Abstract
Purpose: The aim of this paper was to synthesise core-shell nanostructures comprised of mesoporous silica core and a low melting-point polyethylene glycol (PEG) nanoshell with a sharp gel–liquid phase transition for rapid drug release at hyperthermia temperature range. Materials and methods: The phase transition behaviours of PEGs with molecular weights of 1000, 1500, and 2000 Da were analysed using differential scanning calorimetry (DSC) to determine the optimal formulation with phase transition in the hyperthermia range. The ‘graft-to’ method was employed to synthesise core–shell nanostructures using the selected PEG formulation. The drug loading and release behaviours of these nanocarriers were examined by ultra-violet visible spectroscopy (UV-Vis) using doxorubicin as a model drug. Magnetic resonance-guided focused ultrasound (MRgFUS) was also applied as a typical thermal modality to evaluate the rate of drug release from the core-shell nanostructures. Results: The PEG molecular weight of 1500 Da presented the optimal phase transition temperature for thermal-triggered release under hyperthermia conditions. Drug release measurements at different temperatures using UV-Vis methods showed a 20.2 ± 4.3% leakage in aqueous solution at 37 °C after 30 min, while this value was significantly increased to 68.2 ± 3.7% at 50 °C. A 45.5 ± 3.1% drug release was also obtained after sonication of the drug-loaded nanoparticles for 5 × 20 s using MRgFUS. Conclusion: Although the ratio of drug leakage at physiological temperatures was relatively high, the sharp transition temperature, high loading efficiency, and fast drug release at hyperthermia temperature range could make these core-shell nanoparticles prominent for enhancing the efficacy of various hyperthermia modalities in the treatment of cancer tumours.
Introduction
Thermosensitive nanocarriers of therapeutic drugs are increasingly combined with hyperthermia modalities in order to achieve improved drug delivery conditions with minimised systemic toxicity and enhanced local drug dosage in the tumour region [Citation1]. Thermal sensitivity is often governed by a non-continuous alteration in at least one physical characteristic of the material during temperature change accompanied by structural defects which result in facilitated drug release from the nanostructure. The therapeutic drugs need be entrapped within the thermosensitive carrier at physiological temperature and rapidly released within the locally heated tumour to counteract quick clearance by the bloodstream [Citation2].
Thermosensitive nanocarriers can be synthesised using a variety of organic and inorganic biomaterials such as lipids [Citation3–5], self-assembling amphiphilic micelles [Citation6–8], and biocompatible polymers [Citation9–11] which exhibit thermally triggered drug release due to the gel–liquid, micellisation, and coil-globule phase transitions, respectively. In addition to a number of general parameters such as biocompatibility and biodegradability, the clinical efficacy of these thermosensitive nanocarriers in hyperthermia application mainly relies on the temperature range and discontinuity of their physical phase transition mechanisms [Citation12].
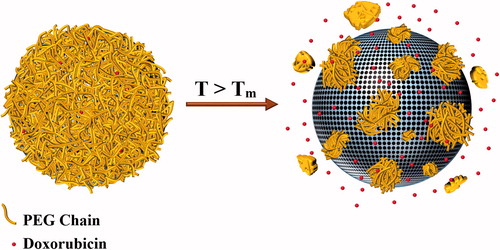
In our previous research we introduced a new mechanism of thermal sensitivity by employing polyacrylamide (PAA) nanoshells which exhibit gelation behaviour at increased temperatures. The gelation temperature of this polymer could be adjusted in the ablation temperature range by controlling the cross-linker/monomer ratio. Therefore, during exposure to the thermal ablation modalities, the encapsulating PAA nanoshell could quickly dissociate and thus facilitate drug release from the nanostructure [Citation13]. However, the crucial limitation of these polymers was their continuous phase transition occurring in a wide temperature range which required relatively high temperatures and irradiation energies to provide the maximum drug release value. In the current research, we aimed to achieve sharper gel–liquid transition temperatures by replacing the PAA layer with a polyethylene glycol (PEG) nanoshell. The phase transition temperature of PEG polymers could be adjusted within the hyperthermia range by controlling their molecular weight. PEGs are hydrophilic and biocompatible polymers which have been widely employed to improve the stealth behaviour of various nanocarriers and decrease their clearance by the reticuloendothelial system (RES) [Citation14–17]. However, the potential of these polymers for application as low melting-point encapsulating shells has not been evaluated. Therefore, different molecular weights of PEG were examined in this study to determine the optimal formulation to offer a melting temperature within the hyperthermia temperature range. The selected PEG was then employed to encapsulate doxorubicin within a mesoporous silica core. It was assumed that the gel–liquid phase transition of PEG shell under hyperthermia conditions could facilitate diffusion of the physically entrapped chemotherapeutics into the surrounding environment. illustrates a schematic of the expected release mechanism from the PEG-coated mesoporous silica nanoparticles (PEG-MSNs) at hyperthermia temperatures.
Materials and methods
Calculation of the optimal PEG molecular weight
Three PEG formulations with molecular weights of 1000 Da (PEG1000), 1500 Da (PEG1500), and 2000 Da (PEG2000) were purchased from Sigma-Aldrich in order to determine a formulation which shows the gel–liquid phase transition close to the hyperthermia temperature range. The phase transition behaviours of these polymers were evaluated using differential scanning calorimetry (DSC; DSC820 with TSO 801RO robot, Mettler-Toledo, Columbus, OH) with a scan rate of 5 °C.min−1. The PEG formulation with the desired range of phase transition temperature was then chosen for synthesis of PEG-MSNs.
Synthesis of core-shell nanoparticles
The PEG-MSNs were synthesised using diamine-terminated PEG, toluene, tetraethyl orthosilicate (TEOS), cetyltrimethylammonium bromide (CTAB), ammonia solution (28%wt), ethanol, and hydrochloric acid aqueous solution (HCl, 37%wt) obtained from Sigma-Aldrich.
The mesoporous silica nanoparticles (MSNs) were firstly activated according to the method proposed in our previous study [Citation13]. The activated particles (AMSNs) were further coated by a PEG shell by covalent bonding. In a typical procedure, equal weight ratios of the MSNs and diamine-terminated PEG (1 g each) were added to 50 mL of toluene. The solution was heated to 80 °C and vigorously stirred for 24 h. The resulting suspension was then centrifuged at 4,000 RCF for 15 min, washed three times with ethanol and distilled water, and freeze-dried for 24 h to obtain the PEG-MSN core-shell nanoparticles.
Material characterisation
The presence of hydroxyl and amine bonds in the AMSNs and diamine-terminated PEG structures as well as the formation of PEG-MSN nanoparticles were confirmed by Fourier transform infrared spectroscopy (FT-IR) (Nicolet 6700, Thermo Scientific, Waltham, MA) at wave number range of 600–4000 cm−1. The particle size distribution was analysed by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZSP instrument (Malvern Instruments, Worcestershire, UK). This instrument was also employed to determine the zeta potential of the synthesised nanoparticles in an aqueous medium with pH 7.0. Transmission electron microscopy (TEM) (Libra 120 kV, Carl Zeiss, Oberkochen, Germany), and atomic force microscopy (AFM) (Ambios AFP-200, Ambios Technology, Santa Cruz, CA) were also employed to visualise the structural morphology of the PEG-MSNs. DSC analysis was again applied on PEG-MSNs to determine the probable changes in the phase transition temperature of the PEG compartment after formation of the core-shell structure.
Drug loading experiments
The drug loading experiments were performed according to the protocol described in our previous research [Citation13]. In brief, the PEG-MSNs were immersed in an aqueous solution containing 2 mg.mL−1 doxorubicin at three different intervals (24, 48, and 72 h) to determine the optimal incubation time resulting in the statistically maximum encapsulation value (Em). Drug loading procedure was performed at room temperature in 25-mL glass tubes without shaking during incubation. The drug-loaded PEG-MSNs were then separated from the supernatant by centrifuging at 16,100 RCF for 60 min. The drug quantity in the aqueous supernatants was further determined using ultraviolet-visible spectroscopy (UV-Vis) (Shimadzu UV-1800, Shimadzu, Tokyo, Japan) at wavelength range of 260–600 nm. The drug loading quantities were calculated by comparing the obtained UV-Vis spectra with that of a reference solution containing 2 mg.mL−1 doxorubicin. The optimal incubation time determined in these experiments was applied in further steps to encapsulate doxorubicin within the PEG-MSNs structure.
Drug release experiments
The drug release studies also followed our procedure described previously [Citation13]. The drug-loaded PEG-MSNs were firstly stabilised in an aqueous solution (2 mg.mL−1) at 37 °C for 7 days. The supernatants were then separated and the ratio of drug leakage was quantified by comparing the UV-Vis spectra of the supernatants with that of a reference solution containing Em mg.mL−1 doxorubicin.
The drug release ratios at increased temperatures were also quantified by preparation of ten similar aqueous solutions containing 2 mg.mL−1 of drug-loaded PEG-MSNs. Each solution was heated in a water bath for 30 min at a designated temperature. Ten temperature values of 30, 37, 40, 45, 50, 55, 60, 65, 70, and 75 °C were chosen in this study to simulate the hyperthermia and thermal ablation conditions as well as to determine the drug release ratios at different temperatures.
After heat treatments, the PEG-MSNs were separated from the supernatants and the drug release percentages were measured by comparing the UV-Vis spectra obtained with that of the reference solution.
Magnetic resonance-guided focused ultrasound (MRgFUS) experiments
The PEG-MSNs were also sonicated using MRgFUS (EX-ablate, Insightec, Haifa, Israel) as a typical thermal modality to evaluate the rate of drug release from their structure. In these experiments a tube containing an aqueous solution of doxorubicin-loaded PEG-MSNs was placed in a thermosensitive tissue-mimicking phantom (TSP) developed previously [Citation18]. The TSP was then sonicated for 5 × 20 s at a frequency of 1.15 MHz with the acoustic energy and acoustic power of 5313 J and 266 W, respectively. The drug release ratios after sonication were further measured by UV-Vis.
Data analysis
All the thermal analyses, drug loading/release tests, and MRgFUS experiments were carried out at least five times and the results were reported as mean ± standard deviation (mean ± SD) using the Statistical Package for Social Sciences (SPSS) software (SPSS 20, SPSS Inc., Chicago, IL). The confidence interval value of 95% was also chosen for performing the comparative studies.
Results
Selection of the optimal PEG molecular weight
The DSC spectra of PEG formulations with different molecular weights are shown in , and their critical temperatures of phase transition are summarised in . By increase of the molecular weight from 1000 Da to 2000 Da, the onset (TO) and peak (TP) phase transitions temperatures were respectively increased from 25.3 ± 2.5 °C and 37.1 ± 2.3 °C to 45.4 ± 1.5 °C and 50.0 ± 1.3 °C, whereas the phase transition intervals decreased from 11.8 °C to 4.6 °C. Therefore, heavier PEG formulations provided a sharper phase transition in contrast to those with lower molecular weights. However, the optimal range of phase transition temperature for hyperthermia application was observed in PEG1500. Although the TP value of PEG1500 was approximately 48.3 ± 1.7 °C, the phase transition initiated at 40.4 ± 1.8 °C which was appropriate for drug release under hyperthermia conditions. Therefore, PEG1500 was selected in order to synthesise the PEG-MSNs for thermally triggered drug release at hyperthermia temperatures.
Figure 2. DSC analyses of PEG formulations with molecular weights of 1000 Da, 1500 Da, and 2000 Da. Increase of the molecular weight results in higher phase transition temperatures.
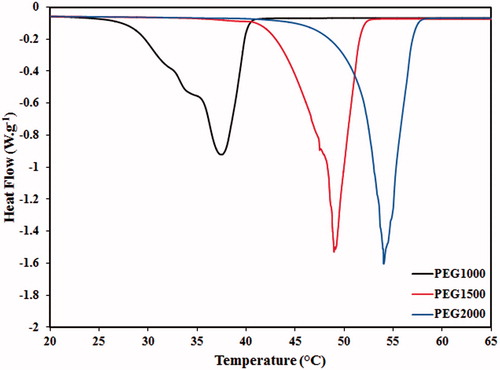
Table 1. The phase transition properties of various PEG molecular weights examined for synthesis of PEG-MSNs.
Material characterisation
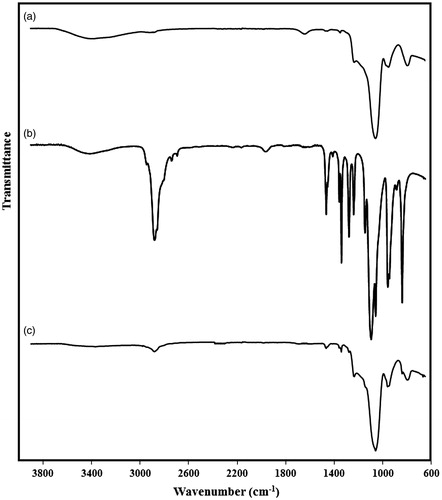
The FT-IR spectra of the AMSNs, diamine-terminated PEG, and PEG-MSNs are presented in . The strong peak observed at 1045 cm−1 in AMSNs represents the Si–O–Si vibration, whereas the wide stretch at wavenumber range of 3200–3600 cm−1 belongs to the absorbed water. A wide stretch was also observed in the FT-IR spectrum of diamine-terminated PEG which probably represents the amine bonds in this polymer. In FT-IR spectrum of the PEG-MSNs, the characteristic peaks of both MSNs and PEG were observed, which proved the presence of both these materials within the synthesised nanoparticles. However, the peak intensities of the MSNs were significantly higher, indicating the dominance of MSNs weight ratio in the core-shell nanostructure.
Figure 3. The FT-IR spectra of the particles at different synthesis stages: (a) activated mesoporous silica (AMSN), (b) diamine-terminated PEG, and (c) PEG-coated mesoporous silica (PEG-MSN) nanoparticles.
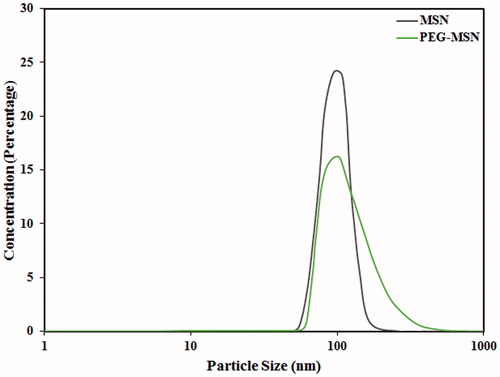
shows typical DLS spectra of MSNs prior to and after PEGylation. Formation of a thin PEG layer on the MSN surfaces resulted in slight but statistically significant increase of the particle size from 95 ± 2 nm to 102 ± 4 nm. Moreover, the zeta potential of the particles was decreased from 8.48 ± 0.33 mV to 4.14 ± 0.42 mV at pH 7.0, probably due to the coverage of surface reactive groups, which act as anchors for PEG conjugation [Citation19].
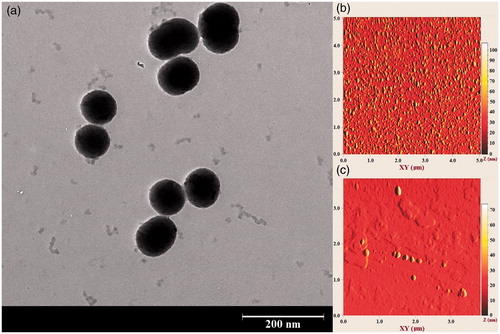
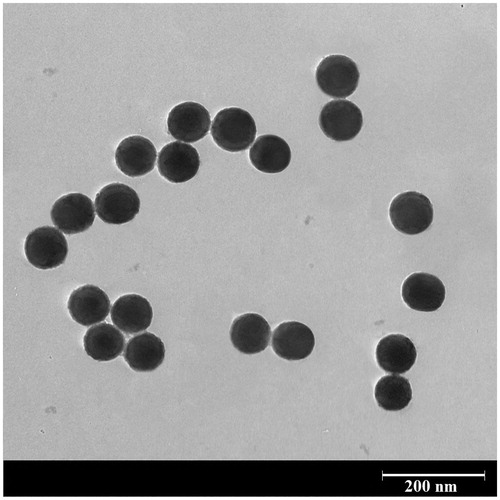
The Transmission electron microscopy (TEM) image of the core-shell nanostructures synthesised using PEG1500 are illustrated in . The MSNs were uniformly covered with a thin PEG layer which not only acted as an encapsulating shell, could also minimise the particle agglomeration in the aqueous solution. However, a relatively low nanoshell thickness was obtained, probably due to the short length of the PEG1500 chains. Although the shell thickness may be increased by utilisation of PEG formulations with higher molecular weights, a significant change in the phase transition temperature makes the heavier polymers impractical for hyperthermia application.
Figure 5. A typical TEM image of the synthesised nanostructure. An ultrathin PEG shell is formed on the surface of mesoporous silica nanoparticles.
The PEG-MSN nanoparticles were again subjected to the DSC analysis in order to determine the probable alteration of the PEG phase transition temperature after formation of the core-shell nanostructure (). The results indicated that interaction with the MSN core had negligible influence on the phase transition temperature of the PEG nanoshell. Although small increases were observed in the mean TO (43.5 ± 2.5 °C) and TP (49.3 ± 2.7 °C) values of the PEG nanoshell, these values were not significantly different from those of pure PEG1500 polymer. The wide peak in the DSC graph of the PEG-MSNs at temperatures above 100 °C might have corresponded to evaporation of the water absorbed by the MSN cores. The TO value of the PEG nanoshell was comparable to that of PAA obtained in our previous study which may minimise the polymer dissociation at physiological temperatures. However, the PEG1500 nanoshell presented a significantly narrower transition temperature range as well as a lower TP value in contrast to the PAA shell (), which may lead to higher release rates and quantities and smaller amounts of thermal energy required for achieving the maximum drug release.
Figure 6. DSC analysis of the core-shell nanoparticles prepared using PEG with molecular weight of 1500 Da. The phase transition temperature of PEG remained unaltered after formation of the core-shell structure. The small inserted graph shows the DSC spectrum in a wider temperature range.
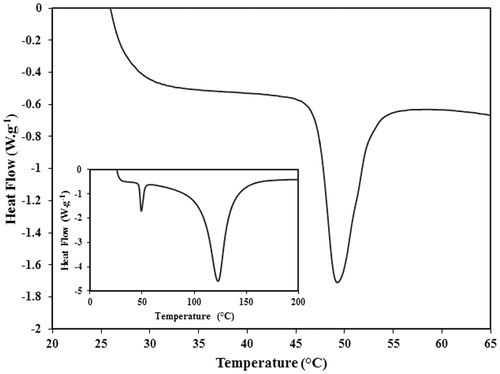
Table 2. A comparison between the drug loading and release characteristics of PEG-MSNs and PAA-MSNs.
Drug loading behaviour
The optimal incubation time needed for encapsulation of doxorubicin in PEG-MSN structure was determined by UV-Vis spectroscopy. The UV-Vis spectra of the supernatants after centrifugal separation of the PEG-MSNs from the doxorubicin solution are illustrated in . The increase of incubation time resulted in reduced UV absorption intensities and thus, decreased doxorubicin concentrations in the supernatant. The amounts of encapsulated doxorubicin in the PEG-MSNs at different intervals are also plotted in . With the increase of incubation time up to 48 h, the average ratio of encapsulated doxorubicin in the nanoparticles was increased (183 ± 14 mg in 1 g of PEG-MSNs) and then, no significant change in this quantity was observed. Although the optimal incubation time was identical to that observed in the PAA-coated MSNs (PMSNs), the amount of encapsulated drug in PEG-MSNs was significantly lower than that of PMSNs (). This might be due to the lower thickness of the PEG shell in contrast to that of PAA which resulted in smaller amounts of drug entrapped within the polymer shell.
Figure 7. The ability of nanoparticles in encapsulation of doxorubicin. (a) UV-Vis spectra of PEG-MSNs after immersion in doxorubicin solution for 0, 24, 48, and 72 h, (b) the quantity of doxorubicin encapsulated in the nanoparticles at various incubation intervals.
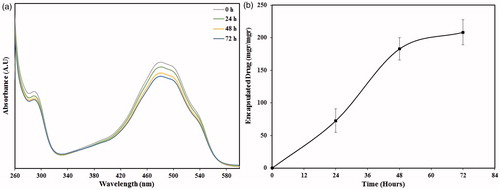
Based on the results obtained, an incubation time of 48 h was used in further experiments to prepare the drug-loaded nanoparticles. Moreover, an aqueous solution containing 366 µg.mL−1 (the average quantity of drug encapsulated in 2 mg of the PEG-MSNs) doxorubicin was prepared and its UV-Vis absorbance at wavelength range of 260–600 nm was recorded as a reference for 100% drug release to quantify the ratio of drug release at 10 different applied temperatures.
Drug release behaviour
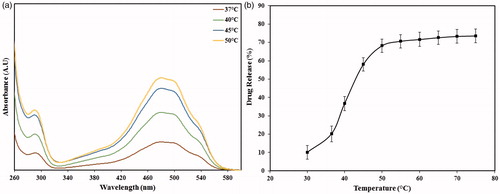
shows the typical absorbance intensities of three specimens heated at 37°, 40°, 45, and 50 °C. The similarity of absorbance spectra at different temperatures indicated the stability of encapsulated doxorubicin during the experiments. The brown spectrum gives an indication of the diffuse-type release of doxorubicin molecules from the PEG-MSNs at 37 °C. The green and blue spectra show that relatively higher amounts of drug molecules could be released at 40° and 45 °C, respectively, probably due to a partial melting of the PEG1500 shell at these temperatures. However, the most intense absorbance was established by the specimens heated at 50 °C. also shows the percentage of drug release at different temperatures from 30° to 75 °C. Incubation of PEG-MSNs at 37 °C resulted in a 20.2 ± 4.3% drug leakage which was not statistically different from that obtained after immersion at a similar temperature for 7 days (23.1 ± 4.5%). Therefore, it may be concluded that the entrapped drug inside the polymer shell could be rapidly released after incubation of the drug-loaded PMSNs into the aqueous solution. According to , the maximum ratio of drug release (68.2 ± 3.7%) was obtained at 50 °C and then no significant increase in this value was observed.
Figure 8. The ability of nanoparticles in release of doxorubicin. (a) UV-Vis spectra of PEG-MSNs heated at 37, 40, and 50 °C, (b) the ratio of drug release from the PEG-MSNs after heating at different temperatures for a period of 30 min.
The TEM images of typical PEG-MSNs after heating at 50 °C () illustrated some small polymer masses detached from the particle surfaces. Therefore, a number of preferred trajectories could be generated which facilitate the diffusion of therapeutic agent into the surrounding environment. Moreover, the TEM and AFM images in indicated that in contrast to the PMSNs, the gel–liquid phase transition of the PEG shell might not result in agglomeration of the nanoparticles, probably due to the low thickness of the polymer shell.
Drug release under MRgFUS exposure
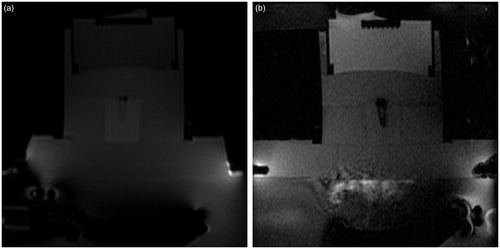
The technique employed for sonication of the doxorubicin-loaded PEG-MSNs with MRgFUS modality is presented in . Heating of the TSP by ultrasound pulses resulted in a dark oval area in the MR images shown in . The temperature patterns of the focal region at different sonication times () also showed a gradual propagation of the heated volume until the entire container reached the maximum temperature at 19 s. In a typical MRgFUS procedure, the temperature of the focal point was raised to over 50° and 70 °C after sonication for approximately 2 and 5 s, respectively (), generating enough thermal energy for initiating the gel–liquid phase transition of the PEG nanoshell.
Figure 10. The protocol employed in MRgFUS sonication of PEG-MSNs. (a–c) Typical magnetic resonance phase images of a thermosensitive phantom (TSP) containing doxorubicin-loaded PEG-MSNs during MRgFUS experiments, (d–g) the changes in temperature patterns during heating process, where higher temperatures are illustrated in red, (h) a typical temperature pattern showing the minimum and maximum rates of temperature change in the focal region during sonication.
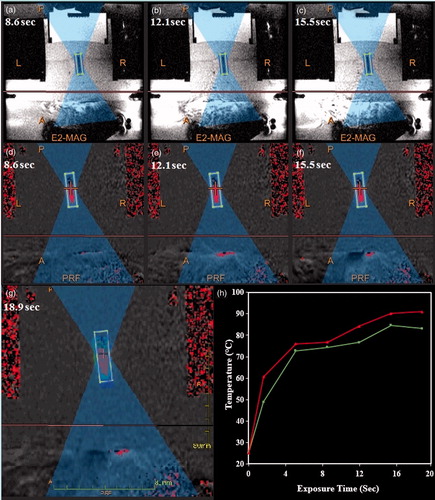
The MR images of a typical TSP containing an aqueous solution of doxorubicin-loaded PEG-MSNs prior and during sonication are illustrated in . The change in the MR contrast parameters of the surrounding TSP during sonication proved the uniform distribution of thermal energy within the container. The UV-Vis analysis indicated a 45.5 ± 3.1% drug release from the PEG-MSNs after MRgFUS sonication for 5 × 20 s. This result showed the potential of these nanoparticles for rapid release of encapsulated therapeutic drugs when used in clinical practice.
Discussion
Thermal-triggered drug release in polymer-based nanoparticles is mostly achieved by coil-globule or micellisation phase transitions at their lower critical solution temperatures (LCSTs). However, most of the polymers which exhibit coil-globule transition possess dubious biocompatibility and biodegradation [Citation20]. Moreover, the LCST of these polymers are often not appropriate for hyperthermia application and must be adjusted by copolymerisation with other hydrophilic or hydrophobic moieties, which may negatively affect their thermosensitive behaviour [Citation21]. The LCST may also depend on the nature and molar concentration of the co-monomers [Citation22], solvent quality [Citation23], and co-solvents or additives present in the solution [Citation24]. For instance, substitution of water with deuterated water results in a 1–2 °C increase in the LCST of poly(N-isopropylacrylamide) (PNIPAM) thermosensitive polymers [Citation25].
Polymers with micellisation behaviour such as poloxamers have also shown potential for application in drug delivery systems and one particular formulation comprised of Pluronics® L61 and F127 is currently undergoing clinical trials in phase III for treatment of metastatic oesophageal adenocarcinoma and other upper gastrointestinal tract cancers [Citation26]. However, weakness of the physical cross-linking between the micelles may cause insufficient in vivo stability for passive targeting of nanocarriers by the enhanced permeability and retention (EPR) effect, which requires a long-term circulation in the bloodstream. In vitro experiments reported a complete dissolution of a 25%wt poloxamer 407 and 50% dissolution of a 35%wt poloxamer 407 into phosphate buffered saline (PBS) medium after 4 h [Citation27]. Therefore, resilience of these hydrogels needs to be improved to achieve long-term retention of the therapeutic drug in the blood circulation.
In order to address the limitations of these thermosensitive polymers, we previously introduced a new mechanism of thermally triggered drug release based on the polymer gelation and dissociation at temperature ranges of 40–60 °C which results in facilitated drug flow into the surrounding environment. The value of drug leakage obtained at physiological temperature was significantly less than that of hollow PNIPAM hydrogel [Citation28] and DNA-capped mesoporous silica nanoparticles [Citation29] (approximately 60% and 30%, respectively). However, because of a wide and continuous transition temperature range, the maximum ratio of drug release in these nanoparticles occurred at temperatures inapplicable in clinical practice.
In the current research, the PAA shell was replaced with PEG in order to achieve a sharper phase transition and obtain the maximum drug release value at temperatures more close to the clinical hyperthermia range. The biocompatibility and biodegradability of PEG has been demonstrated in a number of studies [Citation30,Citation31]. Moreover, the short- and long-term influence of PEGylation on biodegradability of MSNs in different media including aqueous solutions [Citation32], simulated body fluids (SBF) [Citation33], phosphate buffered saline (PBS) [Citation34], and Dulbecco’s modified eagle’s medium (DMEM) with fetal bovine serum (FBS) [Citation32] has been evaluated. Although the biodegradability of these nanoparticles in complex environments is relatively lower than that in aqueous solutions, however, PEGylation shows an overall enhancing effect on MSN biostability, yielding to a decreased loss of mesoporous parameters such as surface area and pore volume [Citation32,Citation33].
The PEG coating could also improve the colloidal stability of MSNs by increasing their hydrophilicity as well as the steric distances, which in turn reduces the attraction forces between the nanoparticles [Citation14]. Moreover, decreased surface charge due to the coverage of active groups by the PEG layer results in decreased protein adsorption (opsonisation) on the particle surfaces [Citation32,Citation34]. Therefore, the PEG shell can endow the nanoparticles with stealth properties, decreasing their clearance by the RES system [Citation32].
The gel–liquid phase transition temperature of PEG is significantly influenced by its molecular weight. Therefore, various PEG molecular weights were firstly examined to determine the formulation with optimal phase transition temperature. The results indicated that the phase transition of PEG1500 which initiated at 40.4 ± 1.8 °C and reached its peak at 48.3 ± 1.7 °C, could be appropriate for hyperthermia application. The phase transition interval of core-shell nanoparticles synthesised using MSNs and PEG1500 (5.8 °C) was significantly narrower than that of PMSNs (23.2 °C). As a result, despite the maximum ratios of drug release not being statistically different in both PMSNs and PEG-MSNs (67.6 ± 2.5% and 68.2 ± 3.7%, respectively), this amount in PEG-MSNs was achieved at approximately 10 °C lower than PMSNs (). However, the ratio of drug release at physiological temperatures was significantly higher in PEG-MSNs in contrast to PMSNs, probably because of the low thickness of the PEG nanoshell which resulted in faster leakage of encapsulated drug at lower temperatures.
The gel–liquid phase transition of PEG-MSNs allowed a rapid drug release when these nanoparticles were exposed to MRgFUS sonication for 100 s. Therefore, these nanocarriers show potential for rapid supply of the encapsulated anticancer drugs in a locally heated tumour. However, a relatively high release ratio at body temperatures must be addressed in further studies to minimise the systemic toxicity of these carriers.
It is also important to note that replacement of the MSN core with superparamagnetic compounds could enhance the contrast properties in MR images and allow nanoparticle navigation using external magnetic fields with appropriate intensities. Moreover, the high thermal conductivity of superparamagnetic cores could increase the melting rate of polymeric shells and lead to higher drug release rates under hyperthermia conditions. However, limited loading capacity for encapsulation of therapeutic agents is the crucial limitation of superparamagnetic materials which may inflict insufficient drug dosage at the tumour site. In a preliminary study, drug-loading values below 50 mg were observed in 1 g of PEG-coated maghemite nanoparticles. Synthesis of maghemite nanoparticles with porous structure could be a particular solution to achieve higher drug loadings combined with the intrinsic advantages of these superparamagnetic materials.
Acknowledgement
The authors would like thank Emad Sadeghian Nezhad and Yus Hafizul from University of Malaya for conducting the UV-Vis and MRgFUS experiments, respectively.
Declaration of interest
This research was supported by the Ministry of Science, Technology, and Innovation (MOSTI) Malaysia (grant no. 06-01-03-SF0587), and a High-Impact Research MoE Grant (UM.C/625/1/HIR/MOE/DENT/14) from the Ministry of Higher Education Malaysia. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 2008;126:187–204
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013;12:991–1003
- Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release 2013;169:112–25
- Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: State of the art review. Int J Nanomed 2014;9:4387–98
- Dicheva BM, Koning GA. Targeted thermosensitive liposomes: An attractive novel approach for increased drug delivery to solid tumors. Expert Opin Drug Deliv 2014;11:83–100
- Croy S, Kwon G. Polymeric micelles for drug delivery. Curr Pharm Des 2006;12:4669–84
- Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed Nanotechnol Biol Med 2010;6:714–29
- Talelli M, Hennink WE. Thermosensitive polymeric micelles for targeted drug delivery. Nanomedicine 2011;6:1245–55
- Ward MA, Georgiou TK. Thermoresponsive polymers for biomedical applications. Polymers 2011;3:1215–42
- Gong C, Qi T, Wei X, Qu Y, Wu Q, Luo F, et al. Thermosensitive polymeric hydrogels as drug delivery systems. Curr Med Chem 2013;20:79–94
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Del Rev 2001;53:321–39
- Dabbagh A, Abdullah BJJ, Abdullah H, Hamdi M, Kasim NHA. Triggering Mechanisms of Thermosensitive Nanoparticles Under Hyperthermia Condition. J Pharm Sci 2015;104:2414–28
- Dabbagh A, Abdullah BJJ, Abu Kasim NH, Abdullah H, Hamdi M. A new mechanism of thermal sensitivity for rapid drug release and low systemic toxicity in hyperthermia and thermal ablation temperature ranges. Int J Hyperthermia 2015;31:375–85
- Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011;6:715–28
- Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Del Rev 2012;64:246–55
- Salmaso S, Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv 2013;2013:374252
- Li Y, Kröger M, Liu WK. Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 2014;35:8467–78
- Dabbagh A, Abdullah BJJ, Abu Kasim NH, Ramasindarum C. Reusable heat-sensitive phantom for precise estimation of thermal profile in hyperthermia application. Int J Hyperthermia 2013;30:66–74
- Andreani T, de Souza AL, Kiill CP, Lorenzon EN, Fangueiro JF, Calpena AC, et al. Preparation and characterization of PEG-coated silica nanoparticles for oral insulin delivery. Int J Pharm 2014;473:627–35
- Patenaude M, Hoare T. Injectable, degradable thermoresponsive poly (N-isopropylacrylamide) hydrogels. ACS Macro Lett 2012;1:409–13
- Kaneko Y, Nakamura S, Sakai K, Aoyagi T, Kikuchi A, Sakurai Y, et al. Rapid deswelling response of poly (N-isopropylacrylamide) hydrogels by the formation of water release channels using poly (ethylene oxide) graft chains. Macromolecules 1998;31:6099–105
- Kujawa P, Tanaka F, Winnik FM. Temperature-dependent properties of telechelic hydrophobically modified poly(N-isopropylacrylamides) in water: Evidence from light scattering and fluorescence spectroscopy for the formation of stable mesoglobules at elevated temperatures. Macromolecules 2006;39:3048–55
- Lien YH, Wu TM. The application of thermosensitive magnetic nanoparticles in drug delivery. Adv Mater Res 2008;47:528–31
- Lutz J-F, Akdemir Ö, Hoth A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly (NIPAM) over? J Am Chem Soc 2006;128:13046–7
- Wang X, Wu C. Light-scattering study of coil-to-globule transition of a poly (N-isopropylacrylamide) chain in deuterated water. Macromolecules 1999;32:4299–301
- Pitto-Barry A, Barry NP. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym Chem 2014;5:3291–7
- Bhardwaj R, Blanchard J. Controlled-release delivery system for the α-MSH analog Melanotan-I using poloxamer 407. J Pharm Sci 1996;85:915–9
- Xing Z, Wang C, Yan J, Zhang L, Li L, Zha L. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter 2011;7:7992–7
- Ruiz-Hernandez E, Baeza A, Vallet-Regí Ma. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011;5:1259–66
- Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 2006;27:2265–74
- Chen Y-C, Lo C-L, Hsiue G-H. Multifunctional nanomicellar systems for delivering anticancer drugs. J Biomed Mater Res A 2014;102:2024–38
- Lin Y-S, Abadeer N, Haynes CL. Stability of small mesoporous silica nanoparticles in biological media. Chem Commun 2011;47:532–4
- Cauda V, Argyo C, Bein T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J Mater Chem 2010;20:8693–9
- Rytkönen J, Miettinen R, Kaasalainen M, Lehto V-P, Salonen J, Närvänen A. Functionalization of mesoporous silicon nanoparticles for targeting and bioimaging purposes. J Nanomater 2012;2012:986562