Abstract
Objective: To identify the types, frequency and impact of asthma triggers and the relationship to asthma control among adults with asthma in Europe. Methods: Adults with self-reported physician-diagnosed asthma receiving maintenance asthma treatment and self-reported exposure to known asthma triggers completed an online questionnaire; a subset completed a diary over 3–4 weeks. Information on asthma control (Asthma Control Test™ [ACT]), asthma triggers, frequency of exposure and behaviours in response or to avoid asthma triggers and the perceived impact on daily life was captured. A post-hoc analysis evaluated the impact of high trigger burden on the frequency of severe asthma exacerbations, hospitalisations and days lost at work/study. Results: A total of 1202 adults participated and 177 completed the diary. Asthma was uncontrolled for the majority (76%) of participants and most (52%) reported exposure to 6–15 asthma triggers. As trigger burden increased, behavioural changes to manage trigger exposure had a significantly increased impact on daily life (p < 0.0001) and job choice (p = 0.002). Participants reporting a high trigger burden (>16) were more likely to report uncontrolled asthma than those with a low trigger burden (1–5). Participants with a high trigger burden had previously experienced on average two more severe asthma attacks during a lifetime (p < 0.001), two more hospitalisations (p < 0.001) and 3.5 more missed days at work or study in the last year due to their asthma (p < 0.001) than those with a low trigger burden. Conclusions: Adults with asthma reporting a high trigger burden (>16 different triggers) experience more severe asthma attacks than those reporting lower trigger burdens.
Introduction
The ultimate aim of asthma treatment is to achieve and maintain clinical control. Asthma control is characterised by the management of symptoms and reduction of predicted future risk, particularly exacerbations [Citation1]. Some asthma exacerbations can be caused by a range of risk factors and irritants, including exercise, allergens, pollutants and occupational exposures, viral infections, medications and other factors such as emotional stress or co-existing medical conditions [Citation1,Citation2]. These risk factors can be collectively referred to as asthma ‘triggers’.
Ongoing exposure to asthma triggers is often a factor in poorly controlled asthma [Citation1,Citation3–7]. Asthma management guidelines and educational materials recommend that healthcare professionals educate patients to identify those triggers that worsen asthma symptoms and lead to loss of control, and take steps to avoid these triggers [Citation1,Citation8–11]. However, there is mixed evidence whether the monitoring and provision of advice, and discussion of environmental control strategies with asthma patients is adequate [Citation12] or suboptimal [Citation12,Citation13]. The reasons why education on trigger identification and avoidance may be suboptimal are unclear but may relate to a perception that little can be done about asthma triggers or that consultations focus on direct clinical management. Another potential reason could be a lack of understanding of what an individual patient considers to be a trigger and the total trigger burden for that patient. Therefore, there is a clear need to improve understanding of the types of triggers that affect asthma patients, how often triggers are encountered, and how the overall burden of asthma triggers impacts disease control.
The current epidemiological study was conducted to identify the types, frequency and impact of asthma triggers, and to investigate the relationship between asthma trigger burden (number and frequency) and asthma control among adults with asthma across five European countries.
Methods
Study design
This was a questionnaire- and diary-based study designed to investigate the type, frequency and impact of asthma triggers among adults with asthma. The study was conducted in five European countries (France, Germany, Italy, Spain and the UK) between November and December 2010. Participants were recruited through a large consumer online panel. Members of the panel were sent an invitation to take part in the survey via an email containing a link to the online questionnaire. The questionnaire contained a series of screening questions and only eligible participants completed the main questionnaire (approximately 25 min in duration). A random subset of participants who completed the questionnaire and who reported that specific events or activities triggered a worsening of their asthma were then asked to complete an online diary at least every 2 d over 3–4 weeks.
Participants
Eligible participants were adults with a physician diagnosis of asthma (self-reported) and currently receiving maintenance asthma treatment and self-reported exposure to known asthma triggers. Eligibility was determined using a series of online screening questions and only those meeting the inclusion criteria proceeded to the online questionnaire. Patients were advised in the email invitation that accessing the online questionnaire provided their consent to partake in the study.
Online survey
The survey was developed based on qualitative research and expert opinion and was designed to describe and quantify common triggers for patients with asthma, understand and quantify coping behaviours in the presence of asthma triggers, and to understand and quantify how patients modify their lifestyles in response to triggers, and the impact this lifestyle modification has on their everyday quality of life. Participants were also asked about the frequency of severe asthma attacks, asthma worsening and use of reliever medication. Participants were then asked to indicate how well controlled they perceived their asthma to be on a scale of 0–10 (0 = very poorly controlled and 10 = completely controlled).
As part of the survey participants completed the Asthma Control Test (ACT) [Citation14]. The previously defined cut-off score of 20 was used to identify participants as having well controlled (ACT score ≥20) or uncontrolled asthma (ACT score <20).
Asthma control was also evaluated in relation to the Global Initiative for Asthma (GINA) guidelines [Citation1]. The GINA guidelines recommend evaluation of asthma control in relation to five characteristics: daytime symptoms, limitation of activities, nocturnal symptoms/awakenings, need for reliever/rescue medication and lung function. Participants were asked to report on the occurrence of day- and night-time asthma symptoms and the frequency with which these affected their planned activities as well as the frequency of rescue medication use in the previous week. Lung function data was not available so the remaining four characteristics were used as a proxy to define the level of asthma control as follows: (1) controlled: daytime symptoms no more than twice a week, no night-time symptoms, no limitation to activities in the previous four weeks and rescue medication use no more than once a week; (2) poorly controlled: daytime symptoms more than twice a week or night-time symptoms or limitation to activities in the previous four weeks or rescue medication use more than once a week; and (3) uncontrolled: three or more features of poorly controlled asthma.
Participants were presented with a list of 36 potential asthma triggers and asked to indicate which had ever resulted in a worsening of their asthma. The list of 36 potential asthma triggers was developed based on qualitative interviews conducted with patients with asthma.
The perceived impact of asthma on the daily lives of participants was derived from the question ‘Overall, how much of an impact do you think asthma has had on your life and the things you are able to do?’. Participants were asked to respond on a Likert-type scale of 1 (very mild) to 10 (very severe).
Participant diary
The diary was developed based on qualitative research. The purpose of the diary was to understand the impact of asthma triggers over a period of time. A random subset of participants were recruited after completing the main online survey. At the end of the survey participants were asked if they wanted to take part in the diary part of the research and an additional online link was then sent to them. Participants were asked to complete online diaries every other day for three weeks. The diaries specifically elicited information on daily exposure to asthma triggers, the impact of exposure to asthma triggers on asthma symptoms, coping strategies and behaviours in relation to exposure to asthma triggers, as well as the impact of these strategies and behaviours on work and home activities.
Statistical analysis
To investigate the relationship between asthma trigger burden (number and frequency) and asthma control an asthma trigger frequency index (ranging from 0 to 1) was calculated using the annual self-reported trigger exposure for all asthma triggers for each individual participant. A cut off of 0.5 was used to stratify patients into high trigger frequency >0.5 and low trigger frequency (≤0.5). Participants were also stratified according to the level of asthma control (ACT or GINA) and their self-reported asthma trigger burden (number of triggers identified by the patient as causing asthma symptoms): very low (1–5), low (6–10), medium (11–15) and high (≥16). Statistically significant differences between groups in relation to trigger burden, level of asthma control and behavioural changes in relation to asthma triggers were determined using Chi-square testing and analysis of variance (Kruskal–Wallis).
Descriptive statistics and multivariate regression analyses are presented on risk factors associated with differences in the mean number of severe attacks in a lifetime, the annual number of times individuals reported hospitalisation in the last year and the annual number of days missed at work or study in the last year due to asthma.
A post hoc, investigational regression analysis was also undertaken in order to examine the impact of a variety of potential covariates on socioeconomic outcomes (Poisson regression), impact of daily life and activities (linear regression) and the number of behavioural changes patients made in order to manage their exposure to known asthma triggers (logistic regression). The following covariates of interest were included in all analyses:
Trigger burden: very low (reference), low, medium, high
Age group: 18–30 years (reference), 31–44 years and >45 years
Dwelling: rural (reference), urban
Country: Germany (reference), UK, Spain, France, Italy
Gender: male (reference), female
Self-reported physician-defined asthma severity: mild (reference), moderate, severe, not informed
Results are reported with 95% confidence intervals.
Results
A total of 1202 adults with asthma were considered eligible to participate in this study and completed the online survey (). Of these, 177 also completed the online diary and provided data on a total of 1947 d.
Table 1. Demographics and disease characteristics.
Asthma control
Asthma was not well controlled for the majority of the 1202 participants. Most (85%) reported experiencing daytime symptoms and 79% reported experiencing night-time symptoms at least once in the past 7 d. In all, 53% of participants indicated that they experienced a worsening of their asthma symptoms at least once a week. The majority of participants (76%) were classed as uncontrolled using the ACT score, while 92% of participants were described as partly controlled or uncontrolled according to the GINA guidelines.
Incidence and frequency of asthma triggers
The number of self-reported asthma triggers varied considerably between participants, ranging from 1 to 5 (13% of participants) to ≥16 (35%); 52% of participants reported exposure to between 6 and 15 triggers. The most common asthma triggers as reported by participants were dust or dusting (72%), colds, flu, infections or sinusitis (69%), coughing (68%), tobacco smoking (60%) and smog or air pollution (58%) ().
Table 2. Self-reported asthma triggers as ranked by the proportion of participants who have ever experienced the trigger and by how frequently the trigger was reported.
Dust and dusting were the most commonly reported triggers and the most frequently experienced (). Colds/flu were commonly reported asthma triggers but were experienced less frequently during the year.
A high asthma trigger burden was associated with a lack of asthma control, as measured by patient-reported control (5.4 for patients with very low and low trigger burdens, 5.8 for patients with medium trigger burden, and 6.5 for patients with high trigger burden) or using the ACT score (). Patients whose asthma was not well controlled (ACT score) made more behavioural modifications than those with controlled asthma. There were statistically significant differences between control groups in terms of the amount and type of housework undertaken, the amount of exercise taken and the choice to remain indoors due to weather conditions (p < 0.05 across groups; Chi-square test) ().
Figure 1. Relationship between trigger burden (exposure and frequency) and asthma control as measured by the ACT score (total participants = 1202). Panel A depicts participants (n = 696 [58%]) with a low to medium asthma trigger burden (<16 self-reported known asthma triggers) and a low total frequency burden (annual frequency index <0.5). Panel B depicts participants (n = 107 [9%]) with a low to medium asthma trigger burden (<16 self-reported known asthma triggers) and a high total frequency burden (annual frequency index ≥0.5). Panel C depicts participants (n = 278 [23%]) with a high asthma trigger burden (≥16 self-reported known asthma triggers) and a low total frequency burden (annual frequency index <0.5). Panel D depicts participants (n = 121 [10%]) with a high asthma trigger burden (≥16 self-reported known asthma triggers) and a high total frequency burden (annual frequency index ≥0.5). The shaded area on each panel depicts well controlled asthma (ACT score >20).
![Figure 1. Relationship between trigger burden (exposure and frequency) and asthma control as measured by the ACT score (total participants = 1202). Panel A depicts participants (n = 696 [58%]) with a low to medium asthma trigger burden (<16 self-reported known asthma triggers) and a low total frequency burden (annual frequency index <0.5). Panel B depicts participants (n = 107 [9%]) with a low to medium asthma trigger burden (<16 self-reported known asthma triggers) and a high total frequency burden (annual frequency index ≥0.5). Panel C depicts participants (n = 278 [23%]) with a high asthma trigger burden (≥16 self-reported known asthma triggers) and a low total frequency burden (annual frequency index <0.5). Panel D depicts participants (n = 121 [10%]) with a high asthma trigger burden (≥16 self-reported known asthma triggers) and a high total frequency burden (annual frequency index ≥0.5). The shaded area on each panel depicts well controlled asthma (ACT score >20).](/cms/asset/51e64fc9-6792-47dc-90f5-2ac12d618a08/ijas_a_846369_f0001_b.jpg)
Figure 2. Asthma control (ACT score) and behavioural changes. This figure depicts the frequency with which participants stratified according to asthma control (ACT score) reported making behavioural changes to avoid or minimise exposure to known asthma triggers.
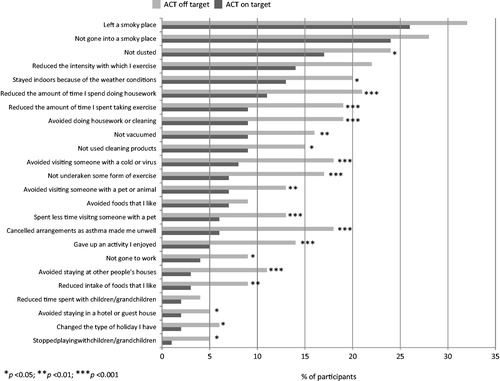
An analysis of the number of triggers to which participants reported being exposed to did not necessarily correspond to the frequency of asthma worsening, with a minority of participants (9%) experiencing few asthma triggers but frequent asthma worsening ().
Burden of asthma triggers
The majority of participants (99%) believed that exposure to known asthma triggers would have a long-term effect on their asthma. One in five participants reported that they made no modifications to their behaviour in response to known asthma triggers until prompted to do so by worsening asthma symptoms.
Participants were asked to rate the severity of asthma worsening in relation to the most commonly reported asthma triggers; all scored 6 or above on a scale of 0 (very mild) to 10 (very severe). The perceived severity of asthma worsening increased as the number of triggers experienced increased.
As the number of known asthma triggers increased, participants reported statistically significantly more severe asthma attacks during their lifetime, more hospitalisations in the previous year, more days missed at work or study in the last year and more symptomatic days and nights per week ().
Table 3. Burden of asthma triggers.
A number of covariates were associated with an increase in the socioeconomic burden of asthma (). Any increase in the level of known asthma triggers above “very low” was positively associated with a statistically significant increase in mean number of severe attacks in a lifetime (p ≤ 0.01), the number of times individuals were hospitalised in the last year (p < 0.001) and the number of days missed at work or study in the last year (p < 0.001) due to asthma. Female gender was associated with an increased number of severe attacks (p < 0.001), hospitalisation (p < 0.001) and missing work (p < 0.001) compared to male gender. Older age (>31 years) was associated with an increased likelihood of hospitalisations and days lost to work or study in the previous year compared with age <30 years. Moderate or severe asthma severity (physician informed and reported by patient) was associated with increased rates of all the three above outcomes (p < 0.001). Patients who reported they had severe asthma had three times the mean number of hospitalisations and four times more days off work than patients who reported they had mild asthma.
Table 4. Covariates associated with an increase in the socioeconomic burden of asthma (Poisson regression analysis).
Participants reporting a high number of known asthma triggers (≥16) reported that their asthma had a significantly greater impact on their overall daily life and job choice compared with those reporting very few, low or medium number of triggers. For overall daily life, asthma impact score was 5.9 compared with 4.5, 4.7 and 5.3, respectively (p < 0.0001 across groups; Kruskal-Wallis test). For job choice, asthma symptom score was 5.2 compared with 3.9, 3.9 and 4.2, respectively (p = 0.002 across groups; Kruskal–Wallis test). Linear regression analyses indicated that participants reporting a medium or high asthma trigger burden were significantly more likely to report an adverse impact on their overall daily life, as were participants in Italy (versus those in Germany; asthma impact score 0.61 points higher, p < 0.001), females (versus males; 0.42 points lower, p < 0.001) and those with moderate or severe asthma (versus mild; asthma impact score 1.33 and 2.36 points, respectively, p < 0.001 for both).
Behavioural changes in response to asthma trigger
Participants who reported that their asthma had impacted their daily life over the previous four weeks also reported making considerable behaviour changes to manage their asthma symptoms, the frequency of which increased as the number of triggers to which participants were exposed increased. The proportion of patients making any behaviour changes ranged from 67% for those with very few triggers to 89% for those with a high trigger burden (p < 0.001 across groups; Chi-square test). A logistic regression analysis showed a significant positive association between the trigger burden and the number of times behavioural changes were made in order to manage exposure to known asthma triggers in the previous four weeks. The higher the burden, the greater the number of changes, with high trigger patients being 10 times more likely to change behaviour up to five times in a week than those with very low number of triggers. No consistent pattern was observed for the other covariates examined.
Discussion
The aim of this large-scale epidemiological questionnaire and diary-based study conducted among adults with asthma across five European countries was to identify the types, frequency and impact of asthma triggers, and to investigate the relationship between trigger burden and asthma control. The majority of participants in the study had uncontrolled asthma and reported 6–15 known asthma triggers. Dust and dusting, colds/flu, sinusitis and coughing were the most common known asthma triggers with dust and dusting, smoking and exercise being the most frequently experienced triggers. An increase in trigger burden was associated with an increased likelihood of uncontrolled asthma, previous severe asthma attacks during a lifetime, more hospitalisations, more missed days at work/study, and behavioural changes to manage trigger exposure.
The number of known asthma triggers reported by participants in this survey varied considerably from just one to more than 16. Many of these triggers have been reported in asthma guidelines [Citation1], by a systematic literature review [Citation2] and documented in the literature [Citation15–24], but the identification of such triggers has typically been through studies focusing on single triggers, not through population-based approaches. However, studies that more fully characterise the wide range of triggers experienced by individual asthma patients, and how frequently they are encountered, have been limited. One such approach was the development of the Asthma Trigger Inventory, a questionnaire designed to measure the main categories of triggers self-reported by asthma patients, with a specific focus on psychosocial triggers [Citation15,Citation25,Citation26]. The most common categories of triggers were climate, physical and air pollution (experienced by >50% of patients), followed by infection, house dust, animal allergy, pollen allergy and psychology (>20 to <50% of patients), then respiration, food, sleep, alcohol, mould and medication (all <20% of patients) [Citation15]. With the exception of mould, which was a much more common trigger in the present survey than in the Asthma Trigger Inventory (51% versus ∼2%), the frequency of triggers common to both datasets were broadly similar.
Some of the triggers perceived by participants in this survey are not considered to be classic asthma triggers, for example coughing, strong odours and lying flat. Although coughing was the third most common trigger (reported by 68% of participants), it is a well-established symptom of asthma and is not listed as an asthma trigger by the GINA guidelines [Citation1]. There is literature to suggest that cough can be a precursor to an asthma exacerbation [Citation27], but this association could be secondary to the worsening of asthma via another trigger (leading to more symptoms, including coughing), rather than coughing being a true trigger. There is also some debate whether strong odours can lead to asthma exacerbations. Strong smells are not listed as an asthma risk factor by GINA [Citation1], but a small-scale study showed that perfume can cause exacerbations and airway obstruction, particularly in patients with severe asthma [Citation28]. There are clearly discrepancies between what patients consider to be triggers and what physicians and guidelines class as triggers. Possible explanations for such discrepancies include the lack of a standardised definition of the term ‘asthma trigger’ [Citation2], a lack of distinction between a trigger and an irritant, and documented gaps between patient perception and probable actual role of a trigger [Citation22,Citation29,Citation30]. Irritants can make the patient feel worse but may not necessarily lead to an exacerbation, while triggers are often thought to cause exacerbations. The present research suggests that patients do not discriminate between an irritant and a trigger and they will categorise any factor that increases symptoms, leads to loss of control, or causes an exacerbation as an asthma trigger. This has important implications for how healthcare professionals listen to and communicate with their patients, and emphasises the need for healthcare professionals to seriously consider all complaints about triggers, irrespective of whether they are real or perceived.
The frequency with which participants reported exposure to triggers varied considerably in this survey, with self-reported exposures ranging from every few months to every few days. These findings are important as there is little information in the literature about how frequently specific asthma triggers are encountered, and they help to characterise the full extent of trigger burden experienced by patients. Although the authors are not aware of comparable reports that assessed the frequency of exposure to a wide range of triggers, those triggers that were experienced most commonly by patients concord with other investigations that aimed to identify common trigger types [Citation2,Citation15]. Those triggers experienced by patients for ≥8 weeks of the year (see ) generally fall into the most important trigger categories reported by a systematic literature review (allergic, physical, environmental) [Citation2] or during the development of the Asthma Trigger Inventory (climate, physical, air pollution, infection, house dust, animal allergy) [Citation15]. The key exception was coughing, which was not reported as a trigger in either the systematic literature review [Citation2] or Asthma Trigger Inventory [Citation15].
Frequent exposure to a large number of triggers can worsen symptoms, cause exacerbations and lead to loss of asthma control [Citation1,Citation3–7], therefore reducing a patient’s exposure to triggers forms part of the overall recommended therapeutic approach to asthma management [Citation1]. However, the relationship between trigger burden and asthma control has not been well documented extensively in the literature. In the present study, a higher trigger burden was associated with more severe attacks in a lifetime, more hospitalisations in the last year, more days absent from work/study in the last year, and larger proportions of patients with uncontrolled asthma, as defined using the ACT (see and ). These findings align with those of a previous study in which patients were asked at the time of hospitalisation for an exacerbation what their usual triggers were and what trigger they felt caused their exacerbation [Citation31]. These patients reported a high trigger burden (mean of 12 triggers) and there were statistically significant correlations between more triggers and more exacerbations, worse quality of life, more hospitalisation due to asthma, and oral corticosteroid use. These collective findings strongly suggest that a greater trigger burden leads to adverse asthma outcomes and worse asthma control, and highlights the importance of patient education to eliminate or mitigate trigger exposure [Citation1].
Asthma guidelines and authoritative groups recommend patients with asthma work to identify triggers that worsen their symptoms and try to reduce exposure to those triggers with the aim of improving asthma control and reducing medication needs [Citation1,Citation8,Citation10,Citation11]. However, it is recognised that a response to triggers is a marker of poorly controlled disease, therefore trigger avoidance should not be used in isolation but rather as part of the holistic approach required to improve disease control overall. In the current study the majority of participants did not report any behavioural avoidance strategies for known environmental triggers, while some did but only after being prompted to do so by worsening asthma symptoms. Further, there was no apparent consistent approach to how patients managed trigger exposure and a wide range of behavioural strategies were reported. Patient education of trigger avoidance and advice on trigger management strategies appear to be logical steps to reduce the impact of triggers, but there are conflicting reports whether these steps are routinely taken [Citation12,Citation13]. Tools for use in the primary care setting that aim to capture specific information on trigger exposure and other reasons for poor asthma control, such as the Asthma APGAR [Citation32] and the Active Life with Asthma tool [Citation33], may help to improve the dialogue between healthcare professionals and patients with respect to triggers. Nonetheless, although some asthma patients do make temporary or permanent lifestyle changes to manage trigger exposure [Citation34], literature reviews [Citation2,Citation35] and task force recommendations [Citation36] have not yielded consistent and conclusive evidence that interventions and trigger avoidance strategies improve symptoms, reduce exacerbations, and increase quality of life and productivity, at least in adults. Although better understanding and identification of asthma triggers, more consistent provision of advice on asthma triggers, and the development of more effective trigger avoidance strategies appear to be needed, it should also be considered whether overall asthma control can be improved to the point where the avoidance of triggers is not necessary.
This study had strengths and limitations. The strengths of the study lie in the relatively large cohort of adults with asthma who took part (n = 1202) and in the validity of the ACT scale as a measure of asthma control both as a written and, as used in the current study, as an online questionnaire [Citation37]. The assessment of asthma control based on the GINA classification is a further benefit. The knowledge gained from the comprehensive characterisation of asthma triggers in this survey can be applied to broader epidemiological assessments of asthma triggers. The results should be considered within the limitations expected of the survey nature of the study design. The results apply to a large population of asthma patients from five European countries who were currently receiving maintenance treatment and who self-reported exposure to known asthma triggers; however, generalising the findings to other populations of asthma patients is not without its limitations. Furthermore, patient assessment of asthma control and trigger burden were based on self reporting with recall periods of up to one year. Finally, verification of self-reported asthma worsening or healthcare service utilisation was beyond the scope of the current study.
Conclusions
The results of the current study support those of previous surveys that suggest asthma control among adults in Europe is suboptimal. Individuals with asthma identify a wide range of environmental and behavioural triggers and often employ avoidance strategies or behavioural changes to manage their exposure. These behavioural changes often have a significant impact on daily life, social life and employment performance and choices. Individuals who perceive that their asthma is worsened on exposure to multiple triggers may be at particular risk for poorly controlled asthma. Physicians should seek to routinely assess the trigger burden for individual patients and offer education and support to enable individuals to manage their exposure while minimising the effect of behavioural changes on their daily lives.
Declaration of interest
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
David Price has served as a consultant to and been on advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Medapharma, Novartis, Napp, Nycomed, Pfizer, Sandoz and Teva; received research funding from UK National Health Service, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer and Teva; received payment for lectures/service on speakers bureaus from Activaero, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Novartis, Merck, Mundipharma, Pfizer and Teva; received payment for manuscript preparation from Merck, Mundipharma and Teva; has patents with AKL Ltd; received payment for development of educational presentations from GlaxoSmithKline; owns shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; and received travel/accommodations/meeting expenses from Boehringer Ingelheim, Napp, Novartis and Mundipharma. Specific for the work presented in the manuscript, David Price received a grant and provision of writing assistance from GlaxoSmithKline. For all of the financial activities listed above, payments were made to David Price’s institution. Peter Dale is the founding director of HEOR Solutions Ltd and was employed by GlaxoSmithKline at the time the study was conducted. Emma Elder is an employee of and holds stock in GlaxoSmithKline. Kenneth R. Chapman has served as a consultant to AstraZeneca, Boehringer Ingelheim, CSL Behring, Hoffman la Roche, GlaxoSmithKline, Grifols, Merck, Novartis and Takeda; received research funding from Amgen, AstraZeneca, CIHR, CSL Behring, GlaxoSmithKline, Merck, Novartis, Pfizer and Takeda; received payment for lectures/service on speakers bureaus from GlaxoSmithKline, Grifols, Merck, Novartis and Takeda; and received payment for development of educational presentations from GlaxoSmithKline, Merck, Novartis and Takeda.
Funding for this research was provided by GlaxoSmithKline.
Editorial support (in the form of development of a draft outline in consultation with the authors, development of a manuscript first draft in consultation with the authors, editorial suggestions to draft versions of this paper, analyses of key data to provide test statistics, assembling tables and figures, collating author comments, copyediting, fact checking, referencing and graphic services) was provided by Dr. Louise Watson and Dr. Tracey Lonergan at EPI PharmaCo Ltd and was funded by GlaxoSmithKline. Further editorial support (in the form of revisions to the paper in response to journal comments) was provided by Dr. David Cutler, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
The open-access fee was paid for by GlaxoSmithKline.
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Available from: http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asthma.html [last accessed 29 May 2013]
- Vernon MK, Wiklund I, Bell JA, Dale P, Chapman KR. What do we know about asthma triggers? A review of the literature. J Asthma 2012;49:991–998
- Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S–12S
- Demoly P, Annunziata K, Gubba E, Adamek L. Repeated cross-sectional survey of patients-reported asthma control in Europe in the past 5 years. Eur Respir Rev 2012;21:66–74
- Göksel O, Celik GE, Erkekol FO, Güllü E, Mungan D, Misirligil Z. Triggers in adult asthma: are patients aware of triggers and doing right? Allergol Immunopathol (Madr) 2009;37:122–128
- Haughney J, Price D, Kaplan A, Chrystyn H, Horne R, May N, Moffat M, et al. Achieving asthma control in practice: Understanding the reasons for poor control. Respir Med 2008;102:1681–1693
- Yawn BP. The role of the primary care physician in helping adolescent and adult patients improve asthma control. Mayo Clin Proc 2011;86:894–902
- American College of Allergy Asthma & Immunology (ACAAI). Common Asthma Triggers. 2010. Available from: http://www.acaai.org/allergist/asthma/asthma-triggers/Pages/default.aspx [last accessed 29 May 2013]
- American Academy of Allergy Asthma & Immunology (AAAAI). Tips to Remember: Asthma Triggers and Management. 2013. Available from: http://www.aaaai.org/patients/publicedmat/tips/asthmatriggersandmgmt.stm [last accessed 29 May 2013]
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, Bowie D, et al. Adult Asthma Consensus Guidelines update 2003. Can Respir J 2004;11(Suppl A):9A–18A
- Teach SJ, Crain EF, Quint DM, Hylan ML, Joseph JG. Improved asthma outcomes in a high-morbidity pediatric population: results of an emergency department-based randomized clinical trial. Arch Pediatr Adolesc Med 2006;160:535–541
- Rank MA, Wollan P, Li JT, Yawn BP. Trigger recognition and management in poorly controlled asthmatics. Allergy Asthma Proc 2010;31:99–105
- Washington D, Yeatts K, Sleath B, Ayala GX, Gillette C, Williams D, Davis S, et al. Communication and education about triggers and environmental control strategies during pediatric asthma visits. Patient Educ Couns 2012;86:63–69
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549–556
- Ritz T, Steptoe A, Bobb C, Harris AH, Edwards M. The asthma trigger inventory: validation of a questionnaire for perceived triggers of asthma. Psychosom Med 2006;68:956–965
- Singh AM, Busse WW. Asthma exacerbations. 2: aetiology. Thorax 2006;61:809–816
- Weiler JM. Exercise-induced asthma: a practical guide to definitions, diagnosis, prevalence, and treatment. Allergy Asthma Proc 1996;17:315–325
- Fauroux B, Sampil M, Quénel P, Lemoullec Y. Ozone: a trigger for hospital pediatric asthma emergency room visits. Pediatr Pulmonol 2000;30:41–46
- Hastert TA, Babey SH, Brown ER, Meng YY. Pets and smoking in the home associated with asthma symptoms and asthma-like breathing problems. Policy Brief UCLA Cent Health Policy Res 2007;Feb(PB2007-2):1–7
- Liangas G, Yates DH, Wu D, Henry RL, Thomas PS. Laughter-associated asthma. J Asthma 2004;41:217–221
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun 2007;21:993–999
- Woods RK, Weiner J, Abramson M, Thien F, Walters EH. Patients' perceptions of food-induced asthma. Aust N Z J Med 1996;26:504–512
- Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am 2005;25:131–148
- Covar RA, Macomber BA, Szefler SJ. Medications as asthma triggers. Immunol Allergy Clin North Am 2005;25:169–190
- Wood BL, Cheah PA, Lim J, Ritz T, Miller BD, Stern T, Ballow M. Reliability and validity of the Asthma Trigger Inventory applied to a pediatric population. J Pediatr Psychol 2007;32:552–560
- Ritz T, Kullowatz A, Kanniess F, Dahme B, Magnussen H. Perceived triggers of asthma: evaluation of a German version of the Asthma Trigger Inventory. Respir Med 2008;102:390–398
- Chang AB, Harrhy VA, Simpson J, Masters IB, Gibson PG. Cough, airway inflammation, and mild asthma exacerbation. Arch Dis Child 2002;86:270–275
- Kumar P, Caradonna-Graham VM, Gupta S, Cai X, Rao PN, Thompson J. Inhalation challenge effects of perfume scent strips in patients with asthma. Ann Allergy Asthma Immunol 1995;75:429–433
- Teeter JG, Bleecker ER. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest 1998;113:272–277
- De Peuter S, Van Diest I, Lemaigre V, Li W, Verleden G, Demedts M, van den Bergh O. Can subjective asthma symptoms be learned? Psychosom Med 2005;67:454–461
- Peterson MG, Gaeta TJ, Birkhahn RH, Fernández JL, Mancuso CA. History of symptom triggers in patients presenting to the emergency department for asthma. J Asthma 2012;49:629–636
- Yawn BP, Bertram S, Wollan P. Introduction of Asthma APGAR tools improve asthma management in primary care practices. J Asthma Allergy 2008;1:1–10
- Kiotseridis H, Bjermer L, Pilman E, Ställberg B, Romberg K, Tunsäter A. ALMA, a new tool for the management of asthma patients in clinical practice: development, validation and initial clinical findings. Prim Care Respir J 2012;21:139–144
- Vernon MK, Bell JA, Wiklund I, Dale P, Chapman K. Asthma control and asthma triggers: the patient perspective. J Asthma Allergy Educ 2013;4:155--164
- National Heart Lung and Blood Institute (NHLBI). National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf [last accessed 29 May 2013]
- Community Preventive Services Task Force. Asthma Control: Task Force Recommendations & Findings. 2008. Available from: http://www.thecommunityguide.org/asthma/index.html [last accessed 29 May 2013]
- Koolen BB, Pijnenburg MW, Brackel HJ, Landstra AM, van den Berg NJ, Merkus PJ, Hop WC, et al. Validation of a web-based version of the asthma control test and childhood asthma control test. Pediatr Pulmonol 2011;46:941–948
