Abstract
Objective. In people with screen-detected type 2 diabetes in primary care, (1) to assess adherence to guidelines, recommending consultation with the GP every three months and treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist when systolic BP was > 120 mmHg and/or diastolic BP was > 80 mmHg, and (2) to identify predictors for adherence. Design. Prospective follow-up of a fixed cohort of patients. Setting. Fifty-four Danish general practices. Subjects and main outcome measures. A total of 361 people with screen-detected type 2 diabetes were followed up for 410 days to assess planned consultations with their GP and recording of BP. Some 226 people, with BP recorded above guideline threshold(s) and where treatment was not already initiated, were followed for up to 410 days to monitor prescription redemption. Results. At 3, 6, 9 and 12 months 80%, 77%, 74%, and 73% of the cohort attended a consultation. A total of 89% of the cohort attended two of the four planned consultations. The probability of redeemed prescriptions for an ACE inhibitor or an angiotensin-II receptor antagonist according to the guideline during the first year following diagnosis was 51%. High initial BP was associated with prescription redemption. No other analysed individual or organisational characteristics were found to be associated with treatment initiation. Conclusion. The consultation attendance was reasonably high, and treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist according to the guideline was found in half of the cases. High initial BP increased the probability of treatment initiation.
Type 2 diabetes is a common disease with micro- and macro-vascular complications appearing while the disease is still symptom-free [Citation1–4]. The purpose of screening is to improve the prognosis by initiating treatment at the asymptomatic stage of the disease. To justify screening, an effective and available treatment must exist [Citation5].
During the last decade the treatment recommendations for type 2 diabetes have changed radically from focusing on treatment of dysglycaemia to multifactorial treatment including treatment of cardiovascular risk factors. Furthermore, thresholds for treatment initiation have become substantially lower [Citation6–9]. Randomized controlled trials concluded that angiotensin-converting enzyme (ACE) inhibitors reduce the risk of vascular complications among people with type 2 diabetes at high risk of complications, irrespective of the initial blood pressure (BP) [Citation8,Citation10]. Thus, this treatment has been recommended to people with screen-detected type 2 diabetes. Adherence to treatment guidelines depends on whether the general practitioner (GP) recommends the treatment, and on whether the patient initially accepts the treatment offered. To our knowledge adherence and predictors of adherence have not been studied in people with screen-detected diabetes, who may be more difficult to motivate due to the lack of symptoms at diagnosis. Accordingly, the aim of the present study was:
(a) to assess adherence to guidelines for consultations with GP and treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist in people with screen-detected type 2 diabetes;
(b) to identify predictors for adherence.
Material and methods
Design, recruitment, and outcome measures
The study was a prospective follow-up of people with screen-detected type 2 diabetes in the setting of 54 general practices from five former counties in Denmark (Copenhagen, Aarhus, South of Jutland, Ribe, and Ringkoebing), and for whom we had prescription data three months prior to the diagnosis. The practices were part of the intervention arm of the Anglo–Danish–Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION) study, Denmark. The ADDITION study is an ongoing international study aiming to evaluate screening procedures for type 2 diabetes in general practice and to evaluate the effect of intensive multifactorial treatment on those people diagnosed with type 2 diabetes [Citation11]. The intervention was directed at the participating GPs and their staff by training in optimal targets for HbA1c, BP, total cholesterol, and optimal pharmaceutical treatment for preventing complications, including an ACE inhibitor or an angiotensin-II receptor antagonist if BP was > 120 systolic and/or > 80 diastolic. Moreover, the intervention group was enhanced to arrange three-monthly consultations for their patients with type 2 diabetes including BP measurements. All this was presented in a written guideline. It was also presented as lectures at the beginning of the study, and in group sessions with specialists later on in the study. Finally, GPs have also received feedback on their patients by way of written reports and practice visits. The screening procedure and diagnostic criteria are presented elsewhere [Citation12].
Less is known about adherence and predictors for adherence to guidelines in people with screen-detected type 2 diabetes.
In this study, half of the target group initiated treatment with an ACE inhibitor or an angiotensin-II receptor antagonist according to the guideline during the first year following diagnosis.
High initial BP increased the probability of treatment initiation.
No other individual or organisational characteristics were found to be associated with treatment initiation.
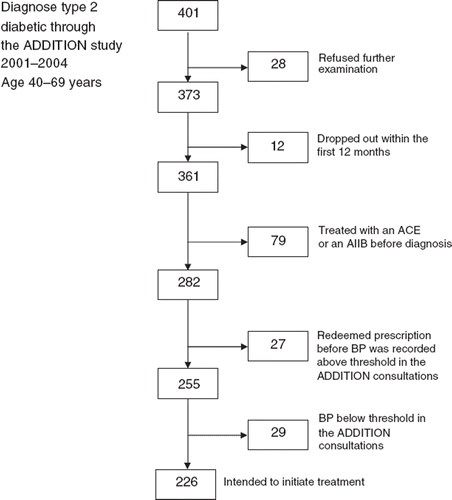
Recruitment to the present study took place from 2001–2004 and the flowchart of the study population is shown in . The outcome measures were derived from the ADDITION study's guideline: consultation every three months, recording of BP by the GP and redeemed prescription for an ACE inhibitor or an angiotensin-II receptor antagonist if BP was > 120 systolic and/or > 80 diastolic during any consultation. The intention was to follow people with screen-detected type 2 diabetes, who were not treated with an ACE inhibitor or an angiotensin-II receptor antagonist before diagnosis. These people were defined as not having a redeemed prescription for an ACE inhibitor or an angiotensin-II receptor antagonist in the 90 days before entering the study, and/or those who claimed not to have received the treatment before entering the study. Planned consultations and recording of BP were followed up for 410 days (12 months + 45 days due to a realistic period of time for annual consultation) from diagnosis in 361 patients. Treatment initiation was followed up for up to 410 days in 226 patients. The median follow-up time from exceeded BP threshold until prescription redemption or end of follow-up was 392 days.
Data
Data on the dependent variables, consultations, and recording of BP in the planned consultations were obtained from case record forms completed by the GPs. Prescription data were obtained from the National Health Service, based on the unique civil registry number assigned to all Danish citizens. Treatment with an ACE inhibitor or an angiotensin-II receptor antagonist was identified by the ATC codes C09A, C09B, C09C, and C09D. Independent variables concerning demographic and social characteristics (gender, age, cohabitation, education, profession) and related to lifestyle and health characteristics (smoking, body mass index (BMI), BP, HbA1c, serum cholesterol, self-rated health (SF36)) were obtained from questionnaires completed by people with screen-detected type 2 diabetes and GPs. Anthropometric measurements were undertaken at baseline following standard operating procedures with height being recorded to the nearest 0.1 cm using a fixed rigid stadiometer and weight in light indoor clothing recorded to the nearest 0.1 kg. BP was recorded in a sitting position after a 10-minute rest with a standard sphygmomanometer in each practice. HbA1c was analysed in venous samples in a central laboratory (University Hospital of Aarhus) using liquid chromatography on a Tosoh machine. Fasting serum samples were analysed in the same laboratory for cholesterol using standard enzymatic methods. Two dimensions, general health and mental health, from the Danish version of the SF-36 questionnaire were used to measure self-rated health [Citation13]. These are measured on a scale from 0 (worst health) to 100 (best health). The independent variables concerning organizational data (number of GPs in the practice, GP's age and gender, number of inhabitants registered in the postcode of the practice, number of patients per GP registered to the practice) were obtained from the National Health Service. The independent variables were divided into categories shown in , , and .
Table I. Treatment initiation1 with an ACE inhibitor or an angiotensin-II receptor antagonist by lifestyle and health characteristics among 226 people with screen-detected type 2 diabetes in 54 general practices in Denmark 2001–2006.
Table II. Treatment initiation1 with an ACE inhibitor or an angiotensin-II receptor antagonist by demographic and social characteristics among 226 people with screen-detected type 2 diabetes in 54 general practices in Denmark 2001–2006.
Table III. Treatment initiation1 with an ACE inhibitor or an angiotensin-II receptor antagonist by general practice characteristics among 226 people with screen-detected type 2 diabetes in 54 general practices in Denmark 2001–2006.
Statistical analysis
The probability of attendance and recording of BP in the planned consultations were estimated by the aid of a flow chart. In addition the probabilities of attendance at two or more, and three or more of the planned consultations were estimated.
Time to treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist from the first time BP was above the threshold(s) in the ADDITION study's guideline was estimated by the Kaplan–Meier method. Censoring occurred by the end of follow-up. Cumulative initiation proportions (CIP) with 95% CI at 410 days were found using the Kaplan–Meier method and Greenwood's formula. Statistical analyses were performed with Intercooled STATA version 10.0 and in Excel version 2003.
Results
Consultations and recording of BP
The probabilities of attendance at the consultations planned at 3, 6, 9, and 12 months were 80% (95% CI 76–84), 77% (73–82), 74% (69–79) and 73% (68–77), respectively. The probabilities of BP being recorded among the people attending the consultations were 99% (97–100), 98% (95–99), 99% (97–100), and 98% (96–100), respectively. A total of 89% (86–92) of the people attended two of the four planned consultations, while 75% (71–80) attended three of the four planned consultations.
Treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist
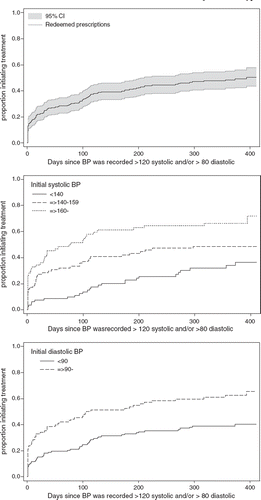
A total of 226 people should have ACE inhibitor or angiotensin-II receptor antagonist treatment initiated according to the BPs recorded in the case record forms and the guidelines of the ADDITION study. Among these, the probability of treatment initiation was 51% (44–58) within the 410 days of follow-up ().
Figure 2. Time to initiate treatment with an ACE inhibitor or an angiotensin-II receptor antagonist among 226 people with screen-detected type 2 diabetes in 54 general practices in Denmark 2001–2006. Upper panel shows results independent of BP level. Middle panel shows results in relation to initial systolic BP and lower panel in relation to initial diastolic BP.
Predictors of treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist
The probability of treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist according to guideline was 72% (58–85), if the initial systolic BP was ≥ 160 versus 36% (26–49), if the initial systolic BP was <140. The difference of 36 percentage points was statistically significant (see ). Equally, the probability of treatment initiation was 66% (55–76) if the initial diastolic BP was ≥ 90, versus 40% (32–49) if the initial diastolic BP was < 90, and the difference of 26 percentage points was also statistically significant. Smoking, BMI, self-rated health, and values for total cholesterol or HbA1c were not found to be associated with treatment initiation. Neither were the demographic and social characteristics: gender, age, cohabitation, education, or employment (see ); or the organizational characteristics: practice type, GP's gender and age, practice setting, or number of patients per GP (see ).
Discussion
Main findings
The high attendance at the planned consultations indicated that the people with screen-detected type 2 diabetes accepted structured consultations. Adherence for recording of BP by the GPs was high, but treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist according to the guideline was found in only half of the cases. As the GPs enrolled themselves into the ADDITION study, the adherence would probably be lower if implemented in general. High initial BP increased the probability of treatment initiation. No other characteristics were found to be associated with treatment initiation.
When considering why the treatment was not initiated according to the guideline, it is unknown whether it was intentional or not, and in which stage of the process it occurred. It could be unintentional, if the message of the guideline did not reach the GPs. High BP was associated with treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist, which might indicate that the message concerning ACE inhibitors’ preventive effect independently of the level of the BP was not converted to clinical actions by the GPs. Prescription of an ACE inhibitor associated with high BP was also seen in studies concerning the use of an ACE inhibitor in heart failure treatment, even though guidelines recommend this as standard therapy for all patients [Citation14,Citation15]. Midlov et al. concluded that GPs accept higher BP levels than recommended in clinical guidelines [Citation16]. The non-compliance could also be intentional. The GPs might abstain from recommending the treatment, if they found polypharmacological treatment not appropriate to offer symptom-free patients [Citation17], or if they were not convinced of the evidence of the treatment. Following a guideline without taking the situation of each individual into consideration is what Zoffmann et al. call a non-situational, disease-orientated perspective. There could be many reasons for not initiating the treatment according to the guideline with the life-orientated perspective taken into consideration [Citation18]. For example, how long will it take people to accept that they are going to initiate lifelong treatment when not feeling ill? Some people are better at seeing potential threats than others and are better at proactive coping, defined as efforts undertaken in advance of a potentially stressful event to either prevent it or modify its form before it occurs [Citation19]. It might be that the people with screen-detected type 2 diabetes declined the treatment offered, perceiving no substantial threat of the fact of having type 2 diabetes. A qualitative study indicated that people with type 2 diabetes perceived their illness as not being serious [Citation20].
Strengths and limitations
A strength of the study was the assessment of different stages of the process of treatment initiation: attendance at consultations, recording of BP, and prescription redemption. However, all feasible stages were not assessed separately. The outcome measure prescription redemption was composed of physician and patient adherence. It indicated that the patient was invited to and attended a planned consultation, the GP recommended the treatment, the patient accepted the offer in the consultation, the GP prescribed the medication, and the patient redeemed the prescription. We were for example not able to determine if there were cases where the GP prescribed medication but the prescription was not redeemed by the patient. Assessing adherence from the first time BP was recorded above threshold in the ADDITION study's guideline was debatable, but the decision was made because of the intention in the ADDITION study of treatment initiation in all people with type 2 diabetes, unless their BP was very low (below 120/80).
A weakness of the study was the unknown influence of other consultations and recorded BPs, outside the ADDITION consultations, but considering different scenarios the range of adherence was between 50–56%.
Future research
When considering implementing screening for type 2 diabetes, further research is suggested with regard to adherence. It makes a difference if the reasons behind non-adherence were related to troubles in implementing the guideline, if the intensive treatment of symptom-free patients was perceived as too much, or if the patients declined the offer. Did the people with screen-detected type 2 diabetes make a conscious decision on treatment initiation? And how were they supported?
Conclusion
The consultation attendance was reasonably high, and treatment initiation with an ACE inhibitor or an angiotensin-II receptor antagonist according to the guideline was found in half of the cases. High initial BP increased the probability of treatment initiation. Reasons for non-adherence are discussed and deserve more attention.
Ethical approval
The Committee on Biomedical Research Ethics and the Danish Data Protection Agency approved the study and its database.
The RCT trial registration number of the ADDITION study is NCT 00237549.
Acknowledgements
This study was supported by the UCSF Lundbeckfund (j.nr. FP 25/2007), the Tryg-Fund, the Snedkermester Sophus Jacobsen and hustru Astrid Jacobsens Fund, the Kristine Petrea Marius Claus and Erik Feldthusens Fund of 5 10 1975, and the Ulla and Mogens Folmer Andersens Fund.
The ADDITION study in Denmark was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and South Jutland, together with the Danish Research Foundation for General Practice, Danish Centre for Evaluation and Health Technology Assessment, the diabetes fund of the National Board of Health, the Danish Medical Research Council, the Aarhus University Research Foundation, and the Novo Nordisk Foundation. The study received unrestricted grants from Novo Nordisk AS, Novo Nordisk Scandinavia AB, Astra Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, Servier Denmark A/S, and HemoCue Denmark A/S.
Conflicts of interest: Knut Borch-Johnsen is head of the Steno Diabetes Center, a hospital integrated in the Danish National Health Care Service, but owned by Novo Nordisk. Knut Borch-Johnsen holds shares in Novo Nordisk Inc.
References
- Laakso M. Dyslipidemia, morbidity, and mortality in non-insulin-dependent diabetes mellitus: Lipoproteins and coronary heart disease in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1997; 11:137–41.
- Spijkerman AM, Adriaanse MC, Dekker JM, . Diabetic patients detected by population-based stepwise screening already have a diabetic cardiovascular risk profile. Diabetes Care. 2002; 25:1784–9.
- Borch-Johnsen K. Type 2 diabetes. Prevention of a public disease and its consequences. Ugeskr Laeger 2004; 166:1316–20.
- Glumer C, Jorgensen T, Borch-Johnsen K, Inter99 study. Prevalences of diabetes and impaired glucose regulation in a Danish population: The Inter99 study. Diabetes Care 2003; 26:2335–40.
- Wilson JMG, Jungner G. Principles and practice of screening for disease Geneva. World Health Organization; 1968.
- Faber OK, Sandahl Christiansen J. Ikke-insulinkrævende diabetes – NIDDM (Non-insulin-dependent diabetes mellitus – NIDDM). Copenhagen: Danish College of General Practitioners; 1998.(in Danish)
- Type 2 diabetes i almen. Diagnose og behandling (Type 2 diabetes in general practice. Diagnosis and treatment). Copenhagen: Danish College of General Practitioners; 2002.(in Danish)
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000; 355:253–9.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–93.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–97.
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, . The ADDITION study: Proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord 2000; 24:6–11.
- Christensen JO, Sandbaek A, Lauritzen T, Borch-Johnsen K. Population-based stepwise screening for unrecognised Type 2 diabetes is ineffective in general practice despite reliable algorithms. Diabetologia 2004; 47:1566–73.
- Bjørner JB, . Dansk manual til SF-36. (Danish manual to SF-36). Lif1997.(in Danish)
- Manyemba J, Mangoni AA, Pettingale KW, Jackson SH. Determinants of failure to prescribe target doses of angiotensin-converting enzyme inhibitors for heart failure. Eur J Heart Fail. 2003; 5:693–6.
- Kasje WN, Denig P, Stewart RE, de Graeff PA, Haaijer-Ruskamp FM. Physician, organisational and patient characteristics explaining the use of angiotensin converting enzyme inhibitors in heart failure treatment: A multilevel study. Eur J Clin Pharmacol 2005; 61:145–51.
- Midlov P, Ekesbo R, Johansson L, Gerward S, Persson K, Nerbrand C, . Barriers to adherence to hypertension guidelines among GPs in southern Sweden: A survey. Scand J Prim Health Care 2008; 26:154–9.
- Olivarius Nde F. Diabetes care today: Not everyone should have intensive multipharmacological treatment. Scand J Prim Health Care. 2004; 22:67–70.
- Zoffmann V, Harder I, Kirkevold M. A person-centered communication and reflection model: Sharing decision-making in chronic care. Qual Health Res 2008; 18:670–85.
- Aspinwall LG, Taylor SE. A stitch in time: Self-regulation and proactive coping. Psychol Bull 1997; 121:417–36.
- Holmstrom IM, Rosenqvist U. Misunderstandings about illness and treatment among patients with type 2 diabetes. J Adv Nurs 2005; 49:146–54.