Abstract
Introduction: Peritoneal carcinomatosis (PC) is increasingly being treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), with or without early post-operative intraperitoneal chemotherapy (EPIC). We compared the morbidities, overall survival (OS) and disease free survival (DFS) between two groups of patients who underwent CRS and HIPEC alone and with EPIC at our institution. Methods: A retrospective review of 111 patients with PC who were treated with CRS + HIPEC or CRS + HIPEC + EPIC in a single institution between January 2008 and April 2014 was performed. EPIC with 5-fluorouracil or paclitaxel was utilised, depending on the primary tumour. Results: Patients who received EPIC had a higher proportion of grade III and above post- operative complications (58% versus 25%; p = 0.048) and a longer duration of hospitalisation (16 days versus 13 days; p = 0.019) than patients without EPIC. There were no significant OS and DFS differences between the EPIC and no EPIC groups (log-rank p = 0.231 and p = 0.144, respectively). Conclusion: The use of EPIC after CRS + HIPEC for PC potentially results in increased morbidity and longer hospitalisation, and is unlikely to affect survival outcomes. Based on our experience, EPIC is not recommended after CRS and HIPEC.
Introduction
Peritoneal carcinomatosis (PC) is increasingly being treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), with significant improvements in overall survival (OS) and disease-free survival (DFS) being reported [Citation1–4]. The aim of this combined treatment is to remove all visible tumours from the peritoneal cavity, and to eliminate the remaining microscopic disease by the administration of heated intra-peritoneal chemotherapy. There are differences pertaining to the administration of the intra-peritoneal chemotherapy that exist between different institutions. The differences include the range of drugs used, the temperature at which the drugs are administered, and whether an open or closed technique is employed during HIPEC. In addition, one of the major differences is the inclusion of early post-operative intraperitoneal chemotherapy (EPIC) delivered after CRS and HIPEC.
EPIC is usually started on the first post-operative day and continued for five days. The rationale for EPIC is that it allows for the administration of intraperitoneal chemotherapy in the early post-operative period, before adhesions are formed, allowing the drugs to be theoretically more effective than if administered in an outpatient setting subsequently. However, this often prolongs the patient’s length of hospital stay and adds to the medical costs. There have been studies which have shown HIPEC and EPIC to be associated with increased morbidity as compared to HIPEC alone [Citation5], and others which have shown HIPEC alone to be superior to EPIC alone [Citation6]. An experimental animal study showed EPIC to have a positive effect on survival, as compared to HIPEC, but in combination HIPEC and EPIC was extremely toxic [Citation7]. To date, the benefits of EPIC remain unclear.
Since January 2000, we have performed 176 CRS and HIPEC procedures at the National Cancer Centre Singapore (NCCS) for patients with PC from colorectal, ovarian, mesothelioma, primary peritoneal, pseudomyxoma peritonei, and other primaries. Prior to November 2012, all patients were planned for EPIC for five days, after CRS and HIPEC. Since then we have ceased EPIC due to a lack of convincing evidence on the benefits of the regimen. We retrospectively reviewed our patients with the aim of evaluating whether EPIC makes any difference to the survival outcomes and morbidities of patients with PC being treated with CRS and HIPEC.
Methods
Patient selection
The study was carried out under the approval of the Centralized Institutional Review Board of the Singapore Health Services. A prospective database of consecutive patients who underwent CRS and HIPEC in NCCS was reviewed. Patients included in the study were treated between January 2008 and April 2014 in order to eliminate potential learning curve bias. Patients who underwent repeat CRS and HIPEC during the study period had their latest operative records selected for analysis.
All patients with PC, who underwent CRS and HIPEC in our institution, were of Eastern Cooperative Group (ECOG) performance status 0 or 1, with no distant metastases. The extent of disease was examined on computed tomography (CT) scan of the abdomen and pelvis, and the feasibility of adequate cytoreduction and tumour clearance was discussed at the multidisciplinary tumour board meetings. The patients were evaluated for the presence of extra-abdominal disease either via a thorax CT scan or a positron emission tomography (PET)-CT scan.
CRS and HIPEC
CRS was performed as described by Sugarbaker and aimed to remove all macroscopic peritoneal disease [Citation8]. Bowel anastomoses were typically performed after HIPEC. HIPEC targeted the microscopic disease, working on lesions less than 3 mm [Citation9,Citation10]. The purpose of CRS was to achieve a complete cytoreduction, with no macroscopic residual disease. Besides the stripping of the parietal peritoneum, individual visceral resections were recorded (i.e. gastrectomy, splenectomy), and documented as individual CRS procedures. The subdiaphragmatic peritoneum was removed when macroscopic disease was visible but was left intact if no gross disease was visualised. All patients who underwent stripping of the subdiaphragmatic peritoneum had placement of chest-tubes on the corresponding side intra-operatively.
All patients received HIPEC with chemotherapeutic agents prescribed by the medical oncologist. The chemotherapeutic agents used for the HIPEC differed based on the organ of the primary tumour, but within the same tumour histology group, the drug prescribed, the duration of the HIPEC, and the temperature at which it was administered remained similar throughout the years. Mitomycin C was the drug provided for colorectal and appendiceal PC, whilst cisplatin was the drug provided for the patients with ovarian neoplasms and mesotheliomas.
At our institution, a closed technique for HIPEC, with the chemotherapy agent diluted in 2–2.5 L of peritoneal dialysis solution at 42 °C was used to distend the abdomen. The Belmont hyperthermia pump was used to deliver the intra-peritoneal chemotherapy agent via a single inflow catheter, and drainage was via four intra-abdominal drains. HIPEC was administered for 60 min in all cases.
Post-operative course and EPIC
Post-operatively, the patients were either transferred to the surgical intensive care unit (SICU) or the high-dependence unit at the anaesthetist’s discretion.
Before November 2012 EPIC was delivered through the four intra-abdominal drains that were left in place after CRS and HIPEC. The initiation of EPIC was a combined decision between the surgical and medical oncology teams, and was based on the patients’ post-operative recovery. If the patient remained well EPIC was initiated by post-operative day 2, and was delivered for 5 days. 5-Fluorouracil (5-FU) was the chemotherapy agent administered for EPIC in our colorectal, appendiceal, and primary peritoneal patients, while paclitaxel was the drug administered for the ovarian cancer and mesothelioma patients.
After November 2012, the delivery of EPIC was stopped. The four intra-abdominal drains were left in place, and gradually removed over the post-operative period when their outputs were minimal.
Morbidity assessments and follow-up
All intra-operative and 30-day post-operative complications were recorded. Post-operative morbidity was evaluated using the common terminology criteria for adverse events version 3.0 of the US National Institutes of Health criteria [Citation11]. All patients were followed up at the outpatient unit at the NCCS at approximately 2 weeks after the surgery, and at least every 3 months thereafter for 1 year. After 1 year, follow-up was every 6 months. CT scans of the thorax, abdomen, and pelvis, together with tumour markers (as appropriate), were performed at each follow-up visit and when clinically indicated. The patients were also followed up with medical oncologists and received adjuvant systemic chemotherapy at their discretion. Events of recurrent disease were recorded.
Statistical analysis
To detect significant differences in characteristics between patients with and without EPIC, categorical characteristics were compared using the chi-square test or Fisher’s exact test, as appropriate. Mann-Whitney U test was used for the comparison of continuous characteristics.
Overall survival (OS) was measured from the date of CRS and HIPEC to the date of death from any cause. DFS was measured from the date of CRS and HIPEC to the date of recurrence or death. Patients who did not develop any of these time-to-event end points were censored at the date of last follow-up. The Kaplan-Meier method was used to estimate all survival distributions, and the log-rank test was used to compare differences between survival curves. Cox proportional hazard models were fitted to estimate hazard ratios (HR) of OS and DFS between patients with and without EPIC.
As EPIC was ceased after November 2012, there was a naturally longer follow-up duration among the patients who received EPIC than the patients without EPIC. To account for this follow-up duration difference between the two patient groups when comparing their survival outcomes, we attempted two analysis adjustments: (1) truncating the follow-up duration of patients with EPIC, and (2) performing a 1:1 matched pair analysis of patients with and without EPIC, with gender, age at CRS and HIPEC (±10 years), and year of CRS and HIPEC (±1 year) as matching variables. For (1), the maximum follow-up duration of patients without EPIC was first determined as 24 months, and patients with EPIC with follow-up duration >24 months were censored at 24 months. For (2), matching was performed based on the greedy matching algorithm using %GMATCH SAS macro (Mayo Clinic, Rochester, MN). Marginal Cox models were fitted to the matched data to estimate the HRs and the corresponding 95% confidence interval (CI) [Citation12].
A two-sided p-value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient and treatment characteristics
There were a total of 111 patients who underwent CRS and HIPEC between January 2008 and April 2014, of whom 42 patients received EPIC and 69 patients did not (). Twenty of the 69 patients who did not receive EPIC underwent CRS and HIPEC before November 2012, with the majority of these 20 patients being rendered unfit for EPIC because of complications from CRS and HIPEC.
Table 1. Patients by whether they received EPIC.
Baseline clinical characteristics of the patients with and without EPIC were comparable (). The most common primary tumour in both groups was ovarian, followed by colorectal and appendix. The other patients included in the study were of primary peritoneal, peritoneal mesothelioma, and one patient with PC from endometrial adenocarcinoma. Most of the patients were of ECOG 0 statuses.
Table 2. Patient and treatment characteristics.
Patients who underwent CRS and HIPEC before November 2012 but did not receive EPIC had significantly more CRS procedures performed (median 3 versus 2; p = 0.030) and a longer duration of surgery (median 582.5 min versus 447.5 min; p = 0.005) than patients who received EPIC. About a third of these patients without EPIC achieved a completeness of cytoreduction (CC) score of 0 (no macroscopic residual disease), which was lower than the corresponding 85% among the EPIC patients. Treatment characteristics between patients who underwent CRS and HIPEC after November 2012 and patients with EPIC were comparable.
Treatment complications and morbidities
A total of 56 patients suffered post-operative complications, of which 37 were in the group without EPIC (). This higher number was mainly due to the 20 patients who did not receive EPIC post-CRS and HIPEC because of complications. Compared with EPIC patients, these 20 patients without EPIC who had undergone a more extensive surgery had a greater amount of blood loss during the surgery (1500 mL versus 1000 mL; p = 0.042), a longer stay in the SICU (4 days versus 1 day; p < 0.001) and a higher proportion requiring post-operative blood transfusion (90% versus 52%; p = 0.004).
Table 3. Treatment complications and morbidities.
Comparing patients who received EPIC with those who underwent CRS and HIPEC after November 2012, patients who received EPIC had a higher proportion of grade 3 and above complications (58% versus 25%; p = 0.048) and a longer duration of hospitalisation (16 days versus 13 days; p = 0.019) than patients without EPIC.
Survival outcomes
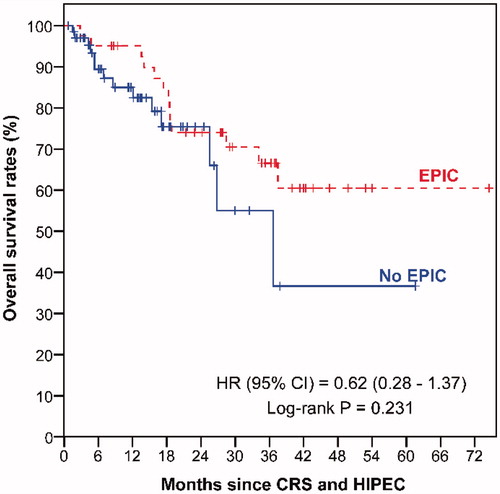
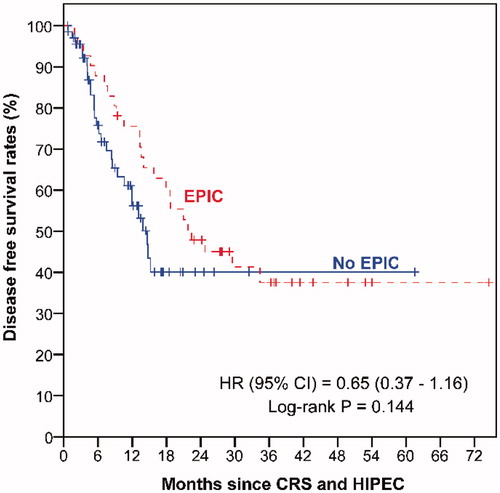
The median follow-up duration was 36.3 months (range 0–74.5 months) for EPIC patients and 13.3 months (range 0.7–61.6 months) for all 69 patients who did not receive EPIC. Patients without EPIC had poorer OS () and DFS () than EPIC patients, although these differences did not reach statistical significance.
The poorer survival outcomes of the 69 patients who did not receive EPIC could be partly attributed to the fact that the group included the 20 patients who had poor post-operative outcomes and were rendered unfit for EPIC post-CRS and HIPEC, even though they were originally planned for EPIC as their surgeries were before November 2012. Amongst patients who underwent CRS and HIPEC before November 2012, those who received EPIC had significantly longer OS (median: not reached versus 26.8 months; p = 0.042) and DFS (median 22.4 months versus 8.5 months; p = 0.035) than those who did not receive EPIC. We repeated the survival comparisons between the EPIC and no-EPIC patients with two modifications to the patient groups: 1) excluding the 20 patients who did not receive their planned EPIC from the no- EPIC patient group ( and ); and 2) reclassifying the 20 patients who did not receive their planned EPIC as having received EPIC in an “intention-to-treat” analysis ( and ).
Figure 3. Kaplan–Meier curves of overall survival by patients with and without EPIC: (a) excluding patients with no EPIC post-CRS and HIPEC before November 2012, (b) reclassifying patients with no EPIC post-CRS and HIPEC before November 2012 as having EPIC, (c) truncating follow-up duration of patients with EPIC to 24 months for patients in (a), (d) truncating follow-up duration of patients with EPIC to 24 months for patients in (b). (e) Matched pair analysis based on patients in (a). (f) Matched pair analysis based on patients in (b).
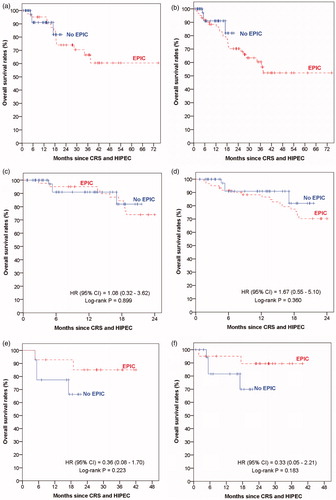
Figure 4. Kaplan–Meier curves of disease-free survival by patients with and without EPIC: (a) excluding patients with no EPIC post-CRS and HIPEC before November 2012, (b) reclassifying patients with no EPIC post-CRS and HIPEC before November 2012 as having EPIC, (c) truncating follow-up duration of patients with EPIC to 24 months for patients in (a), (d) truncating follow-up duration of patients with EPIC to 24 months for patients in (b). (e) Matched pair analysis based on patients in (a). (f) Matched pair analysis based on patients in (b).
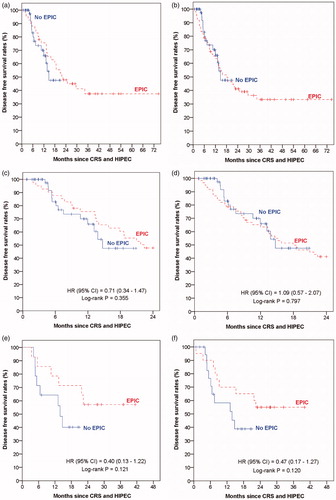
The two modifications clearly showed that EPIC patients had a longer follow-up duration than the no-EPIC patients. To account for this follow-up duration difference between the two patient groups when comparing their survival outcomes, we attempted the two analysis adjustments via the truncation approach and the matching approach as described in the methods section. There were no significant differences in survival outcomes between the two patient groups based on either the truncation approach (OS, and ; DFS, and ) or the matching approach (OS, and ; DFS, and ).
Discussion
There have been no randomised controlled trials evaluating the safety and efficacy of EPIC, and the benefits of the regimen remain unclear. Previous retrospective studies have shown a negative effect of EPIC on complication rates [Citation5,Citation6]. In the Canadian study, a higher rate of high-grade complications was experienced when EPIC was administered, compared to when HIPEC was given without EPIC (44.7% versus 31.0%) [Citation5]. Elias et al. [Citation6] showed that EPIC alone using mitomycin C was associated with a higher rate of high-grade complications when compared to HIPEC alone (26% versus 4%). This latter study suggests that EPIC is associated with greater morbidity even when there is no HIPEC given.
In Glehen et al. [Citation1], a multi-centred study involving 506 colorectal carcinomatosis patients, 271 patients (53.6%) received HIPEC alone, 123 patients (24.3%) had EPIC alone and 112 patients were administered HIPEC and EPIC. The study showed that the use of EPIC with or without HIPEC, was related with a higher rate of complications (OR 1.4; p = 0.032), suggesting that the morbidity associated with EPIC was independent of protocol. In our study, we found similarly that patients who received EPIC suffered more high-grade complications (58% versus 25%; p = 0.048) and stayed in hospital for a longer duration (16 days versus 13 days; p = 0.019) when compared to patients who did not receive EPIC.
The Canadian group is the only other group that recently published on the effects of EPIC on survival outcomes [Citation13]. They focused on the effect of EPIC on patients with colorectal and high-grade appendiceal adenocarcinoma who had undergone CRS and HIPEC. They too, found that EPIC patients suffered from greater morbidity when compared to the patients with HIPEC alone (43.2% versus 19.6%; p = 0.01), without any effect on OS or DFS [Citation13].
EPIC was part of the regime for our patients planned for CRS and HIPEC before November 2012. During this period 32% of the patients did not receive EPIC because of intra- operative and post-operative complications, and as expected, the results showed that these patients underwent a more extensive surgery, with greater blood loss and required a longer SICU stay. These patients would have been predicted to have poorer post-operative and survival outcomes, and would have negatively affected the survival results. In addition, patients who received EPIC naturally had a longer follow-up duration. Hence, in our survival analysis we attempted statistical methods to account for these limitations, and the results showed no difference in OS and DFS between the two groups.
There are several inherent limitations in this retrospective study. Firstly, the survival comparisons between EPIC and no EPIC patients were potentially affected by patient selection bias due to the 20 patients who were planned for EPIC before November 2012 but did not receive EPIC because of intra- and post-operative complications. We attempted to account for this by performing various sensitivity analyses which consistently suggested that there were no survival differences between the two groups of patients. Secondly, our study looks at all patients who underwent CRS and HIPEC, regardless of their histology, which may result in a bias towards or against EPIC for the individual histology.
A randomised controlled study will be necessary to definitely prove the efficacy of EPIC after HIPEC but the numbers needed to treat are likely to be formidable in view of the possible small benefits. However, until one is performed, the experience at our institution strongly suggests that EPIC should not be utilised after CRS and HIPEC.
Conclusion
The use of EPIC after CRS + HIPEC for PC potentially results in increased morbidity and longer hospitalisation, and is unlikely to affect survival outcomes. Randomised studies are required to justify its use following HIPEC. Based on our experience, EPIC is not recommended after CRS and HIPEC.
Declaration of interest
All authors have participated in the research design, analysing of data and writing of the paper and have reviewed the manuscript and approved it for submission. All authors declare no conflict of interest or receive any funding for research. The authors alone are responsible for the content and writing of the paper.
References
- Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol 2004;22:3284–92
- Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–32
- Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315–25
- Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370–5
- McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ. HIPEC + EPIC versus HIPEC alone: Differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol 2013;107:591–6
- Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol 2006;14:509–415
- Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Intraoperative versus early postoperative intraperitoneal chemotherapy after cytoreduction for colorectal peritoneal carcinomatosis: An experimental study. Ann Surg Oncol 2012;19(Suppl 3):475–82
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42
- Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermicintra peritoneal chemotherapy. Cancer Treat Rev 2001;27:365–74
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–74
- Younan R, Kusamura S, Baratti D, Cloutier AS, Deraco M. Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol 2008;98:253–7
- Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc 1989;84:1065–73
- Lam JY, McConnel YJ, Rivard JD, Temple WJ, Mack LA. HIPEC + EPIC vs HIPEC alone: Assessment of survival outcomes for colorectal and high grade appendiceal peritoneal carcinomatosis. Am J Surg 2015;210:424–30