Abstract
Background. The positive correlation between the number of recovered benign lymph nodes and patient prognosis is well established for stage II colon cancer patients. One theory explaining this correlation focuses on potential understaging of cancer specimen, implying that a specimen with few examined lymph nodes is likely to be assigned a lower N-stage than the correct one. Understaging may be the result of an insufficient examination of the specimen post-operatively, whereby few lymph nodes are recovered and potential lymph node metastases are overlooked. This study aims to investigate the association between the total lymph node harvest and the number of lymph node metastases in colon cancer specimen. Material and methods. We studied the original pathology reports of 649 patients diagnosed with T3 adenocarcinoma of the colon at the Department of Clinical Pathology and Genetics at Linköping University Hospital, Linköping, Sweden between the years 2000 and 2008. Patient demographics, specimen staging data, and lymph node recovery data were collected for each case. Results. We found a positive association between the total lymph node harvest and the number of lymph node metastases per specimen. For every additional recovered lymph node 0.17 (95% CI: 0.15–0.19) metastases were detected (p < 0.001). Discussion. Our results support the conclusion that there is no minimum number of recovered lymph nodes at which an accurate determination of nodal status can be assured. Rather than focusing on a recommended minimum number of nodes, efforts should be shifted towards developing methods assuring that colon cancer specimen are dissected in a standardized way that optimizes the lymph node harvest.
The number of lymph node metastases in colon cancer is a widely recognized significant prognostic factor as well as an important determinant for whether or not adjuvant therapy is offered to a patient Citation[1]. Multiple studies have shown a positive association between the total number of recovered lymph nodes in stage II (and in a few studies also stage III) colon cancer specimen and patient prognosis Citation[2–8]. ( explains the group staging system for colorectal cancer.) Positive correlations have been found between the total number of recovered benign lymph nodes in stage II cancer and overall patient survival Citation[2], Citation[3], Citation[5–10], as well as decreased relapse rate Citation[8], Citation[10]. Theories explaining this correlation primarily focus on the risk of understaging, extent of surgical resection, and host-tumour interactions Citation[4], Citation[5]. Understaging implies that a specimen has been assigned a lower N-stage than the correct one. It may be the result of an insufficient examination of the specimen post-operatively, whereby few lymph nodes are recovered and potential lymph node metastases are overlooked. Understaging may also be a consequence of micrometastases which can be difficult to detect on routine light-microscopic examination of H&E coloured slides Citation[11]. Either way, patients risk being diagnosed with lower stage cancers than they actually have. In addition, the surgical procedure can directly affect prognosis, as an inadequate surgical resection of the lymphovascular supply of the specimen would likely result in an insufficient resection of potential metastases. Finally, host factors such as the immune system may also affect lymph node harvest, e.g. by a strong immune response producing enlarged nodes that are easier to find on examination.
Table I. Group staging of colorectal cancer according to the TNM system
Given that multiple studies have found a positive association between lymph node harvest and patient prognosis in stage II cancer, it is reasonable to suspect that lymph node metastases may have been overlooked in specimen where few nodes were examined. Pheby et al. found that the proportion of Duke C stage colorectal cancers was 7.95% higher when an average of 18.18 as opposed to 6.41 nodes per specimen was examined Citation[12]. Goldstein showed that the proportion of specimen with lymph node metastases increased with increased total lymph node harvest for pT3 colorectal cancers Citation[13]. Both of these results indicate that more metastases are found when increasing numbers of lymph nodes are examined.
This study aims to investigate the association between the total lymph node harvest and the number of lymph node metastases in colon cancer specimen. This study is based on material from original pathology reports from a single pathology department in very recent time (oldest material from 2000). This hopefully makes our data more accurate and relevant than data retrieved from database registers, as the latter tend to include records from multiple hospitals and span decades back in time when methods and recommendations regarding colon specimen differed from those in use today.
Material and methods
Data collection
Data was collected from the patient archive of the Department of Clinical Pathology and Genetics at Linköping University Hospital in Linköping, Sweden. The pathology reports of all patients diagnosed with adenocarcinoma of the colon (excluding the rectum) between January 1, 2000 and September 17, 2008 (date of last search) were reviewed. The starting date was chosen because of concomitant alterations in the standardized pathology report whereby the number of recovered lymph nodes was added as an obligatory field. Furthermore, recommendations about lymph node harvesting changed around this time, resulting in that lymph node harvests before this date were low according to newer standards. Data was collected by reading the original pathology reports of all colon cancer cases from the stipulated time frame. The following parameters were recorded in each case: year of diagnosis, patient age at diagnosis, patient gender, TNM stage, tumour differentiation grade, total number of lymph nodes recovered, and the number of lymph node metastases. Specimen containing multiple tumours, specimen with zero recovered lymph nodes and specimen with incomplete reports (regarding above-mentioned parameters) were excluded from further analysis.
Statistics
Mean values were compared using the two-sided Student's t-test. The relationship between the total lymph node harvest and the number of lymph node metastases was first examined graphically. The relationship between the two factors suggested a positive relationship between the two, although with an increasing variation in y-axis with increasing x-value. Therefore weighted linear regression was chosen for statistical analysis. Weighting was carried out according to standard praxis in an optimal manner (weighted value inversely proportionate to the variance of each x-value). A p-value of ≤ 0.05 was considered to be statistically significant.
Results
A total of 999 patients were diagnosed with adenocarcinoma of the colon within the stated time frame. One hundred and twenty five patients were excluded according to the criteria above leaving 874 for further analysis. Of the 125 excluded cases, 29 had multiple tumours, 16 were rectal tumours incorrectly coded as colon tumours, one was a carcinoma in situ, 15 had zero recovered lymph nodes, 55 did not have the number of recovered lymph nodes reported, and nine had otherwise incomplete reports. The 874 remaining cases are presented in .
Table II. Patient demographics (n = 874)
The majority of patients (74.3%) had T3 tumours. As the T-stage (depth of tumour invasion) is an important factor for whether or not a tumour will spread, the data was stratified according to T-stage before any statistical analysis was made regarding lymph node metastases Citation[14]. The sparse number of T1, T2, and T4 tumours made it impossible to achieve any statistically significant results in subgroup analyses. It was therefore deemed necessary to limit analysis to the T3 tumours only. These 649 cases are presented in .
Table III. T3 patient demographics (n = 649)
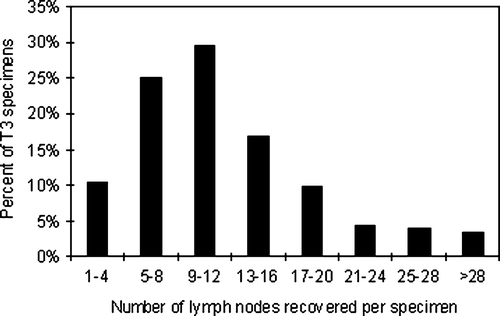
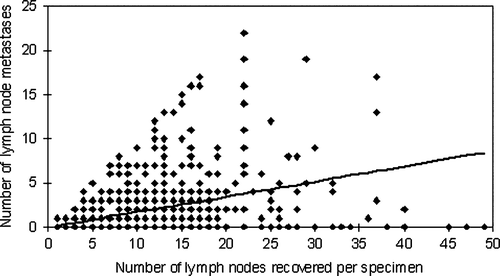
In the 321 T3 cases without lymph node metastases (N0) a mean of 11.6 lymph nodes were examined. In the 328 T3 cases with lymph node metastases (N1 and N2) a mean of 12.5 lymph nodes were examined. This difference did not reach statistical significance. shows the distribution of the T3 specimen according to the number of lymph nodes recovered per specimen. Specimen with 9 – 12 recovered lymph nodes constituted the largest group (29.6% of all T3 specimen). is a scatter plot of the total lymph node harvest versus the number of lymph node metastases per specimen. According to this weighted regression model, 0.17 (95% CI: 0.15–0.19) metastases are detected for each additional recovered lymph node. The number of detected metastases increases significantly with increased total lymph node harvest (p < 0.001).
Discussion
Accurate assessment of the lymph node status of colon cancer specimen is of the utmost importance and demands that sufficient time and resources are allocated to specimen dissection. The most significant factor from the clinical point of view is the distinction between specimen with and without lymph node metastases – stage III and stage II respectively, according to the TNM system Citation[15]. Adjuvant chemotherapy is offered primarily to those with lymph node metastases (stage III), where adjuvant therapy has been shown to be highly beneficial – reducing cancer recurrence and improving survival as opposed to after surgery alone Citation[1], Citation[16]. The point is therefore to ensure that all patients with lymph node metastases are actually offered adjuvant therapy.
Numerous studies have demonstrated a positive correlation between the total number of recovered benign lymph nodes in stage II colorectal cancer specimen and patient survival Citation[2], Citation[3], Citation[5–8]. A recent study by Vather et al. found that the mean number of examined lymph nodes in 216 Duke B cancer specimen differed significantly between patients who died within 5 years (12.8 lymph nodes) and those still alive after 5 years (17.5 lymph nodes). A similar difference was seen between patients with cancer recurrence within 5 years (11.8 lymph nodes) and those without recurrence (17.1 lymph nodes) Citation[8]. However, these results merely show an association between the number of recovered lymph nodes and patient prognosis and do not prove a causal link between finding more lymph nodes in the pathological specimen examination and improved patient prognosis. As mentioned in the introduction finding more lymph nodes could potentially reflect a more radical surgical excision, in which case the improved patient prognosis at higher lymph node harvests could be a result of a more radical excision and a concomitant reduced risk of residual tumour cells. Developing methods for finding more lymph nodes would then assumedly not give much benefit since the problem lies with the surgeon and not the pathologist. Another possible explanation for the association between the lymph node harvest and prognosis is interactions between host and tumour. A strong immune response against the tumour might provide a survival benefit while at the same time producing enlarged lymph nodes that are easier to find on examination. In this case, a high lymph node harvest would reflect a good patient prognosis because of a strong immune response and simply searching for more lymph nodes in a specimen would not contribute much in itself. However, there is the possibility of understaging where high lymph node harvests correlate with good prognosis because of the smaller risk of overlooking metastases in comparison to when fewer nodes are examined. In this case, finding high numbers of lymph nodes in a specimen should provide a benefit.
It is routine practice among pathologists to search for lymph nodes by slicing through the pericolic fat while observing the sliced surfaces and palpating the fat. This technique most often yields ≥ 12 lymph nodes and the search is therefore regarded as adequate for accurate determination of nodal status. However, this assumption can prove highly deleterious as it tends to underestimate the importance of smaller nodes that risk being overlooked. Mönig et al. investigated the correlation between lymph node size and metastatic infiltration in colon cancer. While they found, as is commonly assumed, that malign lymph nodes are on average larger than their benign counterparts, they also showed that a majority (53%) of malign nodes have diameters < 5 mm and that lymph node size can therefore not be considered a reliable indicator of metastatic spread Citation[17]. Richter et al., using an acetone fat clearing method for examining colorectal cancer specimen, established that finding more lymph nodes meant finding more metastases. When initially searching manually for lymph nodes and then applying the clearing method on the same specimen, 15% (out of 188 specimens) were found to have additional or previously undetected metastases and 8% of specimen had to have their N-stage upgraded. Nine specimens were upgraded from N0 to N1 or N2 (from stage II to stage III) Citation[18].
Much time and effort has been spent attempting to identify a minimum number of recovered lymph nodes at which the N-stage of a specimen can be accurately determined Citation[2], Citation[3], Citation[6], Citation[7], Citation[10], Citation[19], Citation[20]. demonstrates that the number of lymph node metastases per specimen increased with increased total lymph node harvest. In agreement with previous studies our results support the idea of there being no reliable cut-off number and that as many lymph nodes as possible should be recovered Citation[8], Citation[13], Citation[21]. Relying on a specific cut-off number could have potentially adverse effects on the accuracy of nodal status determination. Having identified the recommended minimum number of lymph nodes, one may be less inclined to continue the search with the same diligence in the remaining portion of the specimen.
While it is theoretically easy to argue that all lymph nodes in a specimen should be recovered and examined, the two ever-present factors of time and economy must also be considered. It is impossible to manually recover every single lymph node in a specimen within a reasonable time-frame without resorting to special fat-clearing techniques, which in themselves are laborious. Other methods enhancing visualization of lymph nodes, such as simple lymphatic system dye techniques and specialized fixatives such as GEWF (glacial acetic acid, ethanol, distilled water, and formaldehyde) have been described as simple and inexpensive Citation[22–24]. In our opinion, the most worthwhile compromise would be to establish routines to consistently follow methods for how to handle colon specimen, either with standardized protocols for manual specimen dissection or simple visualization techniques.
Conclusion
The number of lymph nodes with metastases increases as the total lymph node harvest increases. There does not appear to be a minimum number of lymph nodes at which an accurate determination of nodal status can be assured. Methods should be developed assuring that colon cancer specimen are dissected in a standardized way that optimizes the lymph node harvest.
Acknowledgements
Statistical analysis was performed in collaboration with Karl Wahlin (assistant professor) and Olle Eriksson (associate professor) at the Division of Statistics, Department of Computer and Information Science at Linköping University, Linköping, Sweden. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chau I, Cunningham D. Adjuvant therapy in colon cancer–what, when and how?. Ann Oncol 2006; 17: 1347–59
- Caplin S, Cerottini JP, Bosman FT, Constanda MT, Givel JC. For patients with Dukes' B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998; 83: 666–72
- Cianchi F, Palomba A, Boddi V, Messerini L, Pucciani F, Perigli G, et al. Lymph node recovery from colorectal tumor specimens: Recommendation for a minimum number of lymph nodes to be examined. World J Surg 2002; 26: 384–9
- Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 2006; 24: 3570–5
- Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer 2005; 41: 272–9
- Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003; 10: 65–71
- Tsai HL, Lu CY, Hsieh JS, Wu DC, Jan CM, Chai CY, et al. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg 2007; 11: 660–5
- Vather, R, Sammour, T, Zargar-Shoshtari, K, Metcalf, P, Connolly, A, Hill, A. Lymph node examination as a predictor of long-term outcome in Dukes B colon cancer. Int J Colorectal Dis 2008, [Epub ahead of print].
- Jestin P, Pahlman L, Glimelius B, Gunnarsson U. Cancer staging and survival in colon cancer is dependent on the quality of the pathologists' specimen examination. Eur J Cancer 2005; 41: 2071–8
- Luna-Perez P, Rodriguez-Ramirez S, Alvarado I, Gutierrez de la Barrera M, Labastida S. Prognostic significance of retrieved lymph nodes per specimen in resected rectal adenocarcinoma after preoperative chemoradiation therapy. Arch Med Res 2003; 34: 281–6
- Iddings D, Ahmad A, Elashoff D, Bilchik A. The prognostic effect of micrometastases in previously staged lymph node negative (N0) colorectal carcinoma: A meta-analysis. Ann Surg Oncol 2006; 13: 1386–92
- Pheby DF, Levine DF, Pitcher RW, Shepherd NA. Lymph node harvests directly influence the staging of colorectal cancer: Evidence from a regional audit. J Clin Pathol 2004; 57: 43–7
- Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: Recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 2002; 26: 179–89
- Kim J, Huynh R, Abraham I, Kim E, Kumar RR. Number of lymph nodes examined and its impact on colorectal cancer staging. Am Surg 2006; 72: 902–5
- Wittekind, C, Greene, F, Hutter, R, M Klimpfinger, L Sobin. TNM Atlas: Illustrated guide to the TNM classification of malignant tumours, 5th ed. Germany: Springer; 2005. p 101–113.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990; 322: 352–8
- Monig SP, Baldus SE, Zirbes TK, Schroder W, Lindemann DG, Dienes HP, et al. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol 1999; 6: 579–81
- Richter D, Lorenz D, Isemer FE, Braun S, Fisseler-Eckhoff A. [Acetone treatment of lymph node preparations in staging colorectal specimens]. Pathologe 2007; 28: 269–72
- Tepper JE, O'Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, 3rd, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001; 19: 157–63
- Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999; 17: 2896–900
- Cserni G, Vinh-Hung V, Burzykowski T. Is there a minimum number of lymph nodes that should be histologically assessed for a reliable nodal staging of T3N0M0 colorectal carcinomas?. J Surg Oncol 2002; 81: 63–9
- Iversen LH, Laurberg S, Hagemann-Madsen R, Dybdahl H. Increased lymph node harvest from colorectal cancer resections using GEWF solution: A randomized study. J Clin Pathol 2008; 61: 1203–8
- Markl B, Kerwel TG, Jahnig HG, Oruzio D, Arnholdt HM, Scholer C, et al. Methylene blue-assisted lymph node dissection in colon specimens: A prospective, randomized study. Am J Clin Pathol 2008; 130: 913–9
- Newell KJ, Sawaka BW, Rudrick BF, Driman DK. GEWF solution: An inexpensive, simple, and effective aid for the retrieval of lymph nodes from colorectal cancer resections. Arch Pathol Lab Med 2001; 125: 642–5