Abstract
Purpose To examine the effectiveness of hyperthermic intravesical chemotherapy (HIVEC™) with mitomycin-C (MMC) for patients with intermediate–high-risk non-muscle invasive bladder cancer (NMIBC). Materials and methods From November 2010 to April 2015, 40 patients with intermediate–high-risk NMIBC received HIVEC™ treatment with a Combat BRS system. Of these patients, 24 received neoadjuvant HIVEC™ treatment (eight weekly instillations) before a transurethral resection of the bladder (TURBT) and 16 received adjuvant HIVEC™ treatment post-TURBT (four instillations weekly + six monthly). The pathological response of each tumour was evaluated after the neoadjuvant treatment. Recurrence rates and adverse effects were evaluated in both groups. Results A total of 40 patients completed the induction therapy: 24 patients received the Neoadjuvant HIVEC™ treatment. Of these patients, 15 (62.5%) showed a complete response. Eight patients (33.3%) showed a partial response, and one patient (4.1%) showed no response at all. The 4-year cumulative incidence of recurrence was 20.8%. The adjuvant HIVEC™ treatment was given to 16 patients. The 2-year cumulative incidence of recurrence was 12.5% for this group. The incidence and severity of side effects were slightly lower in the adjuvant group than in the neoadjuvant group. However, the difference was not statistically significant (p < 0.3). Most of the side effects were low grade and had virtually no effect on the treatment plan, and 97% of patients completed all of the HIVEC™ instillations scheduled. Conclusions The recirculation of hyperthermic MMC using Combat’s HIVEC™ treatment is safe and effective and is capable of achieving good success rates in both neoadjuvant and adjuvant settings. This treatment seems to be appropriate for NMIBC intermediate–high-risk patients who cannot tolerate or have contraindications for standard BCG therapy or in cases in which there are supply issues or shortages of BCG.
Introduction
The standard treatment for non-muscle invasive bladder cancer (NMIBC) is a complete transurethral resection of the bladder (TURBT). However, according to the SEER-Medicare-linked database of the National Cancer Institute (USA), almost 40% of patients with high-risk NMIBC experienced recurrence without progression. Another 33% experienced disease progression, 40% of whom died as a result of the progression [Citation1]. To reduce the high rate of recurrence, intravesical therapy is recommended, specifically intravesical bacillus Calmete–Guerin (BCG), which has been demonstrated to be the most effective therapy for intermediate–high-risk NMIBC.
Shida et al. [Citation2] published the first results for using intravesical mitomycin-C (MMC) to treat NMIBC 45 years ago. Since then, many studies have demonstrated its effects on the reduction of the number of tumour recurrences. However, because of its low absorption through the urothelium, the effectiveness of intravesical MMC is limited compared to BCG [Citation3], with less than 1% of the instilled drug absorbed.
Thus, device-assisted therapies appear to show improved efficacy compared to passive diffusion regimens. A meta-analysis of 22 studies showed a 59% relative reduction in NMIBC recurrence when hyperthermic MMC was used compared to MMC alone [Citation4]. The authors suggest that in the future hyperthermic MMC may become the standard therapy for high-risk patients with recurrent tumours for patients who are unsuitable for radical cystectomy and in cases in which BCG treatment is contraindicated.
Materials and methods
Cohort and data collection
We reviewed a group of 40 patients with intermediate- or high-risk NMIBC treated with HIVEC™ in the Comarcal Hospital of Monforte, Spain, between November 2010 and April 2015. The risk groups were defined using a European Organisation for Research and Treatment of Cancer (EORTC) stratification [Citation5]. Of these patients, 24 were treated with neoadjuvant HIVEC™ prior to a TURBT and 16 were treated with adjuvant HIVEC™ after a TURBT and complete tumour resection. All patients signed an informed consent and were treated according to the principles of the Helsinki Declaration.
The neoadjuvant group consisted of 24 patients who received eight instillations of MMC (80 mg MMC in 50 mL at 43 °C for 1 h), one per week, resulting in a total of 192 instillations of 80 mg of MMC. The group of 16 adjuvant patients received a total of 10 instillations (one per week for 4 weeks followed by one per month for 6 months, of 40 mg in 50 mL at 43 °C for 1 h), resulting in a total of 160 instillations of 40 mg of MMC.
Neoadjuvant group (also called ablative)
The first 15 patients were treated in a pilot study of neoadjuvant HIVEC™ [Citation6] that was approved by the ethics committee of Galicia. Since then, nine additional patients were treated with this protocol after the urology department of the hospital considered the treatment suitable for patients.
Criteria for inclusion in the study group
Part of the neoadjuvant pilot clinical trial (15 patients) [Citation5]
Other inclusion criteria:
Tumour recurrence after MMC (plus absence or shortage of BCG) (four patients)
High surgical risk/age (95 years or over) not suitable for surgery (three patients)
Tumour unlikely to be resected in one TURBT (one patient)
Recurrence after BCG (one patient)
All patients were initially assessed by cystoscopy with cold cup bladder biopsy to establish a diagnosis. An abdominal sonogram and/or CT scan were also performed. A general blood analysis, including coagulation and metabolic profile, was performed. Urine cultures were also performed. Signed informed consent was obtained from all patients. Patients treated off-trial were included under the special circumstances described above, in which the HIVEC™ treatment potentially offered an advantage over the standard treatment. The potential advantages and disadvantages of the HIVEC™ treatment were discussed with the patients. The current standard of care treatment according to guidelines, including TURBT, BCG or radical cystectomy, was also offered. Characteristics of the study population are noted in .
Table 1. Characteristics of the study population.
Treatment schedule
The treatment schedule consisted of eight intravesical instillations of MMC administered once a week at a concentration of 80 mg diluted in 50 mL of distilled water. The solution was heated to a target temperature of 43 °C and recirculated at 200 mL per min at stable pressure. The temperature inside the bladder was maintained at 43 °C ± 0.5 °C for the 60-min duration of the treatment.
Patients were informed of a possible sensation of local and/or systemic heat during the first treatment. An optional prophylactic antibiotic prior to each dose of neoadjuvant HIVEC™ was also considered on medical grounds, as was urine alkalisation with bicarbonate following a local hospital protocol. Restricting fluid intake 8 h prior to the HIVEC™ instillation was strongly recommended.
Tolerance data were recorded in a specific format for each patient before each instillation by assessing any side effects from a previous instillation and during the procedure. Any mild and expected side effects similar to instillation of MMC at room temperature were recorded. Side effects were recorded in each patient’s notes for evaluation at the end of the clinical trial and were treated following local hospital policy. Any serious and/or unexpected side effects were reported to the principal investigator and appropriate authorities as outlined by the Spanish Agency for Medicines and Health Products. Treatment was discontinued if serious adverse effects or an allergy to MMC became evident, if the patient withdrew consent, or if the doctor felt it was in the interest of the patient.
Finally, after eight instillations of HIVEC™, a complete TURBT using a standard bipolar resection was conducted between 7 and 15 days after completion of the HIVEC™ treatment. Tumour size, multi-focality, and location were recorded using a rigid cystoscopy. All visible tumours and residual scars were resected, and deep biopsies were taken from the bladder wall. Mapping bladder biopsies were also consistently taken, even if the bladder was endoscopically negative. All samples were sent for pathological examination.
Partial responsive or non-responsive patients were treated with 15 additional instillations of MMC at room temperature as an adjuvant therapy (four doses once a week plus 11 doses once a month) administered at a concentration of 40 mg diluted in 50 mL of distilled water. Those with a complete response (pT0) received no further treatment.
All patients were followed for a minimum period of 2 years with cystoscopy and urine cytology every 3 months and an abdominal-pelvic ultrasound every 6 months. If macroscopic tumour recurrence or positive cytology was detected, treatment would proceed to a complete TURBT, pathological study and treatment schedule assessed according to local hospital policy and the special features of each case. Safety and efficacy parameters are described in .
Table 2. Safety and efficacy parameters.
Adjuvant group (also called prophylactic)
Adjuvant HIVEC was offered to 19 patients after a complete TURBT as a therapeutic alternative to the standard treatment (BCG or radical cystectomy). Sixteen patients accepted it.
Criteria for inclusion in the study group
Tumour recurrence after MMC (plus absence or shortage of BCG) (8 patients)
Recurrence after BCG (5 patients)
Rejected for radical cystectomy (3 patients)
Treatment schedule
Prior to HIVEC™, a complete TURBT was performed using a standard bipolar resection. Tumour size, multifocality, and location were recorded. All visible tumours were resected, and deep biopsies were taken from the bladder wall. Mapping bladder biopsies were also consistently taken, even if the bladder was endoscopically negative. All samples were sent for a pathological examination.
The treatment schedule consisted of four weekly intravesical instillations of HIVEC™ MMC followed by six instillations administered monthly at a concentration of 40 mg MMC diluted in 50 mL of distilled water. The solution was heated to a target temperature of 43 °C and recirculated at 200 mL per min at stable pressure. The temperature inside the bladder was maintained at 43 °C ± 0.5 °C for the 60-min duration of the treatment. Tolerance data were recorded in a specific format for each patient before each instillation by assessing any side effects from a previous instillation and during the procedure. Any mild and expected side effects similar to instillation of MMC at room temperature were recorded. Side effects were recorded in each patient’s notes for evaluation at the end of the clinical trial and treated following local hospital policy. Any serious and/or unexpected side effects were reported to the principal investigator and appropriate authorities as outlined by the Spanish Agency for Medicines and Health Products. Treatment was discontinued if serious adverse effects or an allergy to MMC were evident, if the patient withdrew consent, or if the doctor felt it was in the interest of the patient.
All patients from both groups were followed for a minimum period of two years with cystoscopy and urine cytology every 3 months and an abdominal-pelvic ultrasound every 6 months. After 2 years of follow-up, a cystoscopy and urine cytology were performed every 6 months and an abdominal-pelvic sonogram every year for a further 3 years. If macroscopic tumour recurrence or positive cytology was detected, the treatment proceeded to a complete TURBT, pathological study and treatment schedule assessed according to local hospital policy and the special features of each case. Safety and efficacy parameters are described in .
HIVEC™ recirculation system
All instillations were conducted with the Combat BRS system V2.0 (Combat Medical, Wheathampstead, UK). The Combat BRS device is a closed, dry, external system that heats the MMC solution and recirculates it in the urinary bladder at a stable pressure, constant temperature of 43 °C ± 0.5 °C, and flow rate of 200 mL/min through a three-way Foley catheter.
A Combat BRS disposable set is composed of four basic elements: an aluminium heat exchanger (placed between the heating plates of the BRS system), temperature probe, tubing and 16F three-way silicone catheter. At the end of each treatment the closed BRS system allows for the removal of the cytotoxic drug into a waste bag supplied. Exposure of both patient and health professional is therefore minimised.
Statistical analysis
For both the neoadjuvant and adjuvant groups, standard statistical outputs were calculated. Continuous variables were summarised with medians and their interquartile range (IQR). Median follow-up time was calculated using a reverse Kaplan-Meier method and tumour recurrence risk using a cumulative incidence function. Confidence intervals (95% CI) for these quantities were calculated using a complementary log transformation.
Results
The ablative and prophylactic groups were assessed for both clinical efficacy and patient safety.
Efficacy: neoadjuvant group
Twenty-four patients received the neoadjuvant HIVEC™ treatment (80 mg of MMC at 43 °C for 60 min, once a week for 8 weeks). Between 7 and 15 days after the HIVEC™ treatment, a TURBT was performed. All visible lesions and the attachment site of the original tumour were biopsied even if they had a normal appearance. In 17 patients a complete elimination of any papillary tumour was observed, although almost all lesion areas had persistent erythema and/or oedema. In the remaining six patients, persistence of tumour lesions was observed but with a > 50% reduction in tumour size. In one case, the reduction was less than 25%.
The pathological study confirmed the absence of a tumour (pT0) in 15 patients, representing a complete response rate (CR) of 62.5%. Eight patients showed tumour persistence but with a reduction in tumour size > 50%, representing a 33.3% partial response (PR). The remaining patient showed little change in tumour load and was considered a non-responder (NR 4.1%). The completely responsive patients showed no remaining tumour cells or any severe tumour cell degeneration with cytoplasmic vacuolisation and nuclear hyperchromia. Peritumoural tissues exhibited inflammatory changes, coagulative necrosis and hyalinisation.
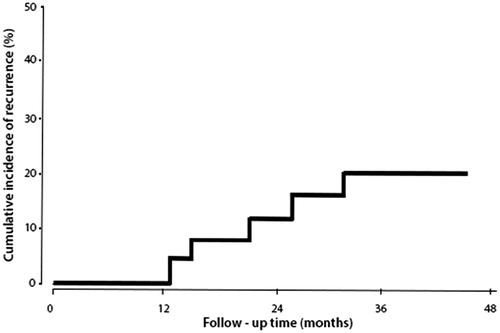
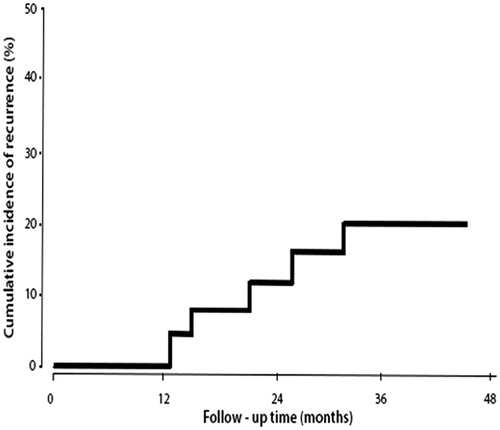
The patients were followed for a median duration of 37 months (95% CI: 12–52 months). During this follow-up period five patients experienced disease recurrence at 3, 15, 19, 27 and 32 months each. All the patients with recurrent tumours had been previously treated with MMC (n = 2) or BCG (n = 3). Of these five patients, three had recurrence at the same stage and grade (n = 2 for T1G3, and n = 1 for T1G3 + CIS). The remaining two patients had an increase in tumour stage and grade from TaG2 to T1G3. These patients were treated conservatively with BCG. The 4-year cumulative incidence of recurrence was 20.8% (95% CI: 4.1–45.3%) (). Four patients died during the follow-up period for reasons unrelated to bladder cancer, which included advanced age (n = 2: 99 and 91 years), acute myocardial infarction (n = 1) and pulmonary embolism (n = 1).
Efficacy: adjuvant group
Sixteen patients underwent a complete TURBT followed by the adjuvant HIVEC™ treatment (four instillations at weekly intervals followed by six instillations monthly, of 40 mg of MMC in 50 mL at 43 °C for 60 min).
The patients were followed for a median duration of 24 months (95% CI: 9–32 months). During the follow-up period, three patients experienced disease recurrence at 7, 15 and 26 months each, and underwent a further TURBT. All the patients experienced recurrence with the same stage and grade (n = 1 for T1G2, n = 1 for T1G3, and n = 1 for CIS,). The first of these patients was re-treated with HIVEC™, and the remaining two were treated with BCG. The 2-year cumulative incidence of recurrence was 12.5% (95% CI: 7.8–19.3%, ). During the follow-up period one patient died of pneumonia and respiratory failure of unknown cause but unrelated to bladder cancer.
Safety: neoadjuvant group
A total of 192 treatments were scheduled. Of these, 184 instillations were completed (95.8%). Eight of the complete treatments were delayed for 1–7 days due to MMC-induced cystitis (n = 4), urinary tract infection (n = 3) and haematuria (n = 1). Seven other instillations ended prematurely because of poor patient tolerance of the treatment resulting in bladder spasms (n = 4) and pelvic pain (n = 3), despite analgesic treatment and/or spasmolytic administration. The observed side effects are presented in and . The most frequent side effects were mild (grade 1) and self-limited (except bladder calcification (n = 1), which required a TURBT to remove the calcifications), de novo urethral stenosis (n = 1), and two cases of bladder contraction which have yet to be fully evaluated and appropriately treated.
Table 3. Side effects according to treatment/patient.
Table 4. Side effects according to treatment/dose.
Safety: adjuvant group
A total of 160 treatments were scheduled. Of these, 158 instillations were completed (98.7%). Of the completed treatments, five were delayed for 1–7 days due to MMC-induced cystitis (n = 2), urinary tract infection (n = 2) and haematuria (n = 1). Three other instillations ended prematurely due to poor tolerance of the treatment by the patients resulting in bladder spasms (n = 2) and pelvic pain (n = 1), despite analgesic and/or spasmolytic treatment. The side effects observed are presented in and . The most frequent side effects were mild (grade 1) and self-limited, with the exception of a de novo urethral stricture which required a direct-vision internal urethrotomy followed by periodic dilatations.
Globally, the incidence and distribution of side effects were slightly lower in the adjuvant group compared to the neoadjuvant group. However, the differences were not statistically significant (p < 0.3). The side effects had virtually no effect on the treatment plan, with an overall 97% completion of the HIVEC™ instillations. A total of 13 instillations (3.8%) had to be delayed for 1–7 days but were subsequently successfully completed without further incident. Another 10 instillations (2.9%) had to be halted prior to the full 60 min. In these cases, 40 min or more had elapsed and were therefore considered effective.
Discussion
The pharmacokinetics of intravesical MMC are mainly related to its low absorption rate, effects of dilution, viscosity [Citation8], urinary pH and exposure time [Citation9]. In fact, less than 30% of the dose is absorbed. In 2001, Paroni et al. [Citation10] observed that hyperthermia produced by microwaves significantly increased MMC absorption after 30, 45 and 60 min (p < 0.008). However, the higher plasma concentrations achieved (67 ng/mL) were six times lower than those needed to be myelosuppressive (approximately 400 ng/mL). Similarly, Milla et al. [Citation11] recently reassessed MMC absorption using recirculating thermo-chemotherapy and observed a significant increase compared to standard normothermic MMC without reaching haematotoxic levels. It is important to understand that hyperthermic MMC is more fully absorbed not only because of the increased permeability of the bladder urothelium but also because of a significant increase in its solubility. At 25 °C, the maximum concentration that can be dissolved is 0.8 mg/mL. This value may reach up to 1.7 mg/mL at 40 °C.
Other groups are combining intravesical chemotherapy with external deep-pelvic hyperthermia, but only a pilot study has been conducted, and more work is necessary to understand its efficacy and safety [Citation12].
Neoadjuvant treatment
Hyperthermia has proven to be more effective than passive intravesical MMC alone for patients with Ta-1 G1–3 NMIBC in comparative studies with ablative and prophylactic treatments. Colombo et al. [Citation13] evaluated the ablative efficacy of neoadjuvant hyperthermia in bladder cancer for the first time in 1998. In that study 19 patients with NMIBC unresectable tumours in a one-stage TURBT in whom a cystectomy was indicated were instead treated with neoadjuvant hyperthermic MMC. After eight doses of hyperthermic MMC per week, a complete TURBT was possible in 16 patients (84%). A histological examination of the specimen demonstrated a tumour absence in 47% of the patients and a > 50% tumour reduction in the other 37%. A cystectomy was performed on the remaining three patients. After an average follow-up of 33 months, eight superficial recurrences were resected without difficulty and without having to remove the bladder.
It is important to note that we do not yet know which hyperthermic regime of MMC is more effective and less toxic. In another study, Colombo et al. [Citation14] compared two groups of 27 patients. Group A received (40 mg weekly for 6 weeks), and group B received (40 mg three times a week for 2 weeks). Of the patients in group B, 7.4% did not complete the treatment due to localised, severe, irritating voiding symptoms. Other side effects were similar in both groups. However, histopathology showed a complete response in 44.4% of patients in group A and 70.4% in group B (p = 0.04)
In 2014 our group [Citation6] reported on a small series of 15 patients treated with eight weekly doses of recirculating neoadjuvant hyperthermic MMC. We achieved a 66.6% complete response rate and 33% partial response rate. As in the previous case, the beneficial effect of HIVEC™ was maintained over time. After 3 years of follow-up, there were only two tumour recurrences (15%); these were treated by a TURBT and an additional adjuvant standard MMC intravesical treatment. The largest study relating to neoadjuvant treatment was presented at the European Congress of Urology (Madrid 2015) by Lüdecke et al. [Citation15]. The study group consisted of 271 patients treated with eight weekly doses of neoadjuvant hyperthermic MMC (40 mg + 40 mg). After a TURBT, 76.1% had a complete response. Another 7.6% showed a partial response. Interestingly, of this group, 80.6% of patients remained free from recurrence after 2 years. It is important to note that this group included 59.8% of patients who had failed BCG. Despite this, the percentages of patients free from recurrence in these groups were 41.7% and 66.7%, respectively, among whom were patients who were already resistant to BCG and those who had had an early relapse after such treatment. It is also important to note that in this study, all the patients who had shown CR were given six further adjuvant treatments of hyperthermic MMC (20 + 20 mg) every 6 weeks for a total of 9 months, which could help patients maintain a high recurrence-free survival rate after 2 years [Citation16].
Adjuvant treatment
A meta-analysis published in 2011 collected a total of 22 studies and noted a 59% reduction in tumour recurrence in a hyperthermic MMC group versus standard MMC [Citation4] at room temperature. Due to the short follow-up, the authors could not draw definitive conclusions about the time to recurrence
Notably, only 0.8% of the patients had tumour progression. Although this figure is lower than that observed with standard MMC, follow-up duration was too short; therefore, no definitive conclusions can be made. In our study, the 2-year cumulative incidence of recurrence was 12.5% (95% CI: 7.8 to 19.3%) with no cases of tumour progression.
The first randomised trial comparing a group of 60 patients treated with hyperthermic MMC using microwave technology versus 72 patients treated with standard BCG was presented by Arends et al. [Citation16] at the European Urology Congress (Madrid 2015). The authors showed how, after 2 years of follow-up, the rate of relapse-free patients was 78% in the hyperthermic group compared to 67% in the BCG group (p = 0.0082).
A recent retrospective study [Citation17] performed with another hyperthermic recirculation system concluded that recirculation of hyperthermic MMC is not as effective as BCG in high-risk NMIBC patients who are BCG naive [Citation18]. Although there was no significant difference in the odds of recurrence, the 2-year recurrence-free interval in the hyperthermic group (76.2%) was lower than in the BCG group (93.9%). There were no differences in progression between hyperthermic MMC and BCG. As the expected recurrence-free rate at 2 years of high-risk NMIBC is 39.9% [Citation1], it seems clear that both treatments, hyperthermic MMC and BCG, are effective in this group of patients. However, BCG treatment has more side effects and more patients are unable to complete the induction period (10.1% BCG (31/306) versus 4.7% with hyperthermic MMC (2/42)). However, this was a retrospective cohort study with an inherent potential for bias, and no subgroup of BCG refractory patients was used for comparison. Both of these factors must be considered when drawing conclusions.
Bladder cancer is the most expensive cancer for public health services to treat from the time of diagnosis to the patient’s death. We evaluated the relationship of cost-effectiveness and budget impact using a neoadjuvant treatment with Combat’s HIVEC™ treatment in patients with NMIBC compared to a standard treatment with BCG and applying values of recurrence and progression risk charts based on internationally accepted values. The expected number of recurrences was reduced from eight to two and progressions from three to zero.
The model was built with published data. The actual costs provide a favourable result for the neoadjuvant Combat HIVEC™ treatment in terms of cost after 3 years, with minimum savings of €687 per patient, demonstrating that neoadjuvant HIVEC™ treatment is a cost-effective therapeutic strategy [Citation19].
Conclusions
Combat’s recirculant HIVEC™ treatment is a safe and effective treatment that appears to be achieving a high level of success in both the neoadjuvant and adjuvant settings. This treatment seems appropriate for NMIBC intermediate–high-risk patients who cannot tolerate or have contraindications for standard BCG therapy or for locations in which there are no BCG supplies.
Disclosure statement
A.S. is a clinical adviser to Combat Medical Ltd and is participating in HIVEC I and HIVEC R studies. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chamie K, Litwin M, Bassett J, Daskivich T, Lai J, Hanley J, et al. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013;119:3219–27.
- Shida K, Shimasaki J, Takahashi H, Kurihara H, Sato J. Therapy and prognosis of bladder tumors – result of injection of mitomycin C into the bladder. Gan No Rinsho 1970;16(7):737–44.
- Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer – indications and limitations. BJU Int 2012;110(Suppl4):12–21.
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol 2011;60:81–93.
- Babjuk M, Burger M, Zigeuner R, Comperat E, Kaasinen E, Palou J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2014. Available at http://www.uroweb.org/guidelines/online-guidelines/
- Sousa A, Inman B, Piñeiro I, Monserrat V, Pérez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. chemotherapy HIVECfor treating and - NMIBC. Int J Hyperthermia 2014;30:166–70.
- Eisenhauera E, Therasseb P, Bogaertsc J, Schwartzd L, Sargente D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Inman BA, Etienne W, Rubin R, Owusu RA, Oliveira TR, Rodriques DB, et al. The impact of temperature and urinary constituents on urine viscosity and its relevance to bladder hyperthermia treatment. Int J Hyperthermia 2013;29:206–10.
- Dalton JT, Wientjes MG, Badalament RA, et al. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res 1991;51:5144–52.
- Paroni R, Salonia A, Lev A, Da Pozzo LF, Cighetti G, Montorsi F, et al. Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol 2001;52:273–8.
- Milla P, Fiorito C, Soria F, Arpicco S, Cattel L, Gontero P. Intravesical thermo-chemotherapy based on conductive heat: A first pharmacokinetic study with mitomycin C in superficial transitional cell carcinoma patients. Cancer Chemother Pharmacol 2014;73:503–9.
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5.
- Colombo R, Da Pozzo LF, Lev A, Salonia A, Rigatti P, Leib Z, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 1998;159:783–7.
- Colombo R, Rocchini L, Suardi N, Benigni F, Colciago G, Bettiga A, et al. Neoadjuvant short-term intensive intravesical mitomycin C regimen compared with weekly schedule for low-grade recurrent non-muscle-invasive bladder cancer: Preliminary results of a randomised phase 2 study. Eur Urol 2012;62(5):797–802.
- Lüdecke G, Schäfer L, Nativ O, Witzsch U, Hanitzsch H, Hasner F, et al. Radiofrequence induced hyperthermia chemotherapy in high-risk NMIBC: Multiinstitutional, international outcome analysis of 271 treated patients with a follow-up time of more than 2 years. Eur Urol Suppl 2015;14:949–949a.
- Arends TJH, Nativ O, Maffezzini M, De Cobelli O, Van Der Heijden AG, Witjes JA. Results of the first randomized controlled trial comparing intravesical radiofrequency induced chemohyperthermia with mitomycin-C versus BCG for adjuvant treatment of patients with intermediate and high risk NMIBC. Eur Urol Suppl 2015;14:944
- Ekin RG, Akarken I, Cakmak O, Tarhan H, Celik O, Ilbey YO, et al. Results of intravesical chemo-hyperthermia in high-risk non-muscle invasive bladder cancer. Asian Pac J Cancer Prev 2015;16:3241–5.
- Ekin RG, Akarken I, Zorlu F, Tarhan H, Kucuk U, Yildirim Z, et al. Intravesical bacillus Calmette-Guérin versus chemohyperthermia for high-risk non-muscle-invasive bladder cancer. Can Urol Assoc J 2015;9:278–83.
- Sousa A, Piñeiro I, Aparici V, Neira P, Monserrat V, Uribarri C. Analysis of budgetary impact of nonmuscle invasive vesical cancer of moderate–high risk by means of neoadjuvant hyperthermic chemotherapy compared to the standard adjuvant treatment with BCG. Arh Esp Urol 2015;68:482–92.