Abstract
Purpose: This study was designed to assess technical success and complications in patients with high-risk soft tissue sarcomas undergoing CT fluoroscopy-guided closed-tip catheter placement before treatment with combined chemotherapy and regional hyperthermia. Materials and methods: This retrospective study comprised all patients referred for insertion of closed-tip catheters for the introduction of thermometry probes before regional hyperthermia treatment at a single university centre from 2010 to 2015. Catheter placements were performed under local anaesthesia and intermittent CT fluoroscopy guidance. Technical success, complication rate, duration of catheter insertion and dose–length product (DLP) were analysed. Technical success was defined as intratumoural catheter placement suitable for subsequent thermometry. Results: A total of 35 procedures were performed on 35 patients (22 men, 13 women). In 34 out of 35 interventions catheters were inserted successfully; in one patient catheter placement was not feasible. No intra-interventional complications occurred. In six patients post-interventional complications were observed – two major (one abscess formation and one severe catheter dislocation) and four minor complications. Technical failure was observed in 11.4% of patients, especially catheter kinking. A total of 55 catheters were placed, with a mean number of 1.7 ± 0.7 per patient. Mean total DLP was 723.2 ± 355.9 mGy*cm. Conclusion: CT fluoroscopy-guided closed-tip catheter placement into high-risk soft tissue sarcomas was characterised by high technical success and relatively low complication rate. While major complications were rarely observed, catheter-kinking preventing successful thermometry represented the most frequent technical failure.
Introduction
Sarcomas represent less than 1% of all solid malignant tumours in adults. They can be classified into more than 50 histological subtypes located in various anatomical regions. However, there are two major entities: soft tissue sarcomas (STS) and malignant bone tumours [Citation1].
Regional hyperthermia (RHT) is one treatment option for patients with high-risk STS (size ≥5 cm, Fédération National des Centres de Lutte Contre le Cancer (FNCLCC) grade 2 or 3, deep to fascia) [Citation2]. Clinical trials suggested that combination of RHT with neoadjuvant and adjuvant chemotherapy (with or without radiotherapy) is beneficial in these patients [Citation3–7]. The therapeutic effect of RHT is well known and based on the following three concepts: a direct thermal cytotoxicity (with more than 42.5 °C), an increased efficacy of both chemo- and radiotherapy, and an immunomodulation (e.g. by the induction of heat-shock proteins) (with 40–44 °C) [Citation8].
Due to the fact that temperature distribution in tissues during electromagnetic radiation is not equal, it is important to monitor temperatures both in the treated tumour region and in neighbouring anatomical structures, defined as thermal mapping. Essential factors influencing temperature distribution are blood flow and tissue-specific energy absorption. In sarcomas, heat distribution is usually heterogeneous due to their chaotic vasculature arrangement and focal areas of necrosis.
There are different possibilities for guidance of the placement of closed-tip catheters within tumours for thermal mapping such as computed tomography (CT) or open surgical guidance [Citation9]. All studies focusing on closed-tip catheter placement guided by CT used sequential CT-guidance [Citation10–13]. In contrast to sequential CT-guidance, CT fluoroscopy-guided interventions can be performed faster, safer, and with a lower effective patient radiation dose [Citation14–16].
Catheter insertion in patients with high-risk STS, especially in those with deep-seated or semi-deep-seated ones, can be a challenging task and implies a potential for complications. Therefore this retrospective analysis was performed at our tertiary university hospital. To the best of our knowledge, this is the first retrospective study aimed at evaluating the technical success of CT fluoroscopy-guided closed-tip catheter placement for thermometry in STS patients, its complications and the radiation exposure of the patients through the interventions.
Materials and methods
Patient selection
In our retrospective single centre clinical analysis, all consecutive patients from April 2010 to February 2015 (n = 35) undergoing CT fluoroscopy-guided closed-tip catheter insertion for RHT were included. All patients referred for closed-tip catheter placement had high-risk STS (size ≥5 cm, histological grade 2 or 3, deep to fascia) considered for neoadjuvant chemotherapy with regional hyperthermia. Indication for RHT was regularly discussed and confirmed within the multidisciplinary institutional tumour board, including oncologists, surgeons, radiation oncologists, pathologists, and interventional radiologists.
We included all consecutive patients who were referred to our radiological department from the hyperthermia unit of the oncological department during the study period. The internationally recognised quality management and assurance guidelines for RHT were respected [Citation17,Citation18]. Patients were included if the aim of the intervention had been to insert a closed-tip catheter into a STS for RHT under CT fluoroscopy guidance. We used the WHO classification for tumours of soft tissue and bone for classification of the various sarcomas [Citation19].
Our clinical ethics board approved this retrospective study regarding the review of clinical patient charts and images. The principles of the Declaration of Helsinki were followed. Informed consent from the patient or a legal guardian to undergo closed-tip catheter placement for RHT was obtained 24 h and immediately before the intervention after extensive explanation of the method and its potential complications.
Regarding exclusion criteria for CT fluoroscopy-guided closed-tip catheter placement the quality improvement guidelines of the Society of Interventional Radiology (SIR) for percutaneous needle biopsy were applied [Citation20]. In particular, relative contraindications included significant coagulopathy that could not be adequately corrected, severely compromised cardiopulmonary function or haemodynamic instability, lack of a safe pathway to the tumour, and inability of the patient to cooperate with or to be positioned for the procedure.
Imaging workup and guidance
In all patients, recent cross-sectional imaging studies obtained by the referring physicians or in-house examinations, such as CT, PET/CT and MRI, were checked by the team of interventional radiologists with respect to the feasibility of the procedure.
All patients underwent pre- and post-interventional CT scans (Somatom Definition AS+ and Somatom Definition Edge, Siemens Healthcare, Erlangen, Germany). Pre-interventional planning CT scans were mostly contrast-enhanced (85.7%) for risk stratification considering peri-interventional complications such as bleeding due to possible effect on vessels during placement. In the remaining cases (14.3%) non-enhanced CT scans were sufficient. Multiplanar reconstructions (MPRs) were made to estimate the extent of the tumour, to detect possible involvement of surrounding anatomical structures and to plan the ideal needle position for closed-tip catheter placement. The position of the catheter was analysed on post-interventional CT images. For CT guidance, a 128-slice CT scanner with CT fluoroscopy (CARE Vision CT, Siemens; 120 kV, 10 to 20 mA) was used. CT fluoroscopy was performed with angular beam modulation (HandCARE™, Siemens). Whole closed-tip catheter placement was performed under intermittent single-shot CT fluoroscopic acquisitions only. The CT-system used in this study comprised three modes for acquisition of CT datasets:
I-Fluoro: For intra-interventional low-milliampere guidance (e.g. using a tube current of 10 mA). The interventional radiologist intermittently acquired single CT fluoroscopic datasets containing three to six images within the detector coverage of 4.6 cm.
I-Sequence: This mode was only exceptionally used for CT acquisitions covering the same scan range (4.6 cm) at a reduced standard tube current of 50 mA providing a slightly reduced CT image quality. The dataset was characterised by a higher image quality e.g. useful for the precise evaluation of the needle position with respect to small vessels.
I-Spiral: This mode provided full diagnostic CT image quality was used for the pre- and post-interventional acquisition of a CT dataset covering the organ region (e.g. lower limb, abdomen) of interest.
With respect to radiation exposure, the interventional radiologist was only exposed to ionising radiation using the i-Fluoro mode for near real-time needle guidance while the i-Sequence and i-Spiral mode were only applied with the interventional team being at the CT workstation. Precautions with respect to radiation protection of the operator during CT fluoroscopy included aprons, thyroid shields, and eyeglasses of 0.5-mm lead equivalent. An additional shield was put onto the lower half of the patient before sterile draping to reduce scattered radiation.
Procedure
TSH, creatinine and coagulation parameters were routinely evaluated before the intervention. All procedures were performed by board-certified radiologists with more than 5 years’ experience in CT-guided interventions, or by junior radiologists under their supervision. The stainless steel hollow needles (Unimed®, Lausanne, Switzerland) utilised were characterised by a diameter of 10.3 gauge (7.5 French) and a length of 16 cm. The closed-tip hyperthermia catheters (Somatex® Medical Technologies, Teltow, Germany) had a diameter of 12.2 gauge (6 French) and a standard length of 35 cm. The patient position and access route chosen depended on the localisation of the tumour, including thoracic, abdominal, pelvic and extremity sarcomas. Pre-interventional antibiosis was not routinely administered. After sterile draping and disinfection of the skin overlying the tumour, local anaesthesia with 10–20 mL of 2% mepivacaine hydrochloride (Scandicain®, AstraZeneca, Wedel, Germany) was applied to the skin and soft tissue along the access route. All procedures were performed under local anaesthesia without the necessity of sedation or general anaesthesia. A small skin incision was made, and the hollow needle was advanced through the soft tissue towards the tumour under intermittent single-shot CT fluoroscopic control. In general, we tried to avoid deep puncture angles as related to the skin surface in order to minimise patient discomfort and sufficient local anaesthesia. After skin incision, the hollow cannula and mandrin were primarily inserted using a direction perpendicular to the skin surface. Secondarily, the cannula was inserted and advanced into the tumour using an angulated direction in order to cover the maximum tumour diameter. The central positioning of the catheters (especially when only one was used) was important. When tumours had both necrotic and solid portions we tried to place the catheters in the solid parts. The insertion was stopped when the needle covered as much of the diameter of the tumour as possible. After removal of the mandrin, a closed-tip catheter was inserted into the cannula, which was subsequently withdrawn. The catheter was fixed in place by sewing a suture fixing the plastic catheter to the skin. Shortly after the procedure a control CT scan was performed to exclude active bleeding and other immediate complications, and to verify a correct intratumoural position of the closed-tip catheter. The duration of the whole procedure was calculated based on the time between the CT topogram and the final control CT scan.
Technical outcome and complications
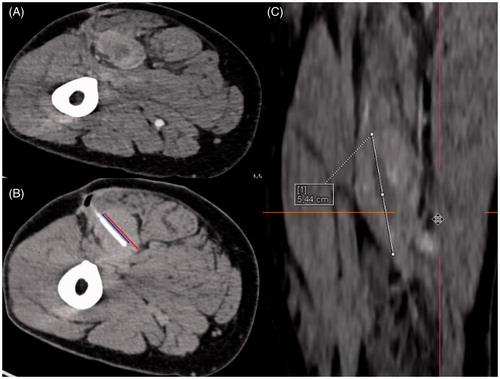
Two interventional radiologists who were experienced in CT fluoroscopy-guided closed-tip catheter placement retrospectively evaluated all CT datasets acquired in all 35 catheter placement sessions performed during the study period for the purpose of obtaining a consensus evaluation of catheter localisation and complications. For the assessment of post-interventional complications, we reviewed follow-up using the patients’ clinical charts within a time interval of 30 days after the intervention. We classified the complications according to the SIR quality improvement guidelines for percutaneous needle biopsy [Citation20]. The SIR standards of practice committee classified the complications by outcome. Successful catheter placement was defined as an intratumoural position without exceeding the tumour margin at the distal end of the closed-tip catheter. Moreover, it is important for ideal temperature monitoring that the inserted catheters comprise as much of the tumour diameter as possible. However, the anatomical location and expansion of a tumour sometimes prevents the interventional radiologist from achieving this goal. Therefore, we defined two ratios: ratio 1 describes the part of the tumour covered by the catheter from its entrance site to the opposite end of the tumour, while ratio 2 characterises the part of the tumour that is comprised by the catheter in relation to the maximum tumour diameter (no matter in which plane). For illustration see . We always tried to place the catheters in solid (i.e. non-necrotic, vital) and central portions of the tumour, and succeeded in the majority of cases.
Figure 1. Illustration of our calculated ratios through the example of a 54-year old man with an undifferentiated sarcoma in the right upper leg. (A) Contrast-enhanced planning CT scan in prone position clearly demarcates the vascularised tumour from the surrounding muscles. (B) Ratio 1 describes the proportion of the intratumoural catheter length (blue line) compared to the maximum tumour diameter in continuation of the catheter position (red line). In this example ratio 1 = 2.23:2.96 cm = 0.75. (C) Ratio 2 describes the proportion of the intratumoural catheter length (blue line in B) compared to the maximum total tumour diameter in any plane. In this example we reconstructed the coronary image via syngo.via® software (Siemens) because the maximum tumour extent is in this plane (white line). Here ratio 2 = 2.23:5.44 cm = 0.41.
Assessment of patient radiation dose
In analogy to Kloeckner et al. [Citation21], CT dosimetry was performed for all procedures using the dose length product (DLP), documented by the CT unit as primary dosimetric quantity data. The DLP is defined as the dose in one CT rotation multiplied with the exposure length in mGy*cm. It is a convenient index for the total dose. We evaluated the DLP of the pre-interventional planning CT scan, the DLP of the sum of all intra-interventional CT fluoroscopic acquisitions and the DLP of the post-interventional control CT scan.
Moreover, we calculated respective effective patient doses for thoracic, abdominal and pelvic sarcomas. For the pre-interventional planning and the post-interventional control CT scan the following formula can be used: E = DLP × t (effective dose = dose-length product × tissue weighting [k] factor). We refer to Deak et al. regarding the respective tissue weighting (k) factors depending on the anatomical region of interest [Citation22].
As so far no k factors have been issued by the ICRP for interventional CT fluoroscopy applications, for this study DLP-to-effective-dose calculation for CT fluoroscopy was performed using a k factor of 0.018 according to the computationally derived model described in detail by Leng et al. [Citation23]. This model gives particular respect to the fact that during the (more or less) stationary intermittent CT fluoroscopy mode, a finite anatomical region, including organs with different weighting factors, is exposed to ionising radiation.
Statistical analysis
For data collection and statistical analysis, the software SPSS version 23.0 (IBM, Chicago, IL, USA) was used. Normally distributed data were usually presented with mean ± SD (range) and non-normally distributed data were usually presented with median (range).
Results
Patient characteristics
A total of 35 patients (22 male, 13 female) with high-risk soft tissue sarcomas underwent RHT between April 2010 and February 2015. The mean age was 52.4 ± 13.6 years (range 24–75 years).
The localisation of the sarcomas varied from patient to patient; 48.6% of our patients suffered from extremity sarcomas (n = 17), 25.7% were affected by pelvic sarcomas (n = 9) and 14.3% by thoracic sarcomas (n = 5), whereas 11.4% had sarcomas in the abdominal region (n = 4). These regions were subsequently subdivided depending on the exact location of the tumour. For more details see .
Table 1. Population characteristics in patients with STS undergoing CT fluoroscopy-guided closed-tip catheter placement (N = 35).
Most of our patients had undifferentiated sarcomas (42.9%; n = 15). The remaining patients had liposarcomas (n = 5), synovial sarcomas (n = 3), malignant peripheral nerve sheath tumours (MPNSTs) (n = 3), myxofibrosarcomas (n = 3), leiomyosarcomas (n = 2), myofibroblastic sarcoma (n = 1), teleangiectatic sarcoma (n = 1), rhabdomyosarcoma (n = 1) and primitive neuroectodermal tumour (PNET) (n = 1).
Intervention characteristics
In total, 55 catheters were inserted during the study period, corresponding to 1.7 ± 0.7 catheters per patient (range 1–4). On average, the catheters remained in place for 4.1 ± 1.8 days (range 2–10 days). The mean duration of the intervention (including the topogram and the pre- and post-interventional CT scans) per patient was 40.8 ± 13.5 min (range 22.3–72.8). The mean duration of the interventional procedure (without the topogram and the pre- and post-interventional CT scans) was 14.5 ± 8.8 min (median 10.4, range 5.2–38.6 min).
Mean total dose length product (DLP) was 723.2 ± 355.9 mGy*cm (range 169–1826 mGy*cm) including the DLPs of the pre-interventional planning scan, intra-interventional CT fluoroscopy, and post-interventional control scan. Mean DLP of CT fluoroscopy was 64.8 ± 81.8 mGy*cm. Moreover, mean effective patient dose in thoracic, abdominal and pelvic sarcomas was 8.0 ± 2.3 mSv, 8.3 ± 1.2 mSv and 11.1 ± 4.5 mSv, respectively. See .
Table 2. Characteristics of CT fluoroscopy-guided closed-tip catheter placement.
Technical outcome, complications and mortality
In 34 out of 35 interventions (97.1%) successful intratumoural catheter placement was achieved. In one patient catheter insertion was technically not possible due to patient non-compliance. This patient received RHT without catheter insertion. In 71.4% of catheter insertions (n = 25) there were neither intra-/post-interventional complications nor technical failures. Mean ratio 1 was 0.96 ± 0.08 and mean ratio 2 was 0.66 ± 0.21. For further details see .
Table 3. Technical outcome.
Closed-tip catheter placement was associated with complications in six cases (17.1%), two major (5.7%) and four minor (11.4%) complications. Technical failure was also observed in four patients (11.4%).
The first major complication was a catheter-associated abscess formation in the left inguinal region of a patient with a STS in the same region. This resulted in subsequent CT-guided drainage after the catheters had been drawn. The second was a severe post-procedural catheter dislocation from the right psoas muscle (where the catheter was initially inserted) along the fascial space into the right upper leg, which consecutively had to be surgically removed. and visualise the patient primarily undergoing correct placement (); subsequently he presented with major displacement of one of two inserted closed-tip catheters (). Since this finding occurred just before the second RHT treatment, only the second catheter was used for thermal mapping in this session. The most frequent technical failure was kinking of the catheter in three patients (8.6%), which led to impeded thermal mapping and necessitated catheter removal. In one case no catheter could be inserted due to patient non-compliance. For an overview see .
Figure 2. Closed-tip catheter placement in a 41-year old mn with a retroperitoneal malignant peripheral nerve sheath tumour (MPNST) (A) Contrast-enhanced planning CT scan shows the extent of the tumour in right psoas muscle. (B) Coronal reconstruction. (C) Intra-interventional CT fluoroscopy image. Final position of the closed-tip catheter.
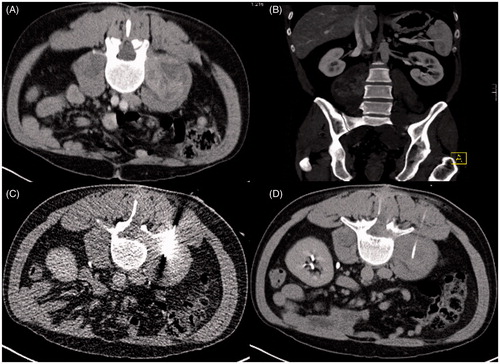
Figure 3. Severe catheter dislocation in the same patient mentioned in . (A–C) Catheter dislocated from its original retroperitoneal intratumoural position down to the upper leg along the fascial space. Arrows indicate the position of the catheter.
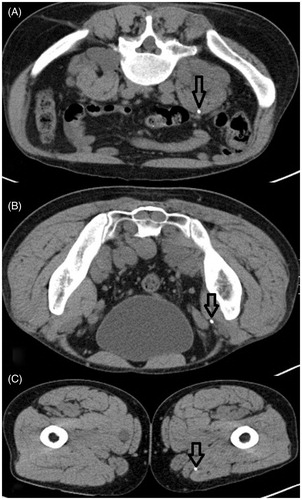
Table 4. Minor and major complications according to SIR quality improvement guidelines (20) and technical failure*.
There was no procedure-associated mortality within 30 days after the intervention.
Discussion
This study demonstrates excellent technical outcome and a low complication rate of CT fluoroscopy-guided closed-tip catheter placement in 35 patients with high-risk STS. In 97.1% of cases (n = 34) intratumoural catheter placement could be performed with only one procedure turning out to be technically unfeasible. In only two cases (5.7%) major complications such as abscess formation and severe catheter dislocation occurred. The most frequent minor complication was catheter kinking (8.7 %). Mean total DLP was 723.2 ± 355.9 mGy*cm and mean total intervention time was 40.8 ± 13.5 min.
In the past, several clinical trials confirmed the efficacy of RHT in high-risk STS. In 1990, Issels et al. demonstrated that RHT and simultaneous chemotherapy could achieve a response rate of 37% in tumours that had turned out to be refractory against previous surgery, radiation and chemotherapy [Citation6]. Following studies (RHT-91 and RHT-95) suggested that both neoadjuvant and adjuvant RHT were effective in those patients [Citation4,Citation7]. In 2010, one randomised phase three clinical study proved that combination of RHT with neoadjuvant chemotherapy is associated with a better outcome on local progression-free survival and disease-free survival than chemotherapy alone [Citation5]. Recently, Angele et al. pointed out that addition of RHT to adjuvant chemotherapy after macroscopically complete tumour resection also resulted in improved local tumour control and DFS [Citation3]. Furthermore, the current European Society for Medical Oncology (ESMO)/European Sarcoma Networking Group clinical practice guidelines underline RHT as a treatment option in STS [Citation2]. Therefore thermometry, whether invasive or non-invasive, should be further evaluated as a tool to ensure adequate hyperthermia treatment in STS.
In invasive thermometry there are basically three options how to insert closed-tip catheters into tumours. By far the most widespread method is via percutaneous CT (fluoroscopy) guidance, the second option is placement during incisional biopsy, and the third one is intraoperative insertion (usually into the tumour bed after incomplete R1 resection) [Citation9].
To our knowledge, there were only a few publications from more than 20 years ago that broached the subject of sequential CT-guided (not CT fluoroscopy-guided) catheter placement in patients designated for hyperthermia treatment [Citation10–13,Citation24]. In the following we compare technical parameters, placement techniques and complications.
Regarding the orientation of catheter placement, Banerian et al. chose access paths ‘to sample the most divergent areas within the tumor page 503’, which was why they often used to insert catheters perpendicular to each other [Citation10]. While our objective was also to encompass as much of the tumour as possible, we chose a different approach by trying to place both catheters along the longest axis of the tumour, which often ended up in parallel insertion of the catheters. To obtain as much thermal data as possible, it is necessary to place the catheters through the whole diameter of the tumour. Feldmann et al. achieved this in only 50% of catheter placements, in 33% their catheters covered only half of the diameter and in 18% they even inadvertently landed in the periphery of the tumour [Citation11]. As opposed to this, we covered more than 70% of the tumour diameter (ratio 1) in 85.7% of cases. Mean ratio 1 of 0.96 and mean ratio 2 of 0.66 illustrate that our catheters comprised most of the tumour diameter in the great majority of cases. It is widely accepted that there is no standard number of catheters to be placed. The more catheters that are inserted, the more information one can get about temperature distribution, but also the more complicated, time-consuming and costly the intervention might become. This is why for most STS after interdisciplinary discussion with the referring oncologists we decided to insert approximately two catheters (mean 1.7 ± 0.7, range 1–4) per treatment area, depending on the tumour size. This is in line with Feldmann et al. [Citation11] and van der Zee et al. [Citation12] who mainly placed one to three catheters in each treatment area. Comparing the hollow needles used, we performed interventions with 10.3 gauge cannulas, while van der Zee et al. used thinner 16 gauge needles. Neither Van der Zee et al. nor we could observe any immediate or late bleeding complications or injury of anatomical structures. Regarding the plastic catheters placed, their lengths had ranged from 25 to 40 cm [Citation11,Citation12], which was comparable to our standard catheter length of 35 cm. In former trials the time period for which the catheters stayed in place was significantly longer (mean 23 days, range 3–6 weeks) [Citation11–13], while we usually removed the catheters after only two RHT sessions (4.1 ± 1.8 days, maximum of 10 days). The comparatively early removal of catheters could explain why we had no cases of external catheter loss, the most frequent complication recorded by both van der Zee et al. and Feldmann et al.
Feldmann et al. reported three cases of acute or early complications, such as bleeding after catheter removal, transient haemorrhage and acute pancreatitis. In contrast, we had no cases of acute complications, and the fact that we mostly performed contrast-enhanced planning CT scans could have played a role. We observed two major complications, one abscess formation leading to CT-guided drainage and one severe catheter dislocation necessitating subsequent surgery. Our most frequent cause for technical failure was kinking of the catheter. In 1993, Feldmann et al. reported that the use of plastic fillers was a way to prevent this problem [Citation11]. Until today there has been only one case of tumour growth along the thermometry catheter trace, recorded in 1992 [Citation24].
All of the previous works described conventional (sequential) CT-guidance as a means of catheter insertion. However, for nearly two decades since the US Food and Drug Administration granted its approval to the first CT fluoroscopy scanner in North America, CT fluoroscopy (CTF) has been replacing sequential CT-guidance more and more in many institutions [Citation14,Citation16,Citation21,Citation25]. Near real-time imaging similar to that of conventional fluoroscopy or ultrasound is made possible by reconstruction of images at a frame rate of approximately 6/s [Citation26,Citation27]. The major advantages with respect to closed-tip catheter placement are 1) the detailed pre-interventional visualisation of the tumour morphology, extent and location in relation to neighbouring risk structures in the contrast enhanced planning CT scan, 2) the intra-interventional visualisation and near-real time of the guiding sheath within the relevant tumour parts, and 3) the post-interventional verification of the correct catheter position and visualisation of potential complications in the control scan. Yet there is also one major disadvantage of CTF as opposed to sequential CT: while conventional CT-guidance is not associated with radiation exposure to the operator, CTF is. In a retrospective study from 2007 to 2011 Kloeckner et al. evaluated dose-length products (DLPs) of 1576 CT-guided interventions (e.g. biopsies, drainage, vertebroplasty) [Citation21]. Of total radiation to the patient 85% could be attributed to diagnostic CT scans, while 15% of radiation exposure occurred during CTF. This is roughly in line with our results, where 91% of the total DLP was attributable to diagnostic CT scans. Comparing our closed-tip catheter placements with different interventional procedures such as CTF-guided abdominal drainage or liver biopsies regarding the mean DLPs (723 mGy*cm versus 802 or 848 mGy*cm respectively), our results are similar to those of Kloeckner et al. Furthermore, they proposed that single-slice quick-check CTF should be preferred over continuous CTF to reduce radiation exposure, and that pre-interventional CT scans should be limited to the region of interest because recent CT images were usually available. In addition it has been reported that CT fluoroscopy can reduce the procedure time when compared to sequential CT guidance [Citation28].
To our knowledge, this is the first study analysing CT fluoroscopy-guided closed-tip catheter placement in RHT treatment in STS. To date there has been no systematic evaluation of DLP or effective patient dose during hyperthermia catheter placement. Moreover, until now no one has assessed procedure time for RHT catheter insertion, albeit our study has some limitations. The analysis has a retrospective design and only included patients from a single institution. In comparison to van der Zee et al. (1987) and Feldmann et al. (1993) our work comprised a smaller patient number. However, while their studies comprised multiple types of tumours (only very few STS), our study included a comparatively high number of STS (n = 35) considering the rareness of the disease (incidence of 4–5/100.000/year) and the even more infrequent indication for RHT.
Conclusion
Our trial showed that CT fluoroscopy-guided closed-tip catheter placement in patients with high-risk STS is a successful, safe and well-tolerated method. Complications occurred in 17.1% of the patients with only 5.7% being major complications such as abscess formation and severe catheter dislocation. Technical failure was an issue in 11.4% of cases; in particular catheter kinking could impede adequate thermometry. Nonetheless, closed-tip catheter placement proved to be an efficient and reliable tool for adequate thermometry in the great majority of patients.
Acknowledgements
Special thanks to Margareta Santl from the Hyperthermia/Oncology Department as well as to Alena Baumann and Vanessa Pfahler from the Radiology Department at Klinikum Grosshadern Munich for their dedicated support.
Declaration of interest
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
References
- Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res 2012;2:14
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl3):iii102–12
- Angele MK, Albertsmeier M, Prix NJ, Hohenberger P, Abdel-Rahman S, Dieterle N, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: A subgroup analysis of a randomized phase-III multicenter study. Ann Surg 2014;260:749–54; discussion:54–6
- Issels RD, Abdel-Rahman S, Wendtner C, Falk MH, Kurze V, Sauer H, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: Long-term results of a phase II study. Eur J Cancer 2001;37:1599–608
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Issels RD, Prenninger SW, Nagele A, Boehm E, Sauer H, Jauch KW, et al. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: A phase II study. J Clin Oncol 1990;8:1818–29
- Wendtner C, Abdel-Rahman S, Baumert J, Falk MH, Krych M, Santl M, et al. Treatment of primary, recurrent or inadequately resected high-risk soft-tissue sarcomas (STS) of adults: Results of a phase II pilot study (RHT-95) of neoadjuvant chemotherapy combined with regional hyperthermia. Eur J Cancer 2001;37:1609–16
- Issels RD. Regional hyperthermia in high-risk soft tissue sarcomas. Curr Opin Oncol 2008;20:438–43
- Schlemmer M, Lindner LH, Abdel-Rahman S, Issels RD. Prinzip, Technik und Indikation der Hyperthermie und Teilkörperhyperthermie [Principles, technology and indication of hyperthermia and part body hyperthermia]. Der Radiologe 2004;44:301–9
- Banerian KG, Roberts JL, Borrego JC, Martinez A. CT-guided thermocouple placement for hyperthermia treatment. Radiographics 1990;10:499–506
- Feldmann HJ, Hoederath A, Molls M, Sack H. Problems associated with CT-guided catheter insertions. Int J Hyperthermia 1993;9:219–25
- van der Zee J, van Rhoon GC, Broekmeijer-Reurink MP, Reinhold HS. The use of implanted closed-tip catheters for the introduction of thermometry probes during local hyperthermia treatment series. Int J Hyperthermia 1987;3:337–45
- Eftekhari F, Bernardino ME, Headley DL, Corry PM. Technical note. Use of CT in the placement of heat monitoring thermocouples for hyperthermia therapy. J Comput Assist Tomogr 1981;5:933–6
- Paprottka PM, Helmberger T, Reiser MF, Trumm CG. CT-Steuerung Fluoroskopie und mehr [Computed tomography guidance: Fluoroscopy and more]. Der Radiologe 2013;53:974–85
- Sarti M, Brehmer WP, Gay SB. Low-dose techniques in CT-guided interventions. Radiographics 2012;32:1109–19; discussion:19–20
- Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology 2001;219:515–20
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: Quality management in regional deep hyperthermia. Strahlenther Onkol 2012;188(Suppl2):198–211
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, Wust P, De Leeuw AC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998;14:125–33
- World Health Organization. WHO Classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC; 2013
- Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010;21:969–75
- Kloeckner R, dos Santos DP, Schneider J, Kara L, Dueber C, Pitton MB. Radiation exposure in CT-guided interventions. Eur J Radiol 2013;82:2253–7
- Deak PD, Smal Y, Kalender WA. Multisection CT protocols: Sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010;257:158–66
- Leng S, Christner JA, Carlson SK, Jacobsen M, Vrieze TJ, Atwell TD, et al. Radiation dose levels for interventional CT procedures. Am J Roentgenol 2011;197:W97–103
- van der Zee J, Veeze-Kuijpers B, Wiggers T, van de Merwe SA, Treurniet-Donker AD. Risk of tumour growth along thermometry catheter trace: A case report. Int J Hyperthermia 1992;8:621–4
- Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, et al. Guidance with real-time CT fluoroscopy: Early clinical experience. Radiology 1996;200:851–6
- Daly B, Templeton PA. Real-time CT fluoroscopy: Evolution of an interventional tool. Radiology 1999;211:309–15
- Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy-guided interventional procedures: Techniques and radiation dose to radiologists. Radiology 2001;220:161–7
- Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: Techniques, results, and radiation exposure. Radiology 1999;212:673–81
