Abstract
Objective. To investigate the prognostic value of subjective memory complaints for all-cause mortality in order to determine whether elderly persons with subjective memory complaints may be regarded as a group of vulnerable patients who need close follow-up. Design. Prospective cohort study. Setting. Primary care units in the central district of Copenhagen, Denmark. Subjects. 758 community-dwellers aged 65 years and older consulting their general practitioner in October and November 2002. Main outcome measures. Information on subjective memory complaints, socio-demographics, and health-related quality of life were collected at the enrolment primary care consultation. Dates of death from all causes occurring during the four-year follow-up were retrieved from the national databases. Cox proportional hazard regression models on time to death (censored after four years) were used to examine the influence of subjective memory complaints on all-cause mortality. Results. 88 patients died during the four-year follow-up. The association between subjective memory complaints and mortality had a statistically not significant hazard ratio (HR) of 0.91, adjusting for known confounders. Statistically significant predictors for mortality were Age (HR = 1.43 for 75–84 years and HR = 3.39 for 85 + years), Sex (HR = 0.51 for women), Mobility (HR = 2.39 for some problems), Self-care (HR = 2.34 for some problems) and Comorbidity (HR = 2.06, 3.19 and 5.89 for a Charlson comorbidity index of 1, 2, or ≥ 3 respectively). Conclusion. In an elderly population presenting for primary care the presence of subjective memory complaints was not significantly associated with an increase in all-cause mortality.
Subjective memory complaints are known to be associated with adverse health outcomes such as dementia, nursing home placement, and increased healthcare utilization.
In an elderly primary care population the presence of subjective memory complaints is not a significant predictor for all-cause mortality.
Introduction
Subjective memory complaints (SMCs) are commonly reported by elderly people [Citation1] and have consistently been associated with depression [Citation2–4], anxiety [Citation5], low education, and female sex [Citation1]. Mixed results on the association between SMCs and decreased cognitive performance have been reported [Citation5–7]. Correspondingly, studies relating SMCs with subsequent impaired cognition or dementia are inconsistent [Citation8–11]. However, these results are predominantly obtained in community surveys. A natural, direct, and timely opportunity to address concerns like SMCs is found in elderly patients visiting their general practitioner (GP). Notably, these patients are different from community dwellers because they have illness or concerns that motivate their visit.
We have previously shown that in a primary care setting, SMCs are predictive of subsequent nursing home placement [Citation12], increased health care utilization [Citation13], and hospital-based dementia diagnosis [Citation14], and may therefore indicate an increased vulnerability in the elderly. Ultimately, vulnerability may manifest itself as a high mortality.
Apart from well-known mortality risk factors such as age, sex, comorbidities, and decreased functional ability, psychometrically measured cognitive performance is also associated with mortality [Citation15,Citation16]. These aspects are to some extent also associated with SMCs and should be treated as potential confounders when investigating their predictive value.
We identified only two studies that address the association of SMCs with mortality, both community surveys. In a large study of US elderly [Citation17], SMCs were associated with lower mortality only for men. This analysis is not adjusted for objective measures of cognitive function. In contrast, a large Israeli study [Citation18] found no association of SMCs with mortality; this analysis is adjusted for objective general mental health.
Patients rarely share their perception of SMCs with their doctor spontaneously, and few GPs actively inquire about SMCs [Citation19]. An understanding of the implications of SMCs may equip the GP with a possibility to identify vulnerability in elderly persons which is not reflected in conventional risk factors. With this aim the present study investigates the predictive value of SMCs for all-cause mortality over a four-year follow-up period in a primary care setting.
Material and methods
Study population
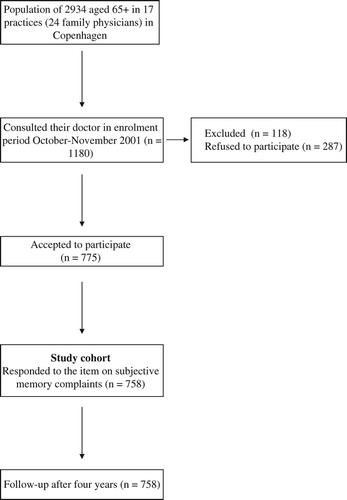
During an enrolment period in October and November 2002 each of the practices in the central district of the municipality of Copenhagen, Denmark (24 GPs with 40 865 patients listed of whom 2934 were 65 or older) asked their patients aged 65 and older, consulting their GP for whatever reason, to participate in the study. All were handed a leaflet with information on the study in which the aims were stated as being to survey the elderly citizen's memory. Patients not able to speak or read Danish, patients living in nursing homes, and patients with severe, acute, or terminal illness or specialist- diagnosed patients with dementia were excluded.
Outcome
The end-point variable was all-cause death within four years from the enrolment period. Information concerning incident deaths was retrieved from the National Health Register [Citation20].
Enrolment data
1. A self-administered participant questionnaire concerning aspects of memory and socio- demographics. Information on SMCs was obtained from the item: “How would you judge your memory?” The response categories were: “excellent”, “good”, “less good”, “poor”, or “miserable”. Patients rating their memory as “less good”, “poor”, or “miserable” were classified as patients with SMCs, while patients rating their memory as “excellent” or “good” were defined as patients without SMCs.
2. A self-administered health-related quality of life assessment. The patients completed the Danish Validated Version of EQ-5D. EQ-5D measures five dimensions – mobility, self-care, usual activities, pain/discomfort, and anxiety/depression – each by three levels of severity [Citation21].
3. A GP- or nurse-administered Mini Mental State Examination (MMSE), a widely distributed test recommended in primary care guidelines as a cognitive screening test [Citation22]. A score lower than 24 is indicative of cognitive impairment [Citation23].
4. A Charlson's Comorbidity Index (CCI) based on register data of hospital visits from the National Patient Registry in the three-year period 1999–2001 before the enrolment period. CCI measures the level of comorbidity with regard to its impact on mortality [Citation24].
5. A short physician questionnaire in which the GP was asked whether the patient complained about his/her memory and whether they thought the patient had cognitive impairment based on the GP's clinical impression.
Statistical analysis
Differences between participants and those refusing to participate or those who were excluded, and differences between participants with and without SMCs, were analysed with chi-squared tests. Influences of various risk factors for mortality – socio-demographics, items from EQ-5D, CCI, MMSE, and SMCs – are assessed as hazard ratios (HR) from Cox proportional hazard regression models on time from enrolment to death or end-of-study (censoring). We assess the effects of potential risk factors individually in simple (i.e. a single predictor) regression models, and jointly in a multiple (i.e. multiple predictors) regression model. In additional analyses interactions between age and SMCs, and sex and SMCs respectively are included in the multiple regression model to investigate whether a predictive value of SMCs differs relative to the participant's age or sex.
Observations with missing values for one or more of the variables involved in an analysis were omitted from that analysis. P < 0.05 is taken as statistically significant.
Ethics
The project was evaluated by the Scientific Ethical Committee for Copenhagen and Frederiksberg Municipalities and approved by the Danish Data Protection Agency, the Danish College of General Practitioners Study Committee, and the National Board of Health.
Results
The study cohort consisted of 775 non-nursing home residents of whom 758 filled out the SMCs item (). The average age of participants at enrolment was 74.8 and 38.6% were males; the mean MMSE was 28.2 (range: 16–30). According to our definition 177 (23.4%) had SMCs at enrolment. Those refusing to participate or excluded from the study cohort were significantly more likely to be males and were, according to the GP, less likely to complain about memory problems. All participants were followed up until the end of October 2006, i.e. for a total of 48–49 months.
A total of 88 deaths were recorded in the cohort in the follow-up period. shows the baseline characteristics of the study cohort stratified by SMCs.
Table I. Characteristics of the study cohort (n = 758) by SMCs.
shows the effects on all-cause mortality for each risk factor individually, and for all factors jointly. SMCs has a non-significant unadjusted HR of 1.26. Many of the other examined risk factors, notably age and comorbidity, are also individually associated with increased mortality risk over the four years of follow-up. SMCs has a non-significant HR of 0.91 adjusted for the potential confounders in the multiple regression analysis, denoting a small decrease in mortality attributable to SMCs.
Table II. Simple (n = 758) and multiple (n = 672) Cox regression analyses of the effects of SMCs and of the effects of potential confounders on all-cause mortality.
The excess risk from SMCs is not seen as different in the three age groups in our analysis (HR = 0.86 for < 75 years, HR = 1.00 for 75–84 years and HR = 0.88 for 85 + years, adjusted by the variables of the multiple regression model); the interaction is rejected (p = 0.97). An interaction of SMCs with sex is also rejected (HR = 0.50 for men and HR = 1.33 for women; p = 0.26).
There is no evidence that the 86 (11.4%) participants who were omitted from the multiple regression analyses because of missing data in the covariates had more SMCs (p = 0.29) or were more likely to die within the four-year follow-up (p = 0.72).
Discussion
This study cannot demonstrate a prognostic value of SMCs for all-cause death among elderly patients visiting their GP. Risk factors that predict death independently are age, sex, self-reported mobility and self-care abilities, and comorbidities, but confounding by these factors does not explain the apparent absence of an SMCs effect.
The failure to find an association of SMCs with increased mortality is in concordance with previous results on community dwellers [Citation17,Citation18]. Furthermore, while Lee [Citation17] observes that SMCs decreases mortality only for men, a non-significant tendency towards a similar sex disparity is seen in the present study; the former is considerably larger and has an older (> 70 years) sample, which may account for the difference in statistical significance. Both community studies [Citation17,Citation18], like the present analysis, identify age, sex, mobility (physical activity, disability), self-care, and comorbidities as primary risk factors for all-cause mortality.
Objective cognitive performance (MMSE) does not relate to all-cause death in the multiple regression analysis; the association is fully explained by confounding from, for example, comorbidities (see ). However, decreased cognitive performance has been shown to be related to adverse health outcomes [Citation1,Citation8,Citation9,Citation25] and was in the present cohort, alongside SMCs, a risk factor for nursing home placement [Citation12] and development of hospital-based dementia [Citation14]. Similarly, anxiety/depression, a predictor for nursing home placement [Citation12], is unrelated to all-cause death. In line with our results, none of the community studies into the association of SMCs and all-cause death identifies objective cognitive performance or depressive symptoms as risk factors.
The use of national registers with high validity ensures that we can account for all the participants at four-year follow-up. The sampling of the participants reflects the population in which the GP had an opportunity to ask questions about SMCs. Hence, this sampling reflects daily clinical practice and is part of the concept of the study.
A GP questionnaire accompanying the participant questionnaire found that 49 (6.6%) participants came forward with memory complaints themselves, for 22 (3.0%) a proxy had informed the GP about memory problems, and for 96 (13.0%) the GP had suspicions of dementia [Citation26]. A more targeted case-finding approach would let the GP inquire about SMCs in these 118 (16.0%) participants only. This does not improve the predictive value of SMCs; 11 (19.3%) of the 57 (48.3%) with SMCs died while 11 (18.0%) of the participants without SMCs also died within four years.
Several limitations must be addressed. This study had an inherent selection bias which may decrease generalizability: only elderly persons who consulted their GP are included, and they may be more vulnerable than elderly persons in general. However, a higher overall mortality in the present cohort compared with the general population would probably result in a less pronounced SMCs effect. Furthermore, we had no information about prevalent medical diagnoses or medication in the participants at enrolment, and had to rely on hospitalizations to assess comorbidities. However, patients with prevalent specialist-diagnosed dementia are excluded from the study. The average MMSE of the patients in the study is 28.2, which may indicate selection of a patient group that has an artificially low tendency for cognitive decline.
In the present study we used a single item to evaluate SMCs. Notably, this item does not allow us to know whether the patient was adjusting the response by comparing with former functioning or with the functioning of peers, and it does not distinguish between short-term and long-term memory. Others have assessed SMCs aggregating several items [Citation27] or also by a single item but with different wording [Citation17,Citation18]. The variation in SMCs prevalence may partly be explained by differing definitions of SMCs, and partly by differing study participants [Citation1].
The omission from multiple regression analyses of relatively few participants because of missing covariate values results in only limited loss of power and is not likely to bias the results as the missing values do not seem to relate to SMCs or death. Adjusted for only those factors that are significant in the multiple regression analysis, SMCs have a non-significant HR of 0.92. The similarity of this additional analysis, which only omits 21 (2.8%) participants, to the original analysis shows that the result is not biased by the omission of participants with missing covariate data. Furthermore, it shows that the result is not an artefact of overfitting.
In conclusion, SMCs cannot be viewed as part of a process to identify patients at high risk of dying. However, SMCs are associated with nursing home placement, increased health care utilization, and dementia, all indicative of increased mortality. The apparent absence of an effect of SMCs on mortality may indicate that, while SMCs indicate decreased future cognitive abilities and increased future need for help, they provide some resilience to death. Then, if elderly patients complain about their memory this may be an indication of some other vulnerability that manifests itself rather as decline in cognitive ability and quality of life, nursing home placement, or increased health utilization.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by a General Practitioners’ Foundation for Education and Development Grant (Grant nr: R56-A369-B186).
References
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000;15:983–91.
- Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: A community study. J Geriatr Psychiatry Neurol 1993;6:105–11.
- Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc 2004;52:263–8.
- Kahn RL, Zarit SH, Hilbert NM, Niederehe G. Memory complaint and impairment in the aged: The effect of depression and altered brain function. Arch Gen Psychiatry 1975;32:1569–73.
- Hanninen T, Reinikainen KJ, Helkala EL, Koivisto K, Mykkanen L, Laakso M . Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatr Soc 1994;42:1–4.
- Gagnon M, Dartigues JF, Mazaux JM, Dequae L, Letenneur L, Giroire JM, . Self-reported memory complaints and memory performance in elderly French community residents: Results of the PAQUID Research Program. Neuroepidemiology 1994;13:145–54.
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. J Am Geriatr Soc 1996;44:44–9.
- Glodzik-Sobanska L, Reisberg B, de SS, Babb JS, Pirraglia E, Rich KE, . Subjective memory complaints: Presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord 2007;24:177–84.
- Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: A longitudinal analysis over 7–8 years. Psychol Med 2001;31:441–9.
- Palmer K, Backman L, Winblad B, Fratiglioni L. Detection of Alzheimer's disease and dementia in the preclinical phase: Population based cohort study. BMJ 2003;326:245.
- Wang L, van BG, Crane PK, Kukull WA, Bowen JD, McCormick WC, . Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc 2004;52:2045–51.
- Waldorff FB, Siersma V, Waldemar G. Association between subjective memory complaints and nursing home placement: A four-year follow-up. Int J Geriatr Psychiatry 2009;24: 602–9.
- Waldorff FB, Siersma V, Waldemar G. Association between subjective memory complaints and health care utilisation: A three-year follow up. BMC Geriatr 2009;9:43.
- Waldorff FB, Siersma V, Vogel A, Waldemar G. Subjective memory complaints in general practice predicts future dementia: A 4-year follow-up study. Int J Geriatr Psychiatry 2012;27:1180–8.
- Lee HB, Kasper JD, Shore AD, Yokley JL, Black BS, Rabins PV. Level of cognitive impairment predicts mortality in high-risk community samples: The memory and medical care study. J Neuropsychiatry Clin Neurosci 2006;18:543–6.
- Smits CH, Deeg DJ, Kriegsman DM, Schmand B. Cognitive functioning and health as determinants of mortality in an older population. Am J Epidemiol 1999;150:978–86.
- Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Community Health 2000;54:123–9.
- Ayalon L. Subjective cognitive functioning as a predictor of all cause mortality in an Israeli national sample of community dwelling older adults. Int J Geriatr Psychiatry 2008;23: 830–6.
- Waldorff FB, Rishoj S, Waldemar G. If you don't ask (about memory), they probably won't tell. J Fam Pract 2008;57: 41–4.
- Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Register: A tool for primary health care research. Dan Med Bull 1997; 44:449–53.
- Rabin R, de CF. EQ-5D: A measure of health status from the EuroQol Group. Ann Med 2001;33:337–43.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc 1992;40: 922–35.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40: 373–83.
- Frisoni GB, Fratiglioni L, Fastbom J, Viitanen M, Winblad B. Mortality in nondemented subjects with cognitive impairment: The influence of health-related factors. Am J Epidemiol 1999;150:1031–44.
- Waldorff FB, Rishoj S, Waldemar G. Identification and diagnostic evaluation of possible dementia in general practice: A prospective study. Scand J Prim Health Care 2005;23:221–6.
- Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006;22:471–85.