Abstract
Purpose: This study was designed to determine the safety, effectiveness and feasibility of contrast-enhanced ultrasound (CEUS)-guided percutaneous microwave ablation (MWA) of renal cell carcinoma (RCC) that is inconspicuous on conventional ultrasound (US).
Materials and methods: A total of 32 RCC nodules in 29 patients (23 men and 6 women) were treated with CEUS-guided percutaneous MWA between January 2010 and September 2014. The median maximum diameter of the nodules was 2.4 cm (interquartile range: 1.8–2.9 cm). The US contrast agent was SonoVue, a second-generation contrast agent. CEUS was applied before the needle was inserted into the tumour, and percutaneous MWA was performed under CEUS-guidance.
Results: In total 31 tumours were successfully visualised via CEUS using 1–2 (1.0–2.0 mL) contrast agent injections, and percutaneous MWA was performed under CEUS-guidance. The technical success rate of CEUS-guided percutaneous MWA of RCC was 96.9% (31/32). The mean number of sessions of CEUS-guided percutaneous MWA for each tumour was 1.2 ± 0.4. The mean duration of energy application for each tumour was 7.3 ± 2.7 min. All patients were followed up for 3–71 months (median 17 months) to observe the therapeutic effects and complications. The therapeutic effects were assessed at follow-up with computed tomography (CT) or magnetic resonance imaging (MRI) and CEUS. There was no local tumour progression and the technique effectiveness rate was 100% (31/31). The complications rate was 6.5% and the major complications rate was 3.2%. We observed one case of pleural effusion and one case of renal subcapsular haemorrhage after the percutaneous MWA procedures.
Conclusion: CEUS-guided percutaneous MWA is a safe, efficient and feasible therapy for patients with RCCs inconspicuous on conventional US.
Introduction
Renal cell carcinoma (RCC) accounts for 3% of malignancies and 80–90% of all malignant renal tumours in adults [Citation1]. Radical nephrectomy and nephron-sparing surgery are now considered to be the standard treatments for early stage kidney cancer (tumour diameter ≤4 cm) [Citation2]. However, there are multiple advantages of percutaneous ablation, including being minimally invasive, decreased operative time, shorter hospital stay, lack of inherent surgery risks and protection of renal function. Thus, percutaneous ablation has become an effective and safe option for kidney tumour treatment [Citation3]. Currently, ablation technologies include cryoablation, radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU) and microwave ablation (MWA). Image-guided percutaneous MWA has been effectively and safely applied to treat renal tumours in select patients [Citation3–6].
Accurate delineation for tumours is mandatory to successfully apply percutaneous ablation, and CEUS is well accepted and used to detect and characterise various tumours because of its capacity to emphasise the macro- and micro-vascularisation of various parenchyma and tumour tissues [Citation7]. Moreover, CEUS can delineate the avascular, necrotic areas from the viable, active, vascularised regions of the tumour [Citation8]. CEUS-guidance has been widely applied during biopsies such as in the liver, lung, lymph nodes, neck lesions and other locations, helping with accurate needle placement [Citation9–11]. In addition, CEUS has been extensively utilised for liver tumours that are undetectable via US [Citation12]. Recently this technique has been reported for the treatment of RCCs, and positive clinical results have been achieved in several reports [Citation13–15]. However, the existing studies have not focused on tumours that are inconspicuous on US or located near to important tissues. Thus, tumours that were inconspicuous on conventional US in US-guided MWA remain a challenge. Our study evaluated the safety, effectiveness, and feasibility of CEUS-guided percutaneous MWA with SonoVue for the treatment of RCCs that were undetected on conventional greyscale US.
Materials and methods
Patients
From January 2010 to September 2014, 29 patients (23 men and 6 women, with a mean age of 66.3 years, SD ±11.9, range 40–84 years) with 32 renal tumours were admitted and underwent CEUS-guided percutaneous MWA treatment at our department (). Of those 29 patients, five had a solitary kidney, and one had a transplanted kidney. The median maximum diameter of the nodules was 2.4 cm (interquartile range 1.8–2.9 cm). Among those tumours 14 were residual tumours and 18 were new lesions. Eleven patients with residual tumours underwent percutaneous MWA 2–3 days before the CEUS guidance. Three patients underwent other forms of treatment, including transcatheter arterial chemoembolisation (TACE) (9 months prior), cryoablation (1 month prior) (), and HIFU (5 months prior).
Figure 1. Images of a 77-year-old man who had CEUS-guided percutaneous MWA of RCC performed after cryoablation. (A) Contrast-enhanced MRI shows a residual tumour (white arrows) around the necrotic tissue (arrowheads) in the left kidney. (B) Residual tumour was undetectable on conventional ultrasound, while the CEUS cortical phase image shows a 6.0 × 4.1 cm tumour in the left kidney (arrows) and there was no enhancement in the necrosis area in the centre of the whole tumour. (C) Enhancement of the tumour in cortical phase (arrow) was not showed in contrast-enhanced MRI 11 months after treatment. (D) Cortical phase of CEUS obtained 6 months after CEUS-guided percutaneous MWA shows complete necrosis of the tumour (arrows).
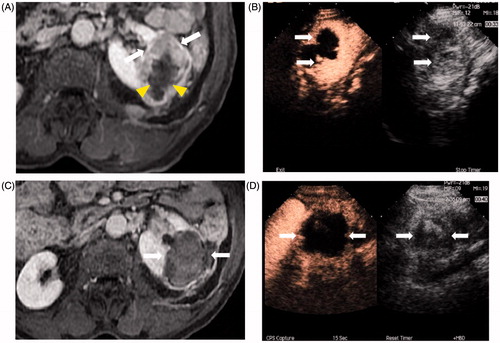
Table 1. Characteristics of patients who had CEUS-guided PMWA.
The interventional radiologists (IRs) graded the conspicuity score with the following 4-point scale before CEUS was performed: 1 = highly identifiable, 2 = identifiable, 3 = likely identifiable, but less confident, and 4 = definitely unidentifiable [Citation16]. The inclusion criteria for this study were as follows. 1) All nodules had a conspicuity score of 3 or 4, and were detectable on contrast-enhanced CT or gadolinium-enhanced MRI and CEUS. 2) The boundary of the tumour(s) and the relationship between the tumour and renal tissue was displayed clearly on CEUS and contrast-enhanced CT/MRI. 3) All of the nodules were diagnosed by CEUS-guided biopsy before CEUS-guided percutaneous MWA. 4) All patients with RCC who refused to undergo surgical resection or who were unsuitable for surgery, or patients with tumours larger than 4 cm were usually not included unless their co-morbidities made them poor candidates for surgery.
Planning US, CEUS and contrast-enhanced CT/MRI were performed prior to the percutaneous MWA procedure in all patients to determine the feasibility of CEUS-guided percutaneous MWA and to evaluate the tumour characteristics, including tumour size, location, blood supply and boundaries. Each participant signed an informed consent form before participating in the study. The study protocol was approved by our hospital’s ethics committee.
Technical equipment
US and CEUS were performed using Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA) with a micro-convex probe (4V1, 2.0–4.5 MHz). The microwave unit (KY-2000; Kangyou Medical, Nanjing, China), which consists of a microwave generator, flexible coaxial cable, water-pump and cool-tip needle antenna, is capable of producing 1–100 W of power at 2450 MHz. The needle antenna has a diameter of 1.9 mm (15-gauge, 1.1-cm antenna tip) and a length of 18 cm. The antennae are coated with Teflon, which is used to prevent adhesion. An automatic biopsy gun with an 18-gauge cutting needle was used to perform the biopsy.
CEUS-guided percutaneous MWA procedure
Patients were placed in a position dependent on the tumour size and location. Two IRs (P.L. and X.L.Y., with 15 years of experience each in MWA) performed the CEUS, biopsy, and percutaneous MWA.
Guided by pre-op contrast-enhanced CT/MRI, the ultrasound probe was placed in the upper, middle or lower pole of the kidney. Then contrast-specific software was used. Next, the IRs adjusted the Mechanical Index (MI) to 0.12–0.18 before CEUS was performed. The US contrast-enhancement agent was SonoVue (Bracco, Milan, Italy). Two doses of 1.0 mL were aspirated from a vial of contrast-enhanced agent. The first dose was injected for the pre-procedural planning during CEUS, and the second dose was used for the CEUS-guided percutaneous MWA. The CEUS agents were shaken well before they were administered intravenously over a period of 2–3 s, followed by one 5-mL bolus of normal saline solution.
Immediately after the injection the IRs searched for and located the area of the target tumour and recorded the enhancement pattern and characteristics of the tumour, which was hyper-enhancement in the cortical phase and hypo-enhancement in the medullar enhancement at the kidney region. Then the target tumour was treated.
If no target tumours were identified within 5 min, CEUS was repeated with another dose to search for the predetermined target or any other potential targets within the kidney. Percutaneous MWA was not performed if the location was not identifiable during the second CEUS. This situation was defined as a CEUS failure. During CEUS the IRs planned the treatment protocol according to the CEUS information. Technical success was defined as tumour visualisation via CEUS, and the MWA procedure was performed under CEUS-guidance.
After CEUS and designing the treatment protocol, patients were sedated with a combination of propofol (Diprivan, Zeneca Pharmaceuticals, Wilmington, DE) and ketamine (Shuanghe Pharmaceuticals, Beijing) via the peripheral vein according to the patient’s weight and height. Before the biopsy needle and antennas were placed, local anaesthesia was administered by injecting 10 mL of 1% lidocaine through the skin into the peritoneum along a predetermined puncture line. If tumours were adjacent to the colon, MWA was performed under CEUS guidance assisted by the artificial ascites technique to prevent colon injury [Citation17]. Saline (500 mL) was infused into the peritoneal cavity, Abdominocentesis was performed with a 16-gauge trocar. During treatment the drip infusion continued to maintain a distance of at least 5 mm between the ablation zone and adjacent organs [Citation4,Citation13].
Biopsy was necessary for all first-time patients who lacked a pathological diagnosis. Following biopsy, the antenna was percutaneously inserted into the tumour along the same needle-path and placed at the desired location. The MWA procedure was performed immediately after the biopsy procedure. Both the biopsy and percutaneous MWA were guided by CEUS. A power output of 50 W (5 min) was routinely used during the procedure. Prolonged microwave emission was applied until the heat-generated hyper-echoic water vapour encompassed the entire tumour. When US showed that the strong echo completely covered the target lesions, reaching the expected treatment range, the treatment was stopped. After the procedure CEUS was performed immediately to confirm that the tumour was completely destroyed. Additional treatments were needed if contrast agent (in or around the tumour) was present. The needle paths were coagulated when the ablation needle was removed to prevent bleeding. The heart rate, electrocardiographic tracing, oxygen saturation, and respiratory rate were continuously monitored, and blood pressure was measured every 5 min during the procedure [Citation18].
Results
Of the 32 tumours, 31 tumours were successfully visualised via CEUS using 1–2 (1.0–2.0 mL) contrast agent injections and one tumour could not be identified. The CEUS success rate was 96.9% (31/32). Of the 31 lesions, 20 nodules were assessed as scale 3, and 11 nodules were assessed as scale 4 and were hyper-enhanced nodules in the cortical phase. All of the CEUS-guided ablated tumours were diagnosed as renal clear cell carcinoma by pathology. The antenna was correctly placed in all lesions, and the percutaneous MWA procedure was performed. The technical success was 100% (31/31). Twenty-five tumours were completely ablated in one session, and six tumours were completely ablated in two sessions. Among the residual lesions, the technique in 13 residual tumours was considered to be effective in one session, and one tumour received additional percutaneous MWA. Among the new lesions, 12 were considered to be completely ablated in one session, and five lesions received additional percutaneous MWA focused on suspicious residue. CEUS-guidance was also applied during the additional MWA. The mean number of CEUS-guided percutaneous MWA sessions for each tumour was 1.2 ± 0.4. The overall mean duration of energy application for each tumour was 7.3 ± 2.7 min (range 4–11 min). In our study, eight lesions were adjacent to the pelvis (), and one lesion was adjacent to the gastrointestinal tract. After one or two sessions of percutaneous MWA (2–3 days after the final ablation), CEUS and contrast-enhanced CT/MRI were performed to assess the procedural effectiveness. All of the tumours were ablated completely and safely.
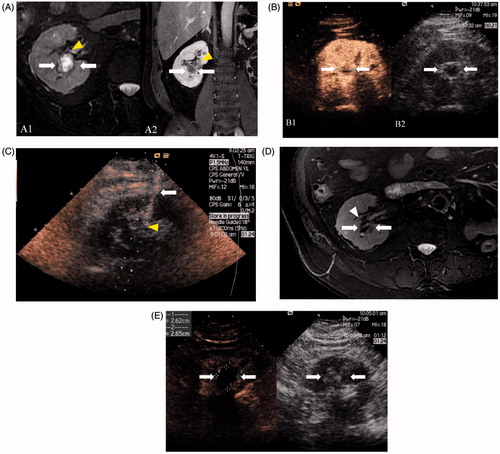
Figure 2. Images in a 68-year-old man with 1.9 × 1.7 cm RCC treated by CEUS-guided percutaneous MWA. (A) MRI shows a complex cystic mass (arrows) adjacent to the pelvis (arrowheads) in the right kidney in T2 and contrast-enhanced MRI shows an enhancement in the tumour solid part (arrows). (B) The tumour boundary and solid part were inconspicuous on conventional US while the CEUS cortical phase image shows a 1.9 × 1.7 cm tumour in the right kidney (arrows) and the tumour boundary was confidently detectable on CEUS. (C) Microwave antenna (arrow) was inserted into the lesion (arrowheads) accurately under CEUS guidance. (D) MRI obtained 14 months after CEUS-guided percutaneous MWA shows complete necrosis of the tumour (white arrows) and pelvis (arrowhead) was not injured. (E) Cortical phase of CEUS obtained 14 months after CEUS-guided percutaneous MWA shows complete necrosis of the tumour (arrow).
The post-operative follow-up period was 3–71 months (median 17 months). The technique was effective in 31 nodules during one or two CEUS-guided percutaneous MWA sessions, and the effectiveness was confirmed by contrast-enhanced imaging 1 month after ablation. Technique effectiveness was defined as tumour visualisation via CEUS and ‘complete ablation’ of the macroscopic tumour as demonstrated by contrast enhanced imaging 1 month after ablation [Citation12]. None of the nodules showed enhancement on CEUS and contrast-enhanced CT/MRI after CEUS-guided percutaneous MWA. The local tumour control rate was 100%. Neck lymph node metastases were observed in one patient during the follow-up US examination, and this patient was treated with US-guided radioactive I125 seed implantation. None of the patients died of RCC recurrence or metastasis.
Routine urine and blood tests were performed within 24 h after ablation. There were no significant differences in the creatinine levels or blood examinations, before or after CEUS-guided percutaneous MWA. The complication rate was 6.5%. Two percutaneous MWA-related complications occurred after treatment. Renal subcapsular haemorrhage was found in one case within 1 day after MWA, and the patient recovered 2 days after medical treatment. One major complication occurred during this study. Pleural effusion occurred in one patient 5 days after MWA, and the symptoms remitted one day after thoracocentesis. The major complications rate was 3.2% (SIR classifications C–E) [Citation19].
Follow up
According to the standardisation of ablation terminology and reporting [Citation19], the technique effectiveness (namely complete ablation) and local tumour progression were assessed. When complete ablation was achieved, routine CEUS and contrast-enhanced CT/MRI were performed to monitor for recurrence or metastasis at 1, 3, 6 and 12 months after MWA (and every 6 months thereafter). All patients were closely monitored for possible complications, including pain, haematuria, infection, and hydrothorax.
Patients with local tumour progression were given the option of additional ablation if they could tolerate it. Patients with additionally ablated tumours were followed up at the intervals defined above. Complications were defined as major or minor according to the SIR classifications.
Discussion
CEUS-guidance facilitates the treatment of tumours that are inconspicuous on conventional US. US invisibility was the main limiting factors for the performance of US-guided thermal ablation of small renal masses, followed by lesion size and steam bubbles [Citation20]. In a comparison study the foremost exclusion criterion for US-guidance was the inability to visualize the lesion [Citation21]. Because of the low resolution of the greyscale between the kidney tissue and isoechoic tumours and the low sensitivity for smaller arteries and arterioles, some small isoechoic nodules cannot be visualised on conventional US [Citation22,Citation23], especially in the deep sections of the renal medulla. It has been reported that US is inferior to CT for the detection of small RCCs [Citation24]. US detected only 70% of RCCs less than 2 cm in diameter, whereas CT detected 95% in a study of 20 patients with kidney tumours.
After the use of various forms of therapies, it can be increasingly difficult to identify the residual tumour with a heterogeneous echotexture coagulation zone by US. In addition, errors in the tumour measurements and the definitions of the tumour margins were generated by US because the indistinct boundaries of infiltrative tumours cannot be determined by conventional US. Therefore, the greater difficulty in treating centrally located lesions could have been considered as a limit of US-guidance in thermal ablation of renal tumours. In a comparison study between US-guided and CT- or MRI-guided ablation, Veltri considered exophytic growth of the lesion to be the only statistically significant predictive factor of possible correlations between complications and percutaneous thermal ablation factors [Citation21]. All of the above limitations make it more difficult to find indiscernible tumours, and they cannot be ablated completely by US-guided ablation. There is also an increased risk of complication.
RCC nodules demonstrated excellent hyper-enhancement in the cortical phase and hypo-enhancement during medullar enhancement on CEUS in our study. Due to the excellent labelling of microvessels during CEUS, the iso-echoic, small RCCs and complex residual tumours, even hypo-vascular tumours, can be differentiated [Citation25]. CEUS is an effective alternative to CT and MRI for the follow-up of renal tumours managed with percutaneous thermal ablation [Citation26]. With the wide application of CEUS, CEUS-guidance is now an option for treating and diagnosing previously invisible HCC tumours [Citation12,Citation27]. From our retrospective study, CEUS-guidance seems to increase indications for the percutaneous MWA treatment of RCCs. In our study, 18 lesions that were missed in conventional US and 14 complex residual tumours that persisted after a series of treatments were identified and treated by CEUS-guided ablation once or repeatedly. The ‘CEUS success rate’ was 96.3%. The technical success and technique effectiveness rates were 100% in our study. Our results seem to be satisfactory compared to the previous study by Carrafiello et al., in which CEUS with SonoVue was utilized for the percutaneous MWA of RCCs [Citation13]. Zhao et al. demonstrated that the accuracy of needle placement in the percutaneous ablation of RCCs increased from 86.1% to 94.6% in a comparative study between US-guided and CEUS-guided percutaneous MWA [Citation14]. However, those studies did not focus on tumours that were inconspicuous in conventional US. The major complications rate of one US-guided percutaneous RFA study was 4.6% [Citation21]. Renal collecting system injuries and colon injuries are the common major complications of renal thermal ablation. Veltri described a patient who developed a renal pelvis leak due to thermal damage mainly due to the lesion location [Citation21]. In our study, tumours located near the kidney, pelvis or colon were ablated completely under CEUS-guidance. One patient had one lesion adjacent to the gastrointestinal tract. In this case, for lesions adjacent to the gastrointestinal tract, percutaneous MWA under CEUS-guidance assisted by artificial ascites effusion was required to obtain a safe puncture tract. Both clearer images of the entire tumour and a safer puncture tract were obtained by using artificial pleural effusion. This technique protects against serious complications such as intraperitoneal haemorrhage and intestinal injury. In this study we mainly analysed the value of CEUS-guided percutaneous MWA for the tumours that were inconspicuous on conventional US and were located near to the important organs.
In retrospective studies, the technical success rate of CT-guided RFA for RCCs ranges from 91–100%. In addition, the major complication and local tumour control rates range from 0–12% and 91–93%, respectively [Citation28–33]. In a large sample study of CT-guided percutaneous thermal ablation of RCCs, the major complication rate was 5.2%, including seven ureteric strictures (3.5%) and one calyceal cutaneous fistula (0.5%) among 210 sessions. Collection system damage is also common even with CT-guidance [Citation34]. We achieved the same complications rate with a higher technique-effectiveness rate because CEUS guidance has an advantage over real-time imaging. Immediately after the injection, the target tumour was detected by continuously sweeping across the kidney tissue, and the location of the lesion was displayed on MRI or CT. Our results were similar to the outcomes of another previously published study regarding US-guided percutaneous MWA for RCCs in our department [Citation4]. However, CEUS-guidance provides a new treatment method for tumours that are unidentifiable on conventional US or are located near to important organs.
This study had two main limitations. First, the study had a small patient sample. A larger sample is needed to further confirm the value of CEUS-guided percutaneous MWA for inconspicuous RCCs via US imaging. Second, this was a retrospective study that lacked control groups. We did not compare the results of CEUS-guided percutaneous MWA with CT/MRI-guided percutaneous MWA.
Conclusions
CEUS-guided percutaneous MWA is an efficient, feasible treatment method for patients with inconspicuous RCCs according to conventional US. CEUS-guided percutaneous MWA protects important tissues or organs near tumours and increase the indications for percutaneous MWA for the treatment of RCCs.
Disclosure statement
This paper is supported by the National Scientific Foundation Committee of China (grant no. 81127006 and 81071210) and the International Cooperation Plan of National Science and Technology Department of China (grant no. 2012DFG32070). The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
- Vander Eeckt K, Joniau S, Van Poppel H. Open surgery for localized RCC. Sci World J 2007;7:742–52.
- Horn JC, Patel RS, Kim E, Nowakowski FS, Lookstein RA, Fischman AM. Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: Technique and initial results. J Vasc Intervent Radiol 2014;25:448–53.
- Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Mu MJ, et al. Us-guided percutaneous microwave ablation of renal cell carcinoma: Intermediate-term results. Radiology 2012;263:900–8.
- Liang P, Wang Y, Zhang DK, Yu XL, Gao YY, Ni XX. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urol 2008;180:844–8.
- Moreland AJ, Ziemlewicz TJ, Best SL, Hinshaw JL, Lubner MG, Alexander ML, et al. High-powered microwave ablation of T1a renal cell carcinoma: Safety and initial clinical evaluation. J Endourol 2014;28:1046–52.
- Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33–59.
- Spârchez Z, Radu P, Zaharia T, Kacso G, Grigorescu I, Badea R. Contrast enhanced ultrasound guidance: A new tool to improve accuracy in percutaneous biopsies. Med Ultrason 2010;12:133–8.
- Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH. Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 2010;20:2047–56.
- Di Vece F, Tombesi P, Ermili F, Sartori S. Contrast-enhanced ultrasound (CEUS) and CEUS-guided biopsy in the diagnosis of lung abscess in a patient with achalasia: Case report. Interv Med Appl Sci 2013;5:31–3.
- Spârchez Z, Radu P, Kacso G, Eniu D, Hica S, Sparchez M. Contrast-enhanced ultrasound guided biopsy of superficial toraco-abdominal and neck lesions. Initial experience in 20 patients. Med Ultrason 2012;14:288–93.
- Liu FY, Yu XL, Liang P, Cheng ZG, Han ZG, Dong BW. Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia 2011;27:555–62.
- Carrafiello G, Mangini M, Fontana F, Recaldini C, Piacentino F, Pellegrino C, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: Preliminary experience. Cardiovasc Intervent Radiol 2010;33:367–74.
- Zhao X, Wang W, Zhang S, Liu J, Zhang F, Ji C, Li X, et al. Improved outcome of percutaneous radiofrequency ablation in renal cell carcinoma: A retrospective study of intraoperative contrast-enhanced ultrasonography in 73 patients. Abdom Imaging 2012;37:885–91.
- Lackey L Jr, Peterson C, Barr RG. Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. Ultrasound Q 2012;28:269–74.
- Kim AY, Lee MW, Rhim H, Cha DI, Choi D, Kim YS, et al. Pretreatment evaluation with contrast-enhanced ultrasonography for percutaneous radiofrequency ablation of hepatocellular carcinomas with poor conspicuity on conventional ultrasonography. Korean J Radiol 2013;14:754–63
- Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41.
- Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Zhang X, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: Intermediate-term results. Radiology 2014;270:880–7.
- Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol 2014;25:1691–705.
- Park BK, Kim CK, Choi HY, Lee HM, Jeon SS, Seo SI, et al. Limitation for performing ultrasound-guided radiofrequency ablation of small renal masses. Eur J Radiol 2010;75:248–52.
- Veltri A, Garetto I, Pagano E, Tosetti I, Sacchetto P, Fava C. Percutaneous RF thermal ablation of renal tumors: Is US guidance really less favorable than other imaging guidance techniques? Cardiovasc Intervent Radiol 2009;32:76–85.
- Forman HP, Middleton WD, Melson GL, McClennan BL. Hyperechoic renal cell carcinomas: Increase in detection at US. Radiology 1993;188:431–4.
- Yamashita Y1, Takahashi M, Watanabe O, Yoshimatsu S, Ueno S, Ishimaru S, et al. Small renal cell carcinoma: Pathologic and radiologic correlation. Radiology 1992;184:493–8.
- Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (≤3 cm) renal masses: Detection with CT versus US and pathologic correlation. Radiology 1996;198:785–8.
- Tamai H, Takiguchi Y, Oka M, Shingaki N, Enomoto S, Shiraki T, et al. Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. J Ultrasound Med 2005;24:1635–40.
- Meloni MF, Bertolotto M, Alberzoni C, Lazzaroni S, Filice C, Livraghi T, et al. Follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: Contrast-enhanced sonography versus contrast-enhanced CT or MRI. Am J Roentgenol 2008;191:1233–8.
- Lorentzen T, Skjoldbye BO, Nolsoe CP. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: Experience with 125 metastases in 39 patients. Ultraschall Med 2011;32:492–6.
- Varkarakis IM, Allaf ME, Inagaki T, Bhayani SB, Chan DY, Su LM, et al. Percutaneous radio frequency ablation of renal masses: Results at a 2-year mean followup. J Urol 2005;174:456–60.
- Arzola J, Baughman SM, Hernandez J, Bishoff JT. Computed tomography-guided, resistance-based, percutaneous radiofrequency ablation of renal malignancies under conscious sedation at two years of follow-up. Urology 2006;68:983–7.
- Zagoria RJ, Hawkins AD, Clark PE, Hall MC, Matlaga BR, Dyer RB, et al. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: Factors influencing success. Am J Roentgenol 2004;183:201–7.
- Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol 2007;189:429–36.
- Sommer CM, Lemm G, Hohenstein E, Bellemann N, Stampfl U, Goezen AS, et al. CT-guided bipolar and multipolar radiofrequency ablation (RF ablation) of renal cell carcinoma: Specific technical aspects and clinical results. Cardiovasc Intervent Radiol 2013;36:731–7.
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: Clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 2003;226:417–24.
- Wah TM, Irving HC, Gregory W, Cartledge J, Joyce AD, Selby PJ. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): Experience in 200 tumours. BJU Int 2014;113:416–28.
