Abstract
Objective. To determine whether a pharmacist-led medications review in primary care reduces the number of drugs and the number of drug-related problems. Design. Prospective randomized controlled trial. Setting. Liljeholmen Primary Care Centre, Stockholm, Sweden. Subjects. 209 patients aged ≥ 65 years with five or more different medications. Intervention. Patients answered a questionnaire regarding medications. The pharmacist reviewed all medications (prescription, non-prescription, and herbal) regarding recommendations and renal impairment, giving advice to patients and GPs. Each patient met the pharmacist before seeing their GP. Control patients received their usual care. Main outcome measures. Drug-related problems and number of drugs. Secondary outcomes included health care utilization and self-rated health during 12 months of follow-up. Results. No significant difference was seen when comparing change in drug-related problems between the groups. However, a significant decrease in drug-related problems was observed in the intervention group (from 1.73 per patient at baseline to 1.31 at follow-up, p < 0.05). The change in number of drugs was more pronounced in the intervention group (p < 0.046). Intervention group patients were not admitted to hospital on fewer occasions or for fewer days, and there was no significant difference between the two groups regarding utilization of primary care during follow-up. Self-rated health remained unchanged in the intervention group, whereas a drop (p < 0.02) was reported in the control group. This resulted in a significant difference in change in self-rated health between the groups (p < 0.047). Conclusions. The addition of a skilled pharmacist to the primary care team may contribute to reductions in numbers of drugs and maintenance of self-rated health in elderly patients with polypharmacy.
The elderly are often prone to drug-related morbidity due to multimorbidity, polypharmacy, and deteriorating organ function. Drug-related problems may cause hospital admissions.
The addition of a skilled pharmacist to the primary care team may contribute to reductions in numbers of drugs and to maintaining self-rated health in elderly patients with polypharmacy.
Introduction
Elderly patients with multiple diseases and polypharmacy risk suffering from drug-related problems (DRPs) [Citation1–4], and a significant proportion of hospital admissions in the elderly are due to adverse drug events (ADEs) [Citation1]. A DRP is defined as any undesirable event experienced by a patient involving or suspected of involving drug therapy and actually or potentially interfering with a desired patient outcome [Citation5]. The definition used in Sweden is “anything that leads to health care utilization, morbidity or mortality” [Citation6], and indicators of prescribing quality for drug treatment in the elderly have been developed by the Swedish National Board of Health and Welfare [Citation7].
Pharmacists included in health care teams in hospitals have helped lower the cost of drugs and reduce hospitalization [Citation8–10]. Medication reviews for elderly patients with polypharmacy in assisted living facilities have produced favourable effects such as fewer falls [Citation11].
In primary health care many different study methods have been used in investigations of effects on health care utilization or quality of life (QoL), but so far none has proven superior [Citation12–19]. However, methods where the pharmacist is part of a team seem to give better results [Citation17]. According to Beney et al. more research is needed since many different methods are used, making it difficult to compare results and to identify the parts of the interventions that are successful [Citation20].
Aims
The primary aim of this study was to assess whether a structured, randomized, and controlled intervention by a pharmacist at a primary health care centre would decrease the number of drugs and the number of DRPs. Secondary aims were to evaluate the impact on self-rated health and health care utilization.
Material and methods
Setting
The study was performed during a 15-month period at a primary care centre in Stockholm serving 24 000 inhabitants.
Subjects
Subjects to be included were persons aged ≥ 65 years with five or more medications who were already scheduled for an appointment with a GP. Patients who were not fluent in Swedish, could not answer for themselves, or had participated in an earlier pilot study were excluded. For all patients fulfilling the inclusion criteria, gender, age, and, when applicable, reason for exclusion, were recorded.
Study design
The study was a randomized controlled trial. We compared patients with a scheduled GP consultation who received normal care with patients who received preparatory structured pharmaceutical advice.
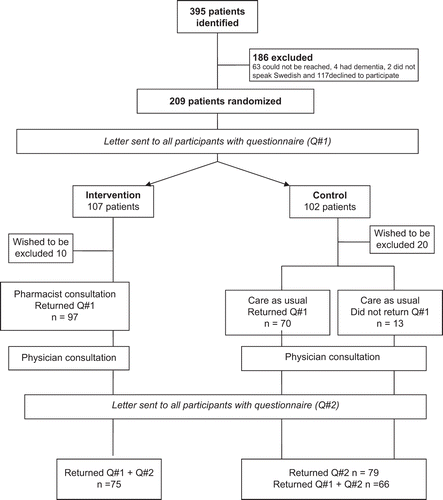
All patients fulfilling the inclusion criteria were contacted by telephone (see ). Those who agreed to participate were sent a questionnaire addressing all their medications (prescription, non-prescription, and herbal drugs) and DRPs and were then randomized. The intervention group met a pharmacist prior to the GP visit and the control group received normal care. After 12 months, all patients were contacted by telephone and were sent a new questionnaire. Control patients were offered a medication review after the conclusion of the trial.
The effect of the intervention was measured 12 months after the intervention in terms of DRPs, utilization of medical services, and self-rated health.
Pharmacist intervention
The medication review was performed by a certified geriatrics pharmacist (CL). The method had been tested in a pilot study (79 patients). It involved a standardized semi-structured protocol that was open for patients’ questions and remarks. Computerized patient records were checked for prescriptions, drug indications, and plans for evaluation. Drugs and dosages were evaluated to correlate with renal function, good practice [Citation7], and the drug formulary [Citation21]. A patient-centred technique was used, focusing on the patients’ questionnaire answers to assess understanding of and concordance with drug treatment. The patients were also asked about prescribers other than their GP, and use of non-prescription and herbal drugs. Concluding pharmaceutical advice was given to patients and entered into the computerized patient record.
Drug-related problems
DRPs were classified based on Beers’ criteria [Citation22] and the structure proposed by Strand et al. [Citation23] using a computer system. Information about DRPs was gathered from the questionnaires at baseline and after 12 months. Data were analysed by an independent certified geriatrics pharmacist (BE), blinded to patient group allocation.
Utilization of medical care
The utilization of medical care was measured as the number of contacts in outpatient care and hospital care during the 12 months following the intervention. Data were extracted from the records of Stockholm County Council using social security numbers, which gave 100% data coverage.
Self-rated health
Patients assessed their health by answering a single question on general self-rated health with the response options “very good”, “good”, “fair”, “bad”, and “very bad” [Citation24].
Statistical analysis
A power analysis was based on the pilot study. To detect a 25% change in DRPs with a power of 80% and an estimated dropout frequency of 10%, the sample size was calculated as 200.
Data are given as means with 95% confidence intervals (CIs), unless otherwise stated. Differences within groups (before vs. after intervention) were analysed by Wilcoxon matched-pairs test. The Mann–Whitney U test was used to test differences between groups. The chi-squared test was used to test for differences in the frequencies of diagnosis between groups. Differences in numbers of primary care visits, hospital admissions, and days hospitalized were analysed by Mann–Whitney U test as they did not fit the Poisson distribution.
Results
Subjects
Between September 2004 and November 2005, 395 persons fulfilling the inclusion criteria were identified, of whom 186 were excluded (). The remaining 209 persons were randomly assigned to the control or intervention group (). Patient characteristics at baseline are given in . The two groups were similar concerning age, sex, and number of diagnoses. There was no significant difference in the prevalence of chronic diagnoses between the groups, apart from for psychiatric disease (). However, patients in the intervention group used a greater number of drugs (8.6 vs. 7.4 drugs per patient, p < 0.05). After 12 months there was a mean reduction in the number of drugs per patient in the intervention group (from 8.6 to 7.9, p < 0.05), but not in the control group, where a mean increase from 7.4 to. 7.5 (not significant) was detected. The change in the number of drugs differed significantly (p < 0.046) between the groups.
Table I. Patient characteristics at baseline for included patients (n = 209) and analysed patients (n = 141).
Table II. Prevalence of chronic diagnoses in 102 control group patients and 107 intervention group patients at baseline.
Drug-related problems
DRPs were analysed in all patients returning both questionnaires (75 patients in the intervention group and 66 patients in the control group) (see ). Significant changes were seen in the before-and-after comparison in the intervention group, but not in the control group (). A between-group analysis of the change in number of DRPs revealed no significant differences (p = 0.72). The mean decrease in number of DRPs was 0.43 (95% CI 0.10, 0.75) in the intervention group and 0.27 (–0.02, 0.57) in the control group. The decrease in the intervention group was mainly due to a significant improvement in compliance (p = 0.048) ().
Table III. Drug-related problems at baseline and 12 months after a pharmacist medication review in primary care patients, for those returning both questionnaires (n = 141).
Utilization of medical care
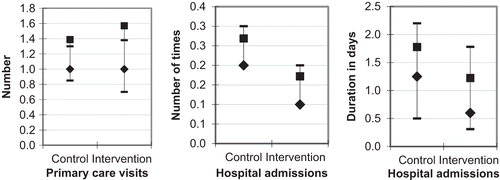
There was no significant difference between the two groups regarding utilization of primary care during the 12-month follow-up period (). Patients in the intervention group were admitted to hospital on fewer occasions compared with the control group (mean 1.7 vs. 2.7, median 1 vs. 2). The length of hospitalisation during the follow-up period was also lower in the intervention group compared with the control group (mean 12 vs. 18 days, median 6 vs. 12.5 days). However, none of the observed differences were statistically significant ().
Figure 2. Primary care visits and hospital admissions (number and duration in days) recorded in the control and intervention groups during one year. Data are shown as the median (♦) with 95% CI (▬) and mean (■) values. None of the investigated parameters showed a statistically significant difference. n = 141 patients (75 in the intervention group and 66 in the control group).
Self-rated health
On a 1–5 scale, there were no significant differences between the groups at baseline (intervention group 2.7 and control group 2.8) regarding self-rated health. Self-rated health remained unchanged in the intervention group, whereas it decreased significantly (p < 0.02) in the control group, resulting in a significant difference in change in self-rated health between the groups (p = 0.047). The mean change was 0.02 in the intervention group (95% CI –0.15, 0.19) and 0.27 (0.06, 0.48) in the control group.
Estimated cost of the intervention
The pharmacist had booked 30 minutes for each consultation, but including time for preparation and follow-up, each patient required approximately two hours. The cost of implementing this intervention in everyday practice was estimated at €79 ($106) per patient, based on the estimated total cost of one clinically trained, experienced pharmacist.
Discussion
This trial showed that a structured medication review with a specially trained pharmacist within a primary care framework may have reduced the number of drugs and prevented a decrease in self-rated health. No significant between-group difference in change in number of DRPs was detected; a within-group reduction was seen in the intervention group, but not in the control group. No effect on hospital admissions was found. Reducing the number of drugs by medication reviews is in line with findings in other studies [Citation25,Citation26].
We found a mean of 1.73 DRPs per patient in the intervention group at baseline (control group 1.37 DRPs per patient), which is less than in other studies [Citation10,Citation19,Citation25,Citation27,Citation28]. This could be explained by patients having more severe illnesses and registration of potential drug problems, rather than only actual problems, in these other studies. The most common DRPs in our study were ADEs and compliance problems. In other studies, common problems were unnecessary drug therapy and need for additional therapy or increased dosages [Citation10,Citation19,Citation26,Citation28]. The difference could be explained by different populations in the studies, i.e. patients in hospitals compared with patients in primary care.
The changes in self-rated health were significantly different between the groups in favour of the intervention group. A few studies have measured the influence of interventions on QoL [Citation18–20,Citation26,Citation29]. These studies used a more extensive instrument and did not show a significant improvement as a result of the intervention.
Our findings are in line with other findings that medication reviews do not decrease health care utilization [Citation18,Citation19,Citation26]. Results of medication reviews are complicated to analyse. It is not known which part or combination of elements in the review plays the key role. Our intervention was based on one pharmacist performing the medication reviews and did not include education of doctors or specified time for team discussions, as did the studies of, for example, Krska et al. [Citation29] and Sorensen et al. [Citation19]. Our intervention took place at a primary care centre, which gave the pharmacist access to full patient records, but limited the study to patients who actually visited the primary care centre. Another limitation is that withdrawal was uneven between the two groups, and that we do not have data concerning medications and DRPs for the patients who withdrew. Therefore we do not know if these patients differ from those who completed the study. With frail, older patients it is also difficult to choose the right follow-up time. We chose 12 months to avoid seasonal differences. However, 12 months is a long time and many things could have happened that would have influenced the results.
Cost-effectiveness is also difficult to assess. The mean total time spent by the pharmacist on each patient in our study was two hours, compared with 20–140 minutes in other studies [Citation10,Citation29]. Studies including home visits [Citation18,Citation19] estimate that two hours is required for each home visit, not including preparation, discussion with the patient's GP, and follow-up. According to Pacini et al. [Citation30], the probability of such interventions being cost-effective is low. The cost of medication reviews mainly depends on the time allotted and it is thus difficult to compare costs between studies. We were unable to show any savings in the form of fewer hospital admissions or less use of primary health care, since this study was powered to detect a reduction of 25% in DRPs and not reductions in health care utilization.
The study was conducted at a primary care centre in an area with many elderly inhabitants. The perspective was on the individual patient's everyday drug use under normal circumstances, focusing on patients’ understanding and possible problems (such as ADEs and interactions), rather than on problems arising in hospitals. The pharmacist was well integrated in the work at the health care centre, and thus had the chance for day-to-day interaction with doctors and other health care personnel. The key to achieving results lay in the pharmacist working with others within a clinical setting, rather than on their own. This is supported by many other studies [Citation8,Citation9,Citation14,Citation16].
All parameters for the intervention group appeared to change in the right direction, i.e. number of drugs, DRPs, self-rated health, and health care utilization. This indicates that this might be a suitable model for primary care. The self-rated health data suggest that medication reviews have positive effects, perhaps improving patients’ understanding of their drug regimen and thereby increasing compliance.
A larger study involving more patients could hopefully show an effect on this and on health care utilization. There is also a need for studies comparing different interventions for optimizing pharmacotherapy in primary health care.
Acknowledgements
The authors would like to thank Eva Hallman (RN), Jennie Björnevik (RN), Henrik Hallberg (MD), and the staff at Liljeholmen Primary Care Centre. They also thank Fredrik Ros for extracting health care data and Professor Sven-Erik Johansson for statistical advice. They are indebted to Stephen Gilliver for his expertise and invaluable advice in proofreading the manuscript.
Funding
The trial was funded by Stockholm County Council, the Stockholm Drug and Therapeutics Committee, and Apoteket AB. BD and JH were employed by Stockholm County Council; CL and BE by Apoteket AB.
Ethics
The study was approved by the Regional Ethical Review Board in Stockholm (application number 04-143/3).
Declaration of interest
There are no conflicts of interest in connection with the paper. The authors alone are responsible for the content and writing of the paper.
References
- Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci 2002;24:46–54.
- Fryckstedt J, Asker-Hagelberg C. Lakemedelsrelaterade problem vanliga pa medicinakuten. Orsak till inlaggning hos nastan var tredje patient, enligt kvalitetsuppfoljning [Drug-related problems common in the emergency department of internal medicine: The cause of admission in almost every third patient according to quality follow-up]. Lakartidningen 2008;105:894–8.
- Odar-Cederlof I, Oskarsson P, Ohlen G, Tesfa Y, Bergendal A, Hellden A, et al. Lakemedelsbiverkan som orsak till inlaggning pa sjukhus. Vanliga medel star for merparten, visar tvarsnittsstudie [Adverse drug effect as cause of hospital admission: Common drugs are the major part according to the cross-sectional study]. Lakartidningen 2008;105:890–3.
- Bakken MS, Ranhoff AH, Engeland A, Ruths S. Inappropriate prescribing for older people admitted to an intermediate-care nursing home unit and hospital wards. Scand J Prim Health Care 2012;30:169–75.
- Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 1990;47:533–43.
- Swedish Council on Health Technology Assessment. Äldres läkemedelsanvändning: hur kan den förbättras?: en systematisk litteraturöversikt [Use of medications in elderly: how can it be improved? A systematic litterature review]. Stockholm: Statens beredning för medicinsk utvärdering; 2009.
- Swedish National Board of Health and Welfare. Indikatorer för utvärdering av läkemedelsanvändningens kvalitet hos äldre [Indicators for the evaluation of quality in drug use of the elderly]. Stockholm, Socialstyrelsens förlag; 2003.
- Bjornson DC, Hiner WO, Jr., Potyk RP, Nelson BA, Lombardo FA, Morton TA, et al. Effect of pharmacists on health care outcomes in hospitalized patients. Am J Hosp Pharm 1993;50:1875–84.
- Koshman SL, Charrois TL, Simpson SH, McAlister FA, Tsuyuki RT. Pharmacist care of patients with heart failure: A systematic review of randomized trials. Arch Intern Med 2008;168:687–94.
- Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: A randomized controlled trial. Arch Intern Med 2009;169:894–900.
- Lidstrom B, Levol R, Sidenvall B, Agnarsson I. Läkemedelsgenomgångar vid särskilda boenden i Uppsala län [Medication review at assisted living facilities in Uppsala]. FoU- Centrum äldre i Uppsala län; 2003.
- Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007;370:173–84.
- Jameson J, VanNoord G, Vanderwoud K. The impact of a pharmacotherapy consultation on the cost and outcome of medical therapy. J Fam Pract 1995;41:469–72.
- Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ 2003;169:17–22.
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ 2001;323:1340–3.
- Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: Systematic review and meta-analysis. Qual Saf Health Care 2006;15: 23–31.
- Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol 2008;65:303–16.
- Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ 2005;330:293.
- Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community: Results of a randomized, controlled effectiveness trial. Br J Clin Pharmacol 2004;58:648–64.
- Beney J, Bero LA, Bond C. Expanding the roles of outpatient pharmacists: Effects on health services utilisation, costs, and patient outcomes. Cochrane Database Syst Rev 2000: CD000336.
- Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: Global relevance. Expert Review Pharmacoeconomics & Outcomes Res 2009;9:65–83.
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: An update. Arch Intern Med 1997;157:1531–6.
- Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP 1990;24:1093–7. Epub 1990/11/01.
- Eriksson I, Unden AL, Elofsson S. Self-rated health. Comparisons between three different measures: Results from a population study. Int J Epidemiol 2001;30:326–33.
- Milos V, Rekman E, Bondesson A, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: A randomised controlled study. Drugs Aging 2013;30:235–46.
- Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care: The POLYMED randomised controlled trial. Age Ageing 2007;36:292–7.
- Blix HS, Viktil KK, Moger TA, Reikvam A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci 2006;28: 152–8.
- Bergkvist Christensen A, Holmbjer L, Midlov P, Hoglund P, Larsson L, Bondesson A, et al. The process of identifying, solving and preventing drug related problems in the LIMM study. Int J Clin Pharm 2011;33:1010–18.
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: A randomized, controlled trial in primary care. Age Ageing 2001;30:205–11.
- Pacini M, Smith RD, Wilson EC, Holland R. Home-based medication review in older people: Is it cost effective? PharmacoEconomics 2007;25:171–80.