Abstract
Background: Hyperthermic isolated limb perfusion (HILP) is a locoregional treatment aimed at avoiding amputation in patients with advanced extremity soft tissue sarcomas (STS). Over the last 25 years, HILP procedure has been implemented to maximise its therapeutic ratio. Methods: A retrospective analysis including 117 patients who underwent HILP from 1989 to 2013 was performed. Three different drug schedules were applied: 1) doxorubicin (n = 47), 2) high dose (3–4 mg) tumour necrosis factor-alpha (TNF-α) plus doxorubicin (n = 30), 3) low dose (1 mg) TNF-α plus melphalan (L-PAM) (n = 40). Tumour response was evaluated by MRI or CT and surgical specimens. Toxicity and local progression-free survival (LPFS) were also evaluated. Results: In total 92 (78.6%) patients had primary, 25 (21.4%) had recurrent and 17 (14.5%) had metastatic disease. The subjects in the three groups were homogeneous for clinical-pathological features. Pathological response was complete in 55 patients (47%), partial in 35 (29.9%), regardless of drug schedule (p = 0.501) and tumour presentation (p = 0.094). Wieberdink III–V toxicity was registered in 19.1%, 20% and 2.5% of patients, respectively (p < 0.051). Twenty-eight patients (23.9%) received adjuvant radiotherapy with no relevant toxicity. Five-year LPFS was 81.6% and 74.2% in patients with primary or recurrent disease, respectively (p = 0.652). After a median follow-up of 36.5 months, the limb sparing rate was 77.8%. Conclusions: HILP performed with different drugs was equally active, either in primary, recurrent or metastatic STS, providing effective limb sparing and durable local control. Low dose TNF-α plus L-PAM had the most favourable toxicity profile. Adjuvant radiotherapy was not associated with relevant toxicity.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of malignant tumours that originate from mesenchymal stem cells and account for approximately 1% of all cancers. About 60% of STS arise in limbs and more than 90% of them can be widely excised preserving the limb [Citation1]. The current treatment of localised STS consists of limb-sparing surgery followed by radiation therapy and/or systemic therapy in selected patients [Citation2]. However, in 5–10% of cases radical resection with adequate surgical margins cannot be accomplished unless by compromising limb functionality or preservation, due to disease extension or major neurovascular involvement. Consequently, these patients are suitable candidates for amputation, even though mutilating surgery does not confer any significant survival improvement [Citation2,Citation3]. Isolated limb perfusion (ILP) was introduced by Creech and Krementz in 1957 [Citation4] and was subsequently implemented by the addition of hyperthermia by Cavaliere et al. (hyperthermic ILP (HILP)) [Citation5,Citation6]. Today, HILP represents an established alternative to limb amputation for patients with locally advanced STS [Citation7–9]. Since its introduction in the clinic, one of the most controversial issues has been the identification of the most effective drugs for the extracorporeal circulation which has led to a progressive evolution of HILP procedure. A variety of anticancer agents have been used over the years: nitrogen mustards, actinomycin, melphalan (L-PAM), doxorubicin, cisplatin and tumour necrosis factor alpha (TNF-α) [Citation9]. Currently, the combination of TNF-α plus L-PAM is used during HILP in STS patients with unresectable disease in order to achieve tumour downsizing and make limb sparing surgery possible [Citation10–12]. The present study reports on a 24-year single institution experience with HILP in STS patients who were otherwise candidates for amputation. The aims of this research were 1) to compare the activity and toxicity of the different drug schedules that were successively adopted over the years at our centre and 2) to provide a long-term report of locoregional disease outcome.
Patients and methods
Patients
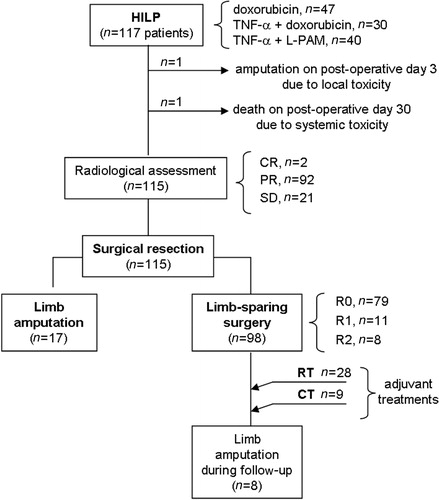
Between February 1989 and September 2013, a total of 117 patients presenting with locally advanced limb STS underwent HILP at the University Hospital and at Veneto Institute of Oncology of Padua (Padova), Italy. Baseline patient characteristics are presented in . All patients were evaluated by a multidisciplinary, pathology-dedicated medical team. The unresectability of tumours was agreed upon the following criteria: disease multifocality, number of recurrences, recurrence in previously irradiated areas, deep tumour location with adherence to bones, nerves or blood vessels. Exclusion criteria for HILP were: clinically relevant peripheral vascular disease, severe cardiac co-morbidities and coagulation disorders, as well as concomitant chemo/radiotherapy or immuno-suppressive therapy. All patients gave informed consent to the use of their data for scientific purposes. The study was authorised by the local institutional review board.
Table 1. Patient characteristics.
Treatments
The patients were treated with three different drugs schedules, according to the available evidence from clinical studies. From 1989 to 1998 HILP procedure was performed with doxorubicin alone (8.5 mg/L of limb volume) [Citation13,Citation14]. In the late 1990s, TNF-α was associated to doxorubicin, according to the favourable results of a phase I–II study [Citation15]. Consequently, from 1999 to 2003 HILP procedure was performed using TNF-α plus doxorubicin. TNF-α dose was 3 or 4 mg for the upper and lower limbs, respectively; doxorubicin dose was 8.5 mg/L limb volume. Subsequently, several studies showed that low dose (1 mg) TNF-α was as effective as the high doses, but was associated with minor toxicity [Citation16,Citation17]. As a result, low dose TNF-α was introduced at our centre in 2004 and we adopted the following schedule: low dose (1 mg) TNF-α plus L-PAM (13 or 10 mg/L of limb volume for the upper and lower limb, respectively). The duration of HILP was 90 min both in the period from 1989 to 1998 and from 1999 to 2003; thereafter, the duration was reduced to 60 min. In fact, 1 h HILP with TNF-α plus L-PAM proved be as safe and effective as 90-min HILP [Citation17]. In all but two patients (in whom target temperature was 41–43 °C) the maximum tumour temperature during the procedure was set at 40–41 °C (borderline true hyperthermia), which is known to be more effective than mild hyperthermia (39–40 °C), but also has less side effects than true hyperthermia (>41–43 °C) [Citation18]. The surgical technique for HILP has been described in detail elsewhere [Citation19]. In brief, the main artery and vein of the affected limb were isolated and encircled with tourniquets. After systemic heparinisation, the tourniquets were tightened as the arterial and venous catheters were inserted into the vessels. Subsequently, the catheters were connected to the extracorporeal circuit. An Esmarch tourniquet was placed at the root of the limb in order to collapse collateral vessels and to prevent systemic drug leakage. 99mTc-albumin was injected into the circuit to measure the perfusate leakage by means of a gamma probe placed over the chest wall of the patient [Citation20]. The flow rate during the perfusion was set at 440 mL/min and 600 mL/min for the upper and lower limb, respectively. Surgical resection was planned 6–8 weeks after HILP and no anticancer treatment was administered during this interval. Magnetic resonance or computed tomography was performed preoperatively to quantify tumour shrinkage as well as to evaluate its surrounding anatomical structures.
Response and toxicity
Radiological response was performed according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1): complete response (CR) indicated tumour disappearance, partial response (PR) indicated tumour shrinkage of at least 30%, stable disease (SD) indicated tumour shrinkage less than 30% or tumour growth less than 20%, progressive disease (PD) indicated tumour increase greater than 20%. The percentage of tumour necrosis after HILP was microscopically assessed by the pathologist and grouped into three categories: complete response (>90% of tumour necrosis), partial response (50–90% of tumour necrosis), and no response (<50% of tumour necrosis). Common toxicity criteria (CTCAE, v 4.0) were used to classify procedure-related adverse events; the Wieberdink scale was also used to describe locoregional toxicity [Citation21].
Statistical analysis
Comparison between groups was performed with Mann Whitney U test or chi-square test, according to variables. Survival analysis was performed with the Kaplan-Meier method, comparison between different cohorts was performed with the log rank test. All tests were double sided and were retained significant for p ≤ 0.05. Analyses were performed with R v3.0.2 for Linux (C-RAN project, Vienna).
Results
Patients
A total of 117 consecutive patients (55 men and 62 women) were evaluated, with a median age of 53 years (range 22–92). The main clinical pathological features of patients who were treated according to the three different drug schedules (doxorubicin, TNF-α plus doxorubicin and TNF-α plus L-PAM) were similar: gender (p = 0.72), age (p = 0.63), tumour size (p = 0.08), tumour location (upper vs lower limb, p = 0.42), disease uni- vs multifocality (p = 0.78), tumour grading (p = 0.84), histotype (p = 0.64), presentation (primary vs recurrent, p = 0.70) and stage (p = 0.67).
Treatment
Out of 117 patients, 47 (40.1%) underwent doxorubicin HILP, 30 (25.6%) TNF-α plus doxorubicin HILP, and 40 (34.3%) TNF-α plus L-PAM HILP. The surgical vascular access was performed through the axillary vessels in 28 (23.9%) patients, the femoral vessels in 67 (57.2%) patients and the external iliac vessels in 22 (18.8%) patients. The median of the maximum temperature of the perfusate during the procedure was 40.5 °C (range 39.2–43.0 °C); the upper threshold of borderline true hyperthermia was exceeded in two patients, in whom the maximum registered temperature was 42 °C and 43 °C, respectively. The median flow rate during the perfusion was 440 (range 200–560) mL/min in the upper limb and 600 (range 300–900) mL/min in the lower limb. The median pH of the perfusate was 7.2 (range 7.0–7.4). The median systemic leakage of 4.8% (range 0.3–12.0); it was higher in patients with an iliac access (median 7.8%, range 0.5–12.0) than in patients with an axillary (2.8%, range 0.3–4.0) or femoral access (1.7%, range 0.8–6.0). Overall, the median hospital stay was 8 days, (range 6–32.0).
Toxicity
Grade I–II Wieberdink toxicities were reported by 80.9%, 80.0% and 92.5% of patients in the doxorubicin, TNF-α plus doxorubicin, and TNF-α plus L-PAM groups, respectively (p = 0.233). Grade III–IV toxicities were more frequent in the two doxorubicin-based schedules (). One patient treated with TNF-α plus doxorubicin had grade IV Wieberdink toxicity and developed a compartmental syndrome which required a fasciotomy to prevent leg ischemia, while another patient treated with the same drug regimen was amputated due to grade V toxicity (). A patient who underwent doxorubicin HILP had grade IV toxicity and underwent fasciotomy. One patient died 30 days after TNF-α plus L-PAM HILP due to bone marrow aplasia which was unresponsive to pharmacological treatment. Details of both locoregional and systemic toxicity are presented in .
Table 2. Tumour response and local toxicity according to drug schedule.
Table 3. Local and systemic toxicity according to drug schedule in 117 sarcoma patients after hyperthermic isolated limb perfusion.
Radiological response
In the 115 evaluable patients, the radiological response after HILP was as follows: complete in two patients (1.7%), partial in 92 patients (80%), and stable disease in 21 patients (18.3%). Two patients were not evaluable due to early amputation on post-operative day 3 and death on post-operative day 30 ().
Surgical treatment and pathologic response
A limb-sparing surgery was feasible in 98/117 patients (83.8%). In those 98 patients, the radicality of resection was R0 in 79 (80.6%), R1 in 11 (11.2%) and R2 in 8 (8.2%) (). In the subgroup of patients with metastatic disease (n = 17) a limb-sparing intervention was performed in 14 cases (82.3%). Overall, pathological response was as follows: complete in 53/115 patients (46.1%), partial in 35/115 (30.4%) and stable disease in 27/115 patients (23.5%). There was no significant difference in tumour necrosis in the surgical specimen according to the drug schedule of HILP (p = 0.501) (). All seven patients with lymphangiosarcoma had a pathologically confirmed CR. On the other hand, we observed some STS histotypes that were poorly responsive to HILP: epithelioid sarcoma (n = 4 patients; of these, two were treated with doxorubicin HILP, the other two with TNF-α plus doxorubicin HILP), clear cell sarcoma (n = 2 patients, both treated with TNF-α plus doxorubicin HILP), osteogenic sarcoma (n = 2 patients; one received TNF-α plus doxorubicin HILP, the other one TNF-α plus L-PAM HILP), and extra-skeletal myxoid chondrosarcoma (n = 4 patients. Two of them underwent TNF-α plus doxorubicin HILP, while two received TNF-α plus L-PAM HILP). None of these patients showed radiological response after HILP and the amount of tumour necrosis in the surgical specimen was <50% in all cases.
Further treatments
After discussion in the frame of a multidisciplinary sarcoma team, 28 (23.9%) patients underwent radiotherapy as a completion treatment after HILP and resection (). The worst local adverse events following radiation were graded G1–G2 in all cases and included skin (dermatitis, erythema, pruritus) or soft tissue (necrosis, infection, fibrosis) toxicity. Nine patients (7.7%) received adjuvant chemotherapy after HILP.
Local control
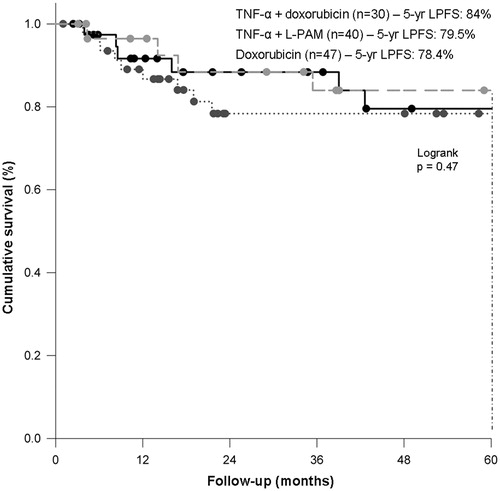
Overall, 26 patients (22.2%) required limb amputation at different times: one patient was amputated three days after HILP with TNF-α plus doxorubicin, due to grade V toxicity; 17 patients were amputated due to non-response to HILP; finally, another eight patients required limb amputation for disease recurrence during the follow-up, after a median interval of 24 months (range 8–33) (). None of the 28 patients who were irradiated after HILP required limb amputation. Overall, after a median follow-up of 36.5 months (range 0.1–270.5) 5-year LPFS was 80.6%, without significant differences among HILP schedules (doxorubicin, 78.4%, TNF-α plus doxorubicin, 84%, TNF-α plus L-PAM, 79.5%, p = 0.47) (). Five-year LPFS was 81.6% and 74.2% in patients with primary or recurrent disease, respectively (p = 0.65).
Figure 2. Local progression-free survival according to HILP drug schedule. HILP, hyperthermic isolated limb perfusion; L-PAM, melphalan; LPFS, local progression-free survival.
In the subgroup of patients with metastatic disease (n = 17), three patients underwent early amputation due to non-response to HILP; in the remaining 14 patients who received a limb sparing surgery, seven developed disease recurrence during follow-up and six of them finally required amputation. As a result, the limb amputation rate in patients with stage IV disease was 9/17 (52.9%).
Survival
Systemic disease progression occurred in 47 patients (40.2%), after a median of 14.6 months (range 4–59) from HILP. Five-year overall survival ranged from 58.6% to 64.5%, in patients with primary and recurrent disease, respectively. Twenty-eight patients (23.9%) were disease-free after 5 years from HILP.
Discussion
The present study summarises a single centre experience with HILP over a 24-year period in patients with advanced extremity STS. Our data confirm that HILP is a highly active and effective treatment. In fact, in our 117 patients the overall pathological response rate was 76.5% and the limb sparing rate was up to 77.8%. During the last three decades, HILP has been continuously implemented and nowadays it represents a highly technically evolved procedure [Citation7,Citation9]. During this phase of progressive improvement, different anticancer drugs have been tested. In particular, at our centre we used three different schedules, i.e. doxorubicin, high dose TNF-α plus doxorubicin, and low dose TNF-α plus L-PAM. The goal of this retrospective analysis was to critically evaluate the toxicity and efficacy of these regimens in STS patients who underwent HILP. According to our experience, this procedure has proved to be safe (although 1/117 patients died because of systemic toxicity of chemotherapy) and its locoregional toxicity profile was easily manageable in most cases. The majority (around 80–90%) of patients reported mainly grade I–II Wieberdink toxicity, without significant differences among the three different drug regimens (). However, the most severe side effects were observed in those treated with the two doxorubicin-based schedules, who experienced grade III–IV locoregional toxicity in 9/47 and 5/30 cases, respectively. Moreover, the single case of grade V Wieberdink toxicity occurred in a patient who underwent HILP with TNF-α plus doxorubicin (). It cannot be excluded that the higher morbidity in patients treated with doxorubicin and with TNF-α plus doxorubicin was also due to the longer duration of the procedure (90 vs 60 min) and to the higher dose of TNF-α (). In fact, according to Deerose et al., reduction of the TNF-α dose and reduction of total HILP time is associated with less local toxicity (from 23% to 14%), while maintaining HILP efficacy [Citation12]. The association of TNF-α plus doxorubicin was previously tested by our group in a small phase II study, where 14 out of 21 patients had moderate regional toxicity and one patient reported severe limb toxicity, although not requiring amputation [Citation13]. However, the small number of enrolled patients did not allow a reliable estimation of the actual rate of doxorubicin-related toxicity. In a more recent study from the Italian Society of Integrated Locoregional Treatment in Oncology (SITILO), 75 patients with limb-threatening STS were treated with the same drug schedule and grade IV–V locoregional toxicity was reported by 21% and 3% of cases, respectively [Citation22].
On the other hand, several studies indicate that HILP with TNF-α plus L-PAM is associated with mainly mild to moderate local toxicity and the occurrence of severe morbidity is rare, with grade V toxicity being reported only in sporadic cases [Citation11,Citation12,Citation17,Citation23–29]. Nevertheless, as shown by the toxicity profile reported by our patients (), HILP may cause a variety of local and systemic adverse events which are well known [Citation30], ranging from mild erythema to soft tissue injury, up to compartment syndrome requiring decompressive fasciotomy or limb amputation; in rare cases, drug leakage into the systemic circulation may cause severe haematological toxicity.
The three drug schedules used in our patients proved to be comparable both in terms of antitumour activity and efficacy. Ninety-eight (83.8%) patients received limb-sparing surgery and the pathological response rate after HILP with doxorubicin, TNF-α plus doxorubicin and TNF-α plus L-PAM was 72.3%, 78.6% and 75.0%, respectively (). The pathological tumour response was complete in 53/115 (46.1%) resected patients and partial in 35/115 (30.4%) patients, without significant differences among the three schedules. These results are comparable to previous series, where the overall response rate ranged from 63% to 96% [Citation7–9], and raise the question about the necessity of surgical resection after a complete clinical response following HILP. However, the discrepancy between the radiological (CT scan or MRI) and pathological complete response rate in our study (1.7% and 46.1%, respectively) underlies the impossibility to predict a pathological CR after HILP and to identify the patients who could be spared from surgical resection. Moreover, especially in patients with a large tumour masses, surgical resection is beneficial in order to remove all the necrotic tissue and avoid possible sovrainfections.
The high tumour response achieved with HILP in our patients, followed by radical resection in most cases, translated into a sustained locoregional disease control in the long term as shown by the fact that 5-year LPFS in the three treatment groups was 78.4%, 84% and 79.5%, respectively. Of note, 84% (98/117) of our patients received limb-sparing surgical treatment, and, of these patients, about 80% achieved R0 resection. These outcomes are in line with those reported in the most recent series, where limb sparing rate ranged from 41–100% [Citation9].
Despite the sustained antitumour activity shown by HILP, some histotypes in our experience were refractory to locoregional chemotherapy. Although small numbers only allow provocative assumptions, we observed that patients with epithelioid sarcoma, clear cell sarcoma, osteogenic sarcoma and extra-skeletal myxoid chondrosarcoma were poorly responsive to HILP. As for patients with epithelioid sarcoma and clear cell sarcoma, our data are in contrast with previous studies [Citation31,Citation32], where these histotypes were responsive to HILP with TNF-α and L-PAM. One plausible explanation could reside in the use of doxorubicin-based regimens in our patients instead of TNF-α and L-PAM. On the other hand, the four patients with extra-skeletal chondrosarcoma who were refractory to HILP in our study were treated with TNF-α plus LPAM.
Considering the high heterogeneity of STS and consequently their variable sensitivity to different anticancer agents, it is arguable that more active drugs should be introduced in order to ensure favourable outcomes after HILP in a greater number of STS patients. At the same time, the identification of mechanism of resistance to drugs as well as a better estimation of limb volume may improve the therapeutic ratio in regional chemotherapy [Citation33,Citation34]. Finally and hopefully, the availability of less invasive and promising locoregional treatments, such as isolated limb infusion (ILI) and electrochemotherapy (ECT) may open new possibilities in the multimodal management of these complex patients [Citation35,Citation36].
Twenty-eight (23.9%) of our patients received adjuvant radiotherapy after HILP and surgical resection due to marginally or microscopically positive resection margins. Unfortunately, due to the long time span of the present analysis as well as to patient heterogeneity and small numbers, it is impossible to evaluate whether radiotherapy had an impact in reducing the risk of local recurrence. We can only observe that the 28 patients in whom HILP was followed by radiotherapy reported only mild local toxicity after irradiation and none of them was amputated for disease recurrence or late treatment-related side effects during follow-up. The value of adjuvant radiotherapy after HILP and surgical resection is still a matter for debate. In fact, while according to some previous experiences the application of external beam radiotherapy seems feasible and reduces the risk of local recurrence [Citation37,Citation38], in other studies it is associated with an increase of soft tissue necrosis or vascular morbidity leading to limb amputation or irreversible functional damage in up to two thirds of patients [Citation39–41]. Waiting for confirmatory studies, two parameters could be of value in balancing the pros and cons of radiotherapy after HILP, i.e. the degree of HILP-induced tumour necrosis and the completeness of surgical resection. According to Deroose et al., in the patients in whom HILP induces > 50% of tumour necrosis and R0 resection is performed, radiotherapy may be safely omitted [Citation42]. This is based on the observation that patients with >50% of tumour necrosis and R0 resection reported similar local outcome (recurrence rate of 0/15 and 1/28 with or without radiotherapy, respectively).
Of note, in our experience HILP conferred a sustained locoregional disease control both in patients with primary or recurrent disease, in whom 5-year LPFS was 81.6% and 74.2%, respectively. Moreover and interestingly, HILP allowed a sustained local control also in patients with metastatic STS, thus confirming its value as a palliative procedure, at least in well-selected patients [Citation28].
Since the different drug schedules used in HILP over the last 24 years proved to be comparable in terms of antitumour activity and efficacy (limb-sparing effect), their selection was dictated by their toxicity profile. Accordingly, since 2004 we have adopted the association of low dose TNF-α and L-PAM, thus improving the local toxicity reported by our patients ().
We are aware that the small numbers and the retrospective nature of this non-comparative study do not allow any firm conclusions to be drawn concerning the real impact of HILP on tumour respectability, limb preservation, disease-free and overall survival. Only a randomised trial comparing HILP vs neoadjuvant chemoradiotherapy could establish the benefit of hyperthermic locoregional chemotherapy on surgical management and patient outcome.
In conclusion, this study confirms that HILP, performed with different drugs, is an active treatment and provides substantial limb-sparing effect as well as durable local control in patients with advanced limb STS, either in those with primary, recurrent or metastatic disease. Adjuvant radiotherapy seems not to increase local toxicity. TNF-α plus L-PAM schedule showed the most favourable therapeutic ratio.
Acknowledgements
The authors thank Cristina Montesco, Roberto Stramare, Maura Digito, Giovanni Scarzello and Umberto Basso for patient care; Paolo Santo del Fiore and Romina Spina for data management.
The authors alone are responsible for the content and writing of the paper.
References
- Cormier JN, Pollock RE. Soft tissue sarcoma. CA Cancer J Clin 2004;54:94–109
- Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb sparring surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–15
- Williard WC, Hajdu SI, Casper ES, Brennan MF. Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann Surg 1992;3:269–75
- Creech O Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958;148:616–32
- Cavaliere R, Ciocatto EC, Giovanella BC, Heidelberger C, Johnson RO, Margottini M, et al. Selective heat sensibility of cancer cells: Biochemical and clinical studies. Cancer 1967;20:1351–81
- Stehlin JS Jr. Hypertermic perfusion with chemotherapy for cancers of extremities. Surg Gynecol Obstet 1969;129:305–8
- Taeger G, Grabellus F, Podleska LE, Müller S, Ruchholtz S. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia 2008;24:193–203
- Bhangu A, Broom L, Nepogodiev D, Gourevitch D, Desai A. Outcomes of isolated limb perfusion in the treatment of extremity soft tissue sarcoma: A systematic review. Eur J Surg Oncol 2013;39:311–19
- Seinen JM, Hoekstra HJ. Isolated limb perfusion of soft tissue sarcomas: A comprehensive review of literature. Cancer Treat Rev 2013;39:569–77
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Liénard D, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996;224:756–64
- Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM. Isolated limb perfusion with melphalan and tumor necrosis factor α for advanced melanoma and soft tissue sarcoma. Ann Surg Oncol 2007;14:230–8
- Deroose JP, Grünhagen DJ, de Wilt JH, Eggermont AM, Verhoef C. Treatment modifications in tumour necrosis factor-α (TNF)-based isolated limb perfusion in patients with advanced extremity soft tissue sarcomas. Eur J Cancer 2015;51:367–73
- Rossi CR, Vecchiato A, Foletto M, Nitti D, Ninfo V, Fornasiero A, et al. Phase II study on neoadjuvant hyperthermic antiblastic perfusion with doxorubicin in patients with intermediate or high grade limb sarcomas. Cancer 1994;73:2140–6
- Di Filippo F, Cavaliere F, Anzà M, Garinei R, Botti C, Perri P, et al. Liposomal doxorubicin in the perfusional treatment of advanced soft tissue limb sarcoma. J Chemother 2004;16:66–9
- Rossi C, Foletto M, Vecchiato A, Menin N, Pizzirani E, Di Filippo F, et al. TNF-alpha and doxorubicin in hyperthermic perfusion for limb sarcoma. Oncol Rep 1996;3:1059–61
- Rossi CR, Mocellin S, Pilati P, Foletto M, Nitti D, Lise M. TNF alpha-based isolated perfusion for limb-threatening soft tissue sarcomas: State of the art and future trend. J Immunother 2003;26:291–300
- Hoven-Gondrie ML, Bastiaannet E, van Ginkel RJ, Suurmeijer AJ, Hoekstra HJ. TNF dose reduction and shortening of duration of isolated limb perfusion for locally advanced soft tissue sarcoma of the extremities is safe and effective in terms of long-term patient outcome. J Surg Oncol 2011;103:648–55
- Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 2003;4:429–37
- Rossi CR, Foletto M, DI Filippo F, Vaglini M, Anza' M, Azzarelli A, et al. Soft tissue limb sarcomas: Italian clinical trials with hyperthermic antiblastic perfusion. Cancer 1999;86:1742–9
- Casara D, Rubello D, Pilati P, Scalerta R, Foletto M, Rossi CR. Optimized procedure of real-time systemic leakage monitoring during isolated limb perfusion using a hand held gamma probe and 99mTc-HSA. Nucl Med Commun 2004;25:61–6
- Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reaction. Eur J Cancer Clin Oncol 1982;18:905–10
- Di Filippo F, Giacomini P, Rossi CR, Santinami M, Garinei R, Anzà M, et al. Hyperthermic isolated perfusion with tumor necrosis factor-alpha and doxorubicin for the treatment of limb-threatening soft tissue sarcoma: The experience of the Italian Society of Integrated Locoregional Treatment in Oncology. In Vivo 2009;23:363–7
- Deroose JP, van Geel AN, Burger JW, Eggermont AM, Verhoef A. Isolated limb perfusion with TNF-alpha and melphalan for distal parts of the limb in soft tissue sarcoma patients. J Surg Oncol 2012;105:563–9
- Grunhagen DJ, de Wilt JH, Graveland WJ, van Geel AN, Eggermont AM. The palliative value of tumor necrosis factor alpha-based isolated limb perfusion in patients with metastatic sarcoma and melanoma. Cancer 2006;106:156–62
- Pennacchioli E, Deraco M, Mariani L, Fiore M, Mussi C, Collini P, et al. Advanced extremity soft tissue sarcoma: Prognostic effect of isolated limb perfusion in a series of 88 patients treated at a single institution. Ann Surg Oncol 2007;14:553–9
- Olofsson R, Bergh P, Berlin O, Engström K, Gunterberg B, Hansson M, et al. Long-term outcome of isolated limb perfusion in advanced soft tissue sarcoma of the extremity. Ann Surg Oncol 2012;19:1800–7
- Lejeune FJ, Pujol N, Liénard D, Mosimann F, Raffoul W, Genton A, et al. Limb salvage by neoadjuvant isolated perfusion with TNF alpha and melphalan for non-resectable soft tissue sarcoma of the extremities. Eur J Surg Oncol 2000;26:669–78
- Olieman AF, van Ginkel RJ, Molenaar WM, Schraffordt Koops H, Hoekstra HJ. Hyperthermic isolated limb perfusion with tumour necrosis factor-αlpha and melphalan as palliative limb-saving treatment in patients with locally advanced soft-tissue sarcomas of the extremities with regional or distant metastases. Is it worthwhile? Arch Orthop Trauma Surg 1998;118:70–4
- van Ginkel RJ, Thijssens KMJ, Pras E, van der Graaf WT, Suurmeijer AJ, Hoekstra HJ. Isolated limb perfusion with tumor necrosis factor alpha and melphalan for locally advanced soft tissue sarcoma: Three time periods at risk for amputation. Ann Surg Oncol 2007;14:1499–506
- Moller GM, Lewis JM, Dessureault S, Zager JS. Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia 2008;24:275–89
- Levy A, Le Péchoux C, Terrier P, Bouaita R, Domont J, Mir O, et al. Epithelioid sarcoma: Need for a multimodal approach to maximize the chances of curative conservative treatment. Ann Surg Oncol 2014;21:269–76
- Grabellus F, Kraft C, Sheu-Grabellus SY, Bauer S, Podleska LE, Lauenstein TC, et al. Tumor vascularization and histopathologic regression of soft tissue sarcomas treated with isolated limb perfusion with TNF-α and melphalan. J Surg Oncol 2011;103:371–9
- Padussis JC, Steerman SN, Tyler DS, Mosca PJ. Pharmacokinetics & drug resistance of melphalan in regional chemotherapy: ILP versus ILI. Int J Hyperthermia 2008;24:239–49
- Beasley GM, Ross MI, Tyler DS. Future directions in regional treatment strategies for melanoma and sarcoma. Int J Hyperthermia 2008;24:301–9
- Vohra NA, Turaga KK, Gonzalez RJ, Conley A, Reed D, Bui MM. The use of isolated limb infusion in limb threatening extremity sarcomas. Int J Hyperthermia 2013;29:1–7
- Campana LG, Bianchi G, Mocellin S, Valpione S, Campanacci L, Brunello A, Donati D, Sieni E, Rossi CR. Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: Results of a non-comparative phase II study. World J Surg 2014;38:813–22
- Olieman AF, Pras E, van Ginkel RJ, Molenaar WM, Schraffordt Koops H, Hoekstra HJ. Feasibility and efficacy of external beam radiotherapy after hyperthermic isolated limb perfusion with TNF-alpha and melphalan for limb-saving treatment in locally advanced extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 1998;40:807–14
- Thijssens KM, van Ginkel RJ, Pras E, Suurmeijer AJ, Hoekstra HJ. Isolated limb perfusion with tumor necrosis factor alpha and melphalan for locally advanced soft tissue sarcoma: The value of adjuvant radiotherapy. Ann Surg Oncol 2006;13:518–24
- Vrouenraets BC, Keus RB, Nieweg OE, Kroon BB. Complications of combined radiotherapy and isolated limb perfusion with tumor necrosis factor alpha ± interferon gamma and melphalan in patients with irresectable soft tissue tumors. J Surg Oncol. 1997;65:88–94
- Hoven-Gondrie ML, Thijssens KM, Van den Dungen JJ, Loonstra J, van Ginkel RJ, Hoekstra HJ. Long-term locoregional vascular morbidity after isolated limb perfusion and external-beam radiotherapy for soft tissue sarcoma of the extremity. Ann Surg Oncol 2007;14:2105–12
- Hoven-Gondrie ML, Thijssens KM, Geertzen JH, Pras E, van Ginkel RJ, Hoekstra HJ. Isolated limb perfusion and external beam radiotherapy for soft tissue sarcomas of the extremity: Long-term effects on normal tissue according to the LENT-SOMA scoring system. Ann Surg Oncol 2008;15:1502–10
- Deroose JP, Burger JW, van Geel AN, den Bakker MA, de Jong JS, Eggermont AM, et al. Radiotherapy for soft tissue sarcoma after isolated limb perfusion and surgical resection: Essential for local control in all patients? Ann Surg Oncol 2011;18:321–7