the terminal tumour volume at the end of the study. One-way ANOVA was conducted after taking the logarithm then the Bonferroni method was used for pairwise comparison. *Dox versus L-Dox and L-Dox + 41 °C TT and L-Dox + 43 °C TT, **L-Dox versus L-Dox + 41 °C TT, p < 0.05.
Effect of treatment times on tumour growth rate
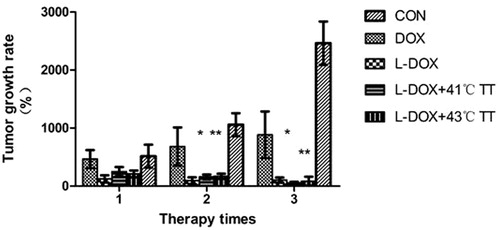
In order to compare the effect of the treatment times on the tumour growth rate, the tumour growth rate was calculated on day 6, 13 and 20 after the start of treatment. No difference in tumour growth was noted between the groups after one treatment; however, after two and three treatments, differences in the tumour growth rates were notable, which suggested that a single treatment may have limited effect with regard to inhibition of tumour growth and that L-Dox treatment combined with hyperthermia requires a course of multiple treatments for effective inhibition of tumour growth ().
Anti-tumour and life extension effects of L-Dox combined with hyperthermia in VX2 tumour-bearing rabbits
At the end of the study the rabbits were euthanised and the tumour tissue was removed, weighed and analysed. The activity of the anti-cancer agents was determined by calculating the inhibition rate (IR = [1 – (average tumour weight, treated/average tumour weight, control)] × 100%) [Citation17]. These results indicated that hyperthermia in combination with L-Dox has a considerable anti-tumour effect ().
Table 2. Tumour weight following excision of the tumour tissue after death (n = 6). One-way ANOVA was conducted after taking the logarithm and the Bonferroni method was used for pairwise comparison. L-Dox combined with hyperthermia significantly increased the anti-tumour rate. *L-Doxvs L-Dox + 41 °C, p < 0.05.
The survival time for each rabbit in each group was recorded and the average survival time for each group noted. The life extension rate was then calculated according to the following formula.
where Dt is the average living days of the treated group and Dc is average living days of the control group. Compared with the control group, the survival time for rabbits treated with L-Dox + 43 °C or L-Dox + 41 °C TT was significantly longer (p < 0.05), with the life extension rate reaching 76.3% and 91.8%, respectively ().
Table 3. Life extension rate of VX2 tumour-bearing rabbits following treatment compared with control animals (n = 6). Log-rank statistics showed that the differences in the survival rates between the groups were statistically significant (p < 0.05).
Effect of thermal dose on the tumour growth rate
We used three rabbits to study the effect of temperature on the tumour growth rate to establish the optimum conditions of hyperthermia. Compared with L-Dox and Dox alone, thermal treatment at 43 °C for 30 min resulted in a smaller gross tumour volume after 23 and 30 days; however, compared with thermal treatment at 41 °C for 30 min in the presence of L-Dox, there was no difference in the tumour growth rate or the rate of tumour progression between those rabbits that received treatment at 41 °C or 43 °C, showing that both temperatures had a similar effect on tumour growth. However, with regard to the life extension rate, values for the L-Dox + 43 °C TT group were slightly lower than the L-Dox + 41 °C TT group but higher than the L-Dox group.
Discussion
Liposomal chemotherapy brings the advantage of minimising systemic toxicity and the destruction of healthy organs and tissues; however, the technique has some drawbacks, such as limited nanoparticle accumulation and low drug bioavailability at the targeted tumour site [Citation18]. Hyperthermia-triggered release of drugs from liposomes can increase the drug concentration within the tumour [Citation19] and several studies [Citation20,Citation21] have suggested that liposomes are a promising tool for the external targeting of drugs to solid tumours when used in combination with local hyperthermia. In this study we used an anti-tumour drug with a long circulation time, namely L-Dox, in white rabbit VX2 tumour models to verify the feasibility of liposomal delivery combined with local hyperthermia in improving the anti-tumour efficacy of Dox. Compared with Dox and L-Dox alone, L-Dox combined with hyperthermia significantly inhibited VX2 tumour growth when administered once a week for a total of 3 weeks.
Hyperthermia
Hyperthermia conditions
It is of great significance to determine the effect of temperature and the conditions of hyperthermia on the release of liposomal drugs. Kong et al. [Citation22] studied the effect of different temperatures (34–42 °C) and hyperthermia conditions (such as time between thermotherapies, drug administration and successive thermotherapy) on nanoparticle extravasation (100 nm liposomes) in athymic nude mouse models with human SKOV-3 ovarian cancer. They found that between room temperature and 39 °C the nanoparticles were unable to extravasate to the tumour tissue space; however, at 40–42 °C, liposome extravasation increased as the temperature increased. Li et al. [Citation16] found that the threshold of liposome (∼85 mm) extravasation triggered by different tumour models was 41 °C for 30 min. Li and co-workers have also [Citation18] presented a novel hyperthermia method, known as two-step hyperthermia, in which the first step is performed at 41 °C for 1 h to promote liposome extravasation and the second step is performed at 42 °C for 1 h to trigger the thermosensitive liposome to release the drug. However, it was found that, compared with one-step hyperthermia, this approach failed to improve tumour growth and prolong survival time.
In clinical hyperthermia, the temperature setting is typically between 41 and 43 °C. To further define the optimal thermal conditions following repeated treatment, we investigated two temperatures, 41 °C for 30 min and 43 °C for 30 min, and their effect on tumour growth. In combination with L-Dox we found that both temperatures exerted similar effects on tumour growth; however, thermotherapy at 41 °C for 30 min significantly increased the life extension rate (L-Dox + 41 °C TT vs L-Dox + 43 °C TT, 91.8% vs 76.3%, respectively). Our previous study [Citation23] found that, compared with a single injection of 5 mg/mL L-Dox, L-Dox + 41 °C TT and L-Dox + 43 °C TT resulted in little differences with regard to the drug concentrations in the plasma, heart and liver, and there was no obvious damage to the surrounding tissues. However, the drug concentration in the tumour tissue with 41 °C thermotherapy was significantly higher than under 43 °C hyperthermia, which may be related to the increase of the life extension rate, suggesting that 41 °C was the most suitable temperature for cancer therapy.
Precise control of the hyperthermia temperature
In the present study we used an ultrasound transducer to heat the tumour site, which was connected to an ultrasound tumour thermotherapy instrument. Suitable temperature measurement methods include thermocouple temperature measurements and magnetic resonance imaging (MRI) scan temperature measurements. As the tumour tissue in our study was located in superficial muscle, we used a T-type thermocouple with a higher cost performance to control the temperature as the AD595 temperature measurement chip can not only amplify a small signal, but it can also record the cold junction compensation for the measuring error of the thermocouple. In order to ensure the temperature of the lesion area stabilised in the target temperature fluctuation range (±0.5 °C) during treatment, the system used an integral algorithm to take the closed-loop PID controller for output energy, which had been calibrated by Shanghai Testing and Inspection Institute for Medical Devices.
During the experiment the device recorded the average temperature information every 60 s and displayed an average of the data via a temperature–time curve. The system needed approximately 5 min to reach the treatment temperature and it should be noted that the temperature was not stable at 41 °C but instead fluctuated around the set temperature point during treatment.
Impact of L-Dox combined with hyperthermia on tumour growth
The effectiveness of chemotherapy drugs depends, in part, on drug delivery to tumour cells at cytotoxic concentrations, as well as tumour heterogeneity and drug distribution in the tumour. These difficulties remain major challenges in liposome chemotherapy [Citation24]. Some studies have considered that, compared with free Dox and despite Doxil® having the higher drug concentration at the tumour site, the release of Dox from Doxil® is slower (< 5% after 24 h); therefore, the biological activity of Doxil® is limited (40–50%) [Citation25,Citation26]. Although Doxil® can reduce acute cardiac toxicity, it is unable to significantly improve treatment efficacy in clinical applications.
Hyperthermia can enhance the anti-tumour effect as a supplementary technique in chemotherapy and radiotherapy [Citation11,Citation26]. As an external stimulus, hyperthermia can increase local blood flow and tissue fluid flow and increase the passive infusion of small molecular-weight compounds, thereby making cancer cells more sensitive to cytotoxic drugs, inhibiting DNA repair and increasing tissue oxygenation [Citation27]. More importantly, hyperthermia can increase the endothelial gap [Citation16] so that the drug can pass through ‘leaking’ tumour vessels, thus permeating into the extravascular extracellular matrix. In addition, hyperthermia can increase local perfusion, promoting stromal micro-convection and further transporting drugs to the tumour tissue site.
In the present study, based on the clinical recommended dosage of doxorubicin, we determined the following treatment plan to establish the anti-tumour effect of L-Dox combined with local hyperthermia: 5 mg/mL was administered as a single dose once per week for a total of 3 weeks. We found that there was a significant difference in the tumour growth rate under L-Dox with hyperthermia compared with L-Dox alone after two and three treatments. This may be due to changes in the pharmacokinetics of the drug caused by hyperthermia, as hyperthermia can lead to changes in the tumour blood supply, which may consequently affect the drug distribution within tumour tissue. Hildebrandt et al. [Citation24] suggested that it may be hyperthermia that causes the changes in fluid and electrolyte balance, as well as pH, thereby changing the solubility and volume distribution of the drug. Therefore, changes in the pharmacokinetics of the drug lead to changes in the action time of the drug.
In addition, Li et al. [Citation16] found that thermosensitive liposome extravasation can be maintained 8 h after hyperthermia. Meanwhile, Kong et al. [Citation22] used a human SKOV-3 ovarian cancer model to show that local hyperthermia (40–42 °C) can increase lipid extravasation, which can be maintained for 6 h after treatment. Therefore, after the end of treatment, due to persistent extravasation after hyperthermia and the longer circulation time of L-Dox itself, the tumour growth rate in the L-Dox + 41 °C TT group of the current study was lower than that of the group treated with L-Dox alone.
Systemic toxicity of Dox
Due to its serious side effects, the use of doxorubicin is restricted. In our study we used weight to reflect the general condition of the rabbits to monitor systemic toxicity [Citation28]. To our surprise, there was significant weight loss in all the animal subjects. The end point of observation, which most of the rabbits in the experiment group reached, was a weight loss rate of 20%.
Theoretically, L-Dox can achieve the purposes of targeted drug delivery to reduce the concentration of Dox in normal tissues and significantly reduce systemic side effects; however, because the weight of the control group also decreased, we believed that there were two probable reasons for the weight loss: 1) a side effect of the drug, and 2) the presence of the tumour. On day 30 the weight loss rate of the rabbits in the L-Dox + 41 °C TT group was less than the weight loss rates in the Dox, L-Dox and L-Dox + 43 °C TT groups (p < 0.05). However, there was no statistically significant difference compared with the control group. Thus, it can be considered that there was no additional systemic toxicity that was specifically associated with the treatment for the L-Dox + 41 °C TT group. In addition, combined with the white blood cell count and lactic dehydrogenase levels, the combination of L-Dox with 41 °C hyperthermia can significantly reduce systemic toxicity and may increase the life extension rate.
Conclusion
In this study we compared treatment with L-Dox or L-Dox combined with hyperthermia to inhibit tumour growth, improve systemic toxicity and extend survival rate. We also explored the optimal settings of hyperthermia and found that 41 °C for 30 min was optimal. These results demonstrate the important role of the liposome combined with hyperthermia in improving the efficacy of anti-tumour drugs, which greatly promotes the transformation of this approach to clinical applications.
Declaration of interest
This study was supported from the Foundation of National Natural Science Foundation of China (No. 81272567). The authors alone are responsible for the content and writing of the paper.
References
- Seynhaeve AL, Dicheva BM, Hoving S, Koning GA, ten Hagen TL. Intact Doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: Evaluated by in vitro/in vivo live cell imaging. J Control Release 2013;172:330–40
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7:653–64
- Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release 2011;153:198–205
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet Genomics 2011;21:440–6
- Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science 2004;303:1818–22
- Duggan ST, Keating GM. Pegylated liposomal doxorubicin: A review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs 2011;71:2531–58
- O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. CAELYX Breast Cancer Study Group. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440–9
- Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001;37:870–7
- Seynhaeve AL, Hoving S, Schipper D, Vermeulen CE, de Wiel-Ambagtsheer Ga, van Tiel ST, et al. Tumor necrosis factor alpha mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res 2007;67:9455–62
- Chrastina A, Massey KA, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011;3:421–37
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al.; European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG); European Society for Hyperthermic Oncology (ESHO). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978;202:1290–3
- Diederich CJ, Wootton J, Prakash P, Salgaonkar V, Juang T, Scott S, et al. Catheter-based ultrasound hyperthermia with HDR brachytherapy for treatment of locally advanced cancer of the prostate and cervix. Proc SPIE Int Soc Opt Eng 2011; 7901:79010O. doi: 10.1117/12.876401
- Kouloulias V, Triantopoulou S, Vrouvas J, Gennatas K, Ouzounoglou N, Kouvaris J, et al. Combined chemoradiotherapy with local microwave hyperthermia for treatment of T3N0 laryngeal carcinoma: A retrospective study with long-term follow-up. Acta Otorhinolaryngol Ital 2014;34:167–73
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000;60:4440–5
- Li L, ten Hagen TL, Bolkestein M, Gasselhuber A, Yatvin J, van Rhoon GC, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release 2013;167:130–7
- Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y, et al. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol 2009;64:53–65
- Li L, ten Hagen TL, Haeri A, Soullié T, Scholten C, Seynhaeve AL, et al. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J Control Release 2014;174:202–8
- Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2012;28:776–87
- Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: State of the art review. Int J Nanomedicine 2014;9:4387–98
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2011;27:156–71
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001;61:3027–32
- Ping X, Angang D, Xia G, Yinzhu Z, Jia L, Guofeng S, Yazhu C. Improved efficacy of liposomal doxorubicin treatment of superficial tumors by thermotherapy. Technol Cancer Res Treat 2015 Apr 16. pii: 1533034615580441. [Epub ahead of print]
- Hildebrandt, B, Wust, P, Ahlers, O, Dieing, A, Sreenivasa, G, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33–56
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clin Pharmacokinet 2003;42:419–36
- Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res 2005;11:6944–9
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA 2011;108:9851–6
- Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: Therapeutic effect in rabbit Vx2 tumours. Int J Hyperthermia 2015;13:1–16