Abstract
Background. To review the currently available therapeutic modalities for radiation retinopathy (RR), including newer investigational interventions directed towards specific aspects of the pathophysiology of this refractory complication. Methods. A review of the literature encompassing the pathogenesis of RR and the current therapeutic modalities available was performed. Results. RR is a chronic and progressive condition that results from exposure to any source of radiation. It might be secondary to radiation treatment of intraocular tumors such as choroidal melanomas, retinoblastomas, and choroidal metastasis, or from unavoidable exposure to excessive radiation from the treatment of extraocular tumors like cephalic, nasopharyngeal, orbital, and paranasal malignancies. After the results of the Collaborative Ocular Melanoma Study, most of the choroidal melanomas are being treated with plaque brachytherapy increasing by that the incidence of this radiation complication. RR has been reported to occur in as many as 60% of eyes treated with plaque radiation, with higher rates associated with larger tumors. Initially, the condition manifests as a radiation vasculopathy clinically seen as microaneurysms and telangiectases, with posterior development of retinal hard exudates and hemorrhages, macular edema, neovascularization and tractional retinal detachment. Regrettably, the management of these eyes remains limited. Photodynamic therapy, laser photocoagulation, oral pentoxyphylline and hyperbaric oxygen have been attempted as treatment modalities with inconclusive results. Intravitreal injections of anti-vascular endothelial growth factor such as bevacizumab, ranibizumab and pegaptanib sodium have been recently used, also with variable results. Discussion. RR is a common vision threatening complication following radiation therapy. The available therapeutic options are limited and show unsatisfactory results. Further large investigative studies are required for developing better therapeutic as well as preventive treatment strategies.
Radiation retinopathy (RR) is a chronic and progressive condition that may result from the exposure to any source of radiation including: external beam radiation, plaque brachytherapy, proton beam radiation, helium ion radiotherapy, and gamma knife radiotherapy [Citation1–5]. RR may be secondary to the treatment of intraocular tumors such as choroidal melanomas, retinoblastomas, and choroidal metastasis or from unavoidable exposure to excessive radiation from the treatment of cephalic, nasopharyngeal, orbital, and paranasal tumors among other malignancies [Citation2,Citation6–8].
Following the Collaborative Ocular Melanoma Study (COMS) therapeutic options for choroidal melanomas, the most common primary malignancy of the eye, have shifted from enucleation of the eye to plaque brachytherapy for medium-size melanomas since the survival rate has been found to be similar [Citation9,Citation10]. This shift towards globe salvaging strategies has increased the use of radiation and consequently increased its complications with the incidence of RR ranging from 3 to over 20% [Citation11–13]. A recent retrospective study reported an incidence of RR with associated retinal neovascularization (proliferative RR) of 5.8% at five years and 7% at ten and 15 years in 3 841 eyes treated with plaque radiotherapy for uveal melanoma [Citation6].
The development of RR has always been related to the total dose of radiation administered to the retina, accepting 35 Gy as the upper limit of safe total dosage [Citation14,Citation15]. Although cases of radiation retinopathy have been reported after much lower levels of irradiation [Citation16,Citation17]. Several studies involving the use of hyperfractionated radiotherapy and reducing the radiation given by plaque brachytherapy from 85 Gy, as indicated by the COMS to a range of 56–69 Gy were shown to have the same survival and local tumor control [Citation18,Citation19]. On the other hand, several studies have found various risk factors for developing RR, such as shorter tumor distance from the optic nerve, preexistent diabetes mellitus, and young age, unrelated to the amount of radiation given [Citation12,Citation20].
Pathogenesis
Retinal vascular endothelial cell damage is believed to initiate the development of RR [Citation21]. The structure of the heterochromatic nuclear DNA of endothelial cells permits less enzymatic repair, rendering dividing retinal vascular cells more susceptible to ionizing radiation [Citation22]. In addition, a small percentage of non-mitotic cells will suffer immediate death if enough radiation is absorbed during the initial insult. Cell death stimulates division and migration of cells in the vicinity to repair the discontinuity in the vessel wall. Migratory cells may also succumb to the effects of radiation during mitosis leading to a vicious cycle that might trigger the clotting cascade due to incompetence of the vascular endothelium leading to clinically observable retinopathy in the form of microaneurysms, telangiectases, neovascularization, vitreous hemorrhage, macular edema and tractional retinal detachment [Citation23–25] (). From a clinical standpoint, RR can be classified as non-proliferative and proliferative, both of which may be associated with macular edema that has been associated with a worse visual prognosis [Citation26].
Management
The management of RR remains to be challenging. The following discussion will address currently available therapeutic options including newer, investigational interventions directed towards specific aspects of the pathophysiology of this refractory complication.
Retinal laser photocoagulation
Light Amplification by the Stimulated Emission of Radiation (LASER) can produce thermal denaturation of tissue when a high intensity laser source of appropriate wavelength is absorbed by hemoglobin and other ocular tissues [Citation27,Citation28]. There is a broad spectrum of indications for laser photocoagulation in ocular diseases such as diabetic retinopathy, branch retinal vein occlusions, and treatment of retinal tears [Citation29–32]. Photocoagulation may also be employed as a prophylaxis against neovascular glaucoma and to treat macular edema secondary to RR [Citation33–36] ().
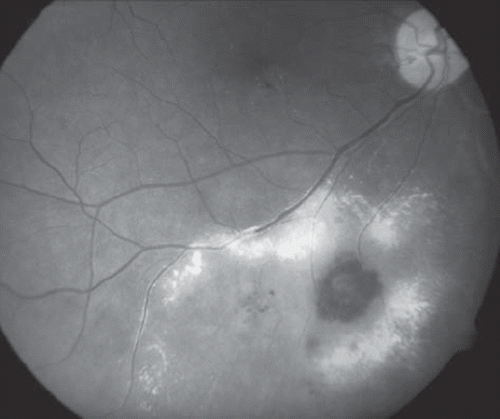
Figure 2. Fundus photograph of the right eye of a patient treated with 125I plaque for a choroidal melanoma showing microaneurysms, telangiectases, and retinal neovascularization secondary to radiation retinopathy. Note the laser photocoagulation scars.
As for RR, several studies have explored the effectiveness of this treatment modality. Finger et al. investigated the use of sector argon laser photocoagulation in early stages of RR (mean laser sessions of 2.75) where they observed regression of RR in 64% of the eyes that had developed RR and found that 18.75% of the “high risk” patients who were treated prophylactically developed RR [Citation36].
Laser photocoagulation has been used for the treatment of macular edema secondary to RR. Hykin et al. found that 42% of the affected eyes experienced at least an improvement of one line on the Snellen chart at six months when compared to the observational group; however the difference at 12 and 24 months was not significant [Citation37]. In the same token, Kinyoun et al. observed an improvement in the visual acuity in 67% of the eyes after an average of one retreatment for recurrent macular edema, concluding that this treatment modality might be more effective in preventing further visual deterioration rather than restoring vision [Citation34,Citation35]. In addition to that, panretinal photocoagulation was found to be effective in the treatment of the retinopathy following radiation for head and neck tumors [Citation38].
Retinal photocoagulation remains the gold standard in the treatment of most forms of ischemic retinopathies; however the beneficial effect for prophylactic photocoagulation in RR remains unjustified in the absence of larger randomized, controlled studies especially that not all patients treated with radiation will develop RR. This raises the question if it is justified to ablate the retina surrounding the tumor prophylactically.
Photodynamic therapy
Photodynamic therapy (PDT) is a two step process in which an intravenous infusion of verteporfin is followed (typically 15 minutes later) by irradiance with a 689 nm laser for about 83 seconds. Verteporfin binds to low density lipoproteins in the plasma during this process, which are then preferentially bound by choroidal neovascular membranes (a neovascular network of new blood vessels in the choroid). Application of laser energy results in formation of toxic oxygen species that induce thrombosis of choroidal neovessels [Citation39,Citation40]. PDT was approved by the United States Food and Drug Administration (US FDA) in 2000 for the treatment of predominantly classic subfoveal lesions associated with age-related macular degeneration.
A small study showed the effect of PDT in four patients with RR related macular edema. All eyes demonstrated improvement in visual acuity [Citation41]. A case study by Lee et al. reported visual improvement six months after PDT in a patient with choroidal neovascular membrane secondary to external beam radiation [Citation42].
The use of PDT in RR has been insufficiently investigated so far, with the few studies done showing a beneficial effect; however, more robust evidence is required.
Corticosteroids
Inflammation is implicated in the pathogenesis of an increasing number of ocular diseases. Corticosteroids are frequently employed in order to inhibit migration and activation of inflammatory cells. Corticosteroids block the pathways of selectins, integrins, Inter-Cellular Adhesion Molecule 1 (ICAM-1), tumor necrosis factor-α and monocyte chemo-attractive protein-1 (MCP-1) at various levels [Citation43]. Corticosteroids also have a direct angiostatic effect by up-regulating the extracellular-matrix protein plasminogen activator inhibitor, and reducing vascular permeability [Citation43]. These characteristics may provide a greater benefit than therapeutic modalities that block a single molecule or pathway.
Since the early 1980s, intravitreal triamcinolone acetonide has been used to treat vitreo-retinal proliferation [Citation44]. In preclinical studies triamcinolone inhibited cytokine-induced upregulation of ICAM-1 by endothelial cells and reduced hypoxia-induced upregulation of VEGF by cultured retinal pigment epithelium cells [Citation45]. As for its use for RR treatment, Shields et al. reported the effect of intravitreal triamcinolone (4 mg/1 mL) in a prospective, non-randomized, single-center case series of 31 patients with visually symptomatic radiation-induced macular edema after plaque radiotherapy. They reported that after intravitreal injection of triamcinolone acetonide, visual acuity was stable or improved in 91% of patients by one month and 45% by six months. There was also a decrease in the mean central subfield foveal thickness measured by optical coherence tomography (OCT) [Citation46]. Suttler et al. also reported the benefit of triamcinolone use in a patient who developed RR six years after external beam irradiation (5 400 rad [54 Gy] in 30 fractions [5 fractions per week]) for a left parotid gland carcinoma [Citation47].
Despite the potential benefits, intravitreal injection of triamcinolone actetate is associated with side effects, including glaucoma, cataracts, retinal detachment, and endophthalmitis [Citation48,Citation49]. This has lead to investigating periocular triamcinolone in an effort to avoid or reduce the incidence of these side effects.
Horgan et al. evaluated the potential benefit of periocular depot triamcinolone in the prevention of macular edema secondary to 125I plaque for uveal melanoma. They found that this treatment modality significantly decreased the risk of macular edema after plaque radiotherapy, but did not significantly alter the rate of vision loss at 24 months of follow-up [Citation50]. Recently, a larger prospective, randomized, controlled clinical trial conducted by this same group, in patients with uveal melanoma treated with 125I plaque showed that periocular injection of triamcinolone (40 mg/1 mL) given at the time of the plaque application and four and eight months later effectively reduced the risk of macular edema and moderate vision loss for 18 months [Citation51].
Anti-vascular endothelial growth factor agents (anti-VEGF)
A strong relationship has been found between vascular endothelial growth factor (VEGF) and the development of diabetic retinopathy, exudative age-related macular degeneration, and ocular ischemic diseases.
VEGF is a protein secreted under hypoxic conditions that promotes vascular leakage and angiogenesis [Citation52]. Animal models have demonstrated the development of the pathologic changes characteristic of diabetic retinopathy following intravitreal injection of VEGF [Citation53,Citation54].
Bevacizumab. Bevacizumab (Avastin, Genentech) is a potent monoclonal antibody that blocks all VEGF-A isoforms. Bevacizumab was the first anti-VEGF therapy approved by the US FDA for the treatment of colorectal, breast, and lung cancer [Citation55]. After the results of preliminary studies with a similar molecule, ranibizumab (Lucentis, Genentech) in the treatment of exudative age-related macular degeneration, ophthalmologists were motivated to use bevacizumab off-label, both systemically and intravitreally, to treat this disease as well as other forms of choroidal neovascular membranes, neovascular glaucoma and diabetic retinopathy [Citation56–59]. Following that, the efficacy of bevacizumab for the treatment of RR was investigated ().
Figure 3. Optical coherence tomography image in a patient with radiation retinopathy following 106Ru brachytherapy for choroidal melanoma. (A) Note the increased foveal retinal thickness with evident cystic spaces. (B) Image of the same patient taken 12 weeks after two intravitreal injections of bevacizumab. Note the significant decreased in the retinal thickness and resolution of the cystic spaces.
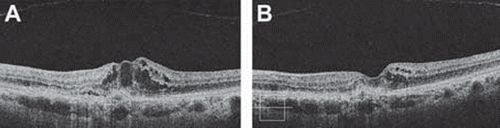
A retrospective case series of ten consecutive patients evaluated the effect of intravitreal bevacizumab on macular edema related to RR after brachytherapy for choroidal melanoma. These patients were treated with a single intravitreal injection of bevacizumab after the development of macular edema. The mean visual acuity improved from 20/100 to 20/86 at six weeks and 20/95 at four months. Mean foveal thickness measured by OCT was 482 μm before injection, 284 μm six weeks after injection, and 449 μm four months after injection [Citation60].
Gupta et al. evaluated the safety and efficacy of 1–2 intravitreal injections of bevacizumab in an interventional case series of five patients who developed radiation related macular edema after 106Ru for choroidal melanoma. Two of the five patients showed resolution of macular edema while the other three patients who had a longstanding disease showed no improvement after a single suggesting that younger patients with shorter duration of macular edema benefited the most [Citation61].
Finger et al. evaluated the effectiveness of intravitreal bevacizumab for radiation retinopathy in six patients who underwent plaque radiotherapy. Following periodic (every six to eight weeks) intravitreal bevacizumab (1.25 mg in 0.05 mL), there was a decrease in macular edema, improvement or maintenance of visual acuity and reduced hemorrhage and retinal edema [Citation62]. Shortly thereafter, the same group published results of 21 patients consistent with the first one whereby reduction in retinal hemorrhage, exudation, and edema was noted after the treatment and visual acuities were stable or improved in 86% with 14% of patients regaining two or more lines of visual acuity [Citation63]. No ocular or systemic side effects were observed in both studies.
Vasquez et al. reported the use of intracameral bevacizumab in a patient with neovascular glaucoma and exudative retinal detachment following 125I brachytherapy for a choroidal melanoma. The end result following the intracameral injection showed a resolution of the retinal detachment, and decrease of the intraocular pressure (with topical medication), however, the visual acuity did not improve [Citation64].
Additional case reports describe improvement in the central subfield foveal thickness, ocular neovascularization and visual acuity after the injection of intraocular bevacizumab following 106Ru and stereotactic radiotherapy [Citation65–67].
Ranibizumab. Ranibizumab (Lucentis, Genentech, Inc) is a small antibody fragment synthesized to have an increased affinity (100 times greater) for all isoforms of VEGF-A, which reduces the vascular permeability and angiogenesis in vivo and in vitro [Citation68]. Ranibizumab is US FDA approved for the treatment of choroidal neovascularization secondary to age-related macular degeneration [Citation69].
Its use for the treatment of RR is also under study and seems to be promising. Dunavoelgyi et al. described a case of a 72-year-old patient with a juxtapapillary choroidal melanoma treated with sterotactic linear accelerator who developed medically uncontrolled neovascular glaucoma, optic neuropathy and a bullous retinal detachment with subretinal exudates. Intravitreal ranibizumab (0.05 mg) injection was followed by significant decrease in ocular neovascularization and a drop in intraocular pressure at two weeks and resolution of iris neovascularization and retinal detachment were evident at 24 weeks [Citation70].
A recent phase 1, open-label, Genentech-sponsored study of five consecutive patients with RR related macular edema, secondary to the treatment with 103Pd for uveal melanoma, showed visual acuity improvement in four of five patients and decrease in the foveal thickness in all cases after monthly intravitreal ranibizumab (0.5 mg) injections for at least four cycles [Citation71].
Pepagtanib sodium. The first drug developed as a selective blocker of a VEGF isoform was pegaptanib sodium (Macugen, (OSI) Eyetech, Inc.). It acted as an anti-VEGF RNA aptamer that binds selectively to VEGF165 [Citation72]. This drug was initially approved by the US FDA for the treatment of neovascular AMD, but has also been used in the treatment of proliferative diabetic retinopathy [Citation73,Citation74].
Recently, Querques et al. published a case of a 63-year-old woman who developed RR 14 months after the treatment with a 106Ru episcleral plaque for a choroidal melanoma, characterized by capillary changes and retinal hemorrhages and exudates around the fovea. She was treated with laser photocoagulation but still developed neovascularization of the optic disc and retinal exudation involving the macula. Following intravitreal injection of 0.3 mg of pegaptanib sodium [Macugen; (OSI) Eyetech and Pfizer Inc., Melville, New York, USA], an improvement in the visual acuity and the macular exudation and optic disc neovascularization were observed at one month and maintained to the last follow-up (six months) [Citation75].
Most of the studies published in the literature suggest that anti-VEGF agents may have a role in the treatment of radiation retinopathy, especially in the treatment of macular edema, and ocular neovascularization, although few studies demonstrate improvement in visual acuity. Because of the potential side effects, and the need for multiple injections to achieve sustained results, the role of bevacizumab and other anti-VEGF medications in the long-term management of radiation retinopathy has not been yet fully elucidated.
Other therapies
Application of some other modalities of treatment for radiation retinopathy, like hyperbaric oxygen therapy and oral pentoxifylline have been reported but more evidence is required to prove their efficacy and safety.
Hyperbaric oxygen therapy has shown success in the treatment of various ocular pathologies like vasculopathies, avascular scleral necrosis, vascular cystoid macular edema and radiation-induced optic neuropathies based on the concept that it improves oxygenation and acts as a sensitizer of hypoxic cells [Citation76]. In terms of its use in radiation retinopathy, one reported case describes a 68-year-old woman with choroidal melanoma who developed 106Ru-induced RR and optic neuropathy. The patient was treated with hyperbaric oxygen (2 h 100% O2 at 2 atm for 20 sessions) for two months with significant improvement in her visual field and fundus examination [Citation77]. However, another report describing its use simultaneously with the radiotherapy revealed severe vaso-occlusion of the retinal vessels that might be due to a synergistic vasoconstrictive effect of hyperbaric oxygen and radiation [Citation78].
Another reported therapeutic modality with favorable outcome is oral pentoxifylline. It is a methyl-xanthine derivative approved for the treatment of peripheral vascular diseases and thought to help in the management of radiation-induced fibrosis of soft tissues [Citation79]. Its effect is based on the ability of the drug to cause vasodilation and decrease blood viscosity, thus improving blood flow and oxygenation. As for its use in radiation retinopathy, a report presents a 28-year-old woman who underwent stereotactic radiosurgery for the treatment of a left temporal lobe medulloblastoma and developed radiation retinopathy. Treatment with oral pentoxifylline (400 mg, 3 times daily) for eight months showed normal reperfusion of the capillary beds and significantly improved visual acuity [Citation80].
Summary
Retinopathy is a significant cause of morbidity in patients treated with radiation. Established, successful long-term management strategies for this condition are lacking. Most of the current therapies have been copied from the treatment of other ischemic retinopathies especially diabetic retinopathy. However, the vascular damage observed in RR occurs at the level of the endothelial cells, while in diabetes the damage occurs mainly at the level of the pericytes, which may explain the poor results obtained in the treatment of RR [Citation23,Citation24].
Retinal photocoagulation remains the gold standard in the treatment of most forms of ischemic retinopathies. Although some reports demonstrate a beneficial effect for prophylactic photocoagulation in RR, larger randomized, controlled studies are needed to corroborate this hypothesis. In addition to that, not all patients treated with radiation will develop RR. This raises the question of whether prophylactically ablating the healthy surrounding retina would be justified. Alternative treatment options such as corticosteroids, have also been employed in the treatment of RR. A recent study that included 163 patients with uveal melanoma treated with 125I plaque showed that periocular injection of triamcinolone (40 mg/1 mL) given at the time of the plaque application and four and eight months later effectively reduced the risk of macular edema and moderate vision loss for 18 months [Citation51].
Intravitreal anti-VEGF-A medications have been widely used in ischemic ocular diseases. There have been several studies, mostly case reports that showed a benefit in using these drugs in the treatment of RR. However, most of the currently used medications, such as corticosteroids, bevacizumab, ranibizumab and pegaptanib, only have a temporal effect, requiring multiple subsequent treatments. Currently, there is an ongoing interventional, randomized, single blinded study, the Treatment of Radiation Retinopathy Trial (TORR), on patients treated for uveal melanomas. The aim of the study is to demonstrate the superiority of intravitreal ranibizumab (0.5 mg) or triamcinolone acetonide (4.0 mg) treatment to no treatment, in the mean change from baseline in best-corrected visual acuity (http://clinicaltrials.gov/ct2/show/NCT00811200). This might offer more powerful clinical evidence about treatment options. However, an important point to take into consideration before treating patients with RR is that some have resolution of the ocular pathology without treatment making it difficult to judge the effect of the intervention. Undoubtedly, a multi-center collaborative effort will be needed in order to design clinical trials with sufficient statistical power to evaluate the safety, efficacy, and role of emerging pharmacotherapeutic agents. The development of preventive strategies remains paramount to avoidance of this potentially blinding condition.
Acknowledgements
No funding support to disclose/no proprietary or financial interests to disclose.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Methods of Literature Search
A MEDLINE search of the English language literature from 1971 to present was conducted. The search terms used were: Radiation retinopathy, radiation maculopathy, choroidal melanoma, uveal melanoma, retinoblastoma, intracranial tumors, photodynamic therapy, laser photocoagulation, corticosteroids, bevacizumab, ranibizumab, pegaptanib, and VEGF inhibitors.
References
- Finger PT, Chin KJ, Duvall G, Palladium-103 for Choroidal Melanoma Study Group. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology 2009;116:790–6, 796.e1.
- Krema H, Somani S, Sahgal A, Xu W, Heydarian M, Payne D, . Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol 2009;93:1172–6.
- Gragoudas ES, Seddon JM, Egan K, Glynn R, Munzenrider J, Austin-Seymour M, . Long-term results of proton beam irradiated uveal melanomas. Ophthalmology 1987; 94:349–53.
- Haas A, Pinter O, Papaefthymiou G, Weger M, Berghold A, Schrottner O, . Incidence of radiation retinopathy after high-dosage single-fraction gamma knife radiosurgery for choroidal melanoma. Ophthalmology 2002;109:909–13.
- Levy RP, Fabrikant JI, Frankel KA, Phillips MH, Lyman JT, Lawrence JH, . Heavy-charged-particle radiosurgery of the pituitary gland: Clinical results of 840 patients. Stereotact Funct Neurosurg 1991;57:22–35.
- Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology 2010.
- Egbert PR, Donaldson SS, Moazed K, Rosenthal AR. Visual results and ocular complications following radiotherapy for retinoblastoma. Arch Ophthalmol 1978;96:1826–30.
- Amoaku WM, Archer DB. Cephalic radiation and retinal vasculopathy. Eye (Lond) 1990;4(Pt 1):195–203.
- Shields CL, Shields JA, Milite J, De Potter P, Sabbagh R, Menduke H. Uveal melanoma in teenagers and children. A report of 40 cases. Ophthalmology 1991;98:1662–6.
- Phillpotts BA, Sanders RJ, Shields JA, Griffiths JD, Augsburger JA, Shields CL. Uveal melanomas in black patients: A case series and comparative review. J Natl Med Assoc 1995;87:709–14.
- Shields CL, Naseripour M, Cater J, Shields JA, Demirci H, Youseff A, . Plaque radiotherapy for large posterior uveal melanomas (> or =8 mm thick) in 354 consecutive patients. Ophthalmology 2002;109:1838–49.
- Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol 1999;117:609–14.
- Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol 1999;127:579–89.
- Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology 1982; 89:1494–501.
- Chacko DC. Considerations in the diagnosis of radiation injury. JAMA 1981;245:1255–8.
- Elsas T, Thorud E, Jetne V, Conradi IS. Retinopathy after low dose irradiation for an intracranial tumor of the frontal lobe. A case report. Acta Ophthalmol (Copenh) 1988;66:65–8.
- Lopez PF, Sternberg P, Jr, Dabbs CK, Vogler WR, Crocker I, Kalin NS. Bone marrow transplant retinopathy. Am J Ophthalmol 1991;112:635–46.
- Moore RF. Choroidal sarcoma treated by the intraocular insertion of radon seeds. Br J Ophthalmol 1930;14:145–52.
- Saconn PA, Gee CJ, Greven CM, McCoy TP, Ekstrand KE, Greven KM. Alternative dose for choroidal melanoma treated with an iodine-125 radioactive plaque: A single-institution retrospective study. Int J Radiat Oncol Biol Phys 2010.
- Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology 1999;106:1571–7; Discussion 1577–8.
- Archer DB. Doyne Lecture. Responses of retinal and choroidal vessels to ionising radiation. Eye (Lond) 1993;7(Pt 1):1–13.
- Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol 1994;5:59–65.
- Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy–clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond) 1991;5(Pt 2):239–51.
- Irvine AR, Alvarado JA, Wara WM, Morris BW, Wood IS. Radiation retinopathy: An experimental model for the ischemic–proliferative retinopathies. Trans Am Ophthalmol Soc 1981;79:103–22.
- Midena E, Segato T, Valenti M, Degli Angeli C, Bertoja E, Piermarocchi S. The effect of external eye irradiation on choroidal circulation. Ophthalmology 1996;103:1651–60.
- Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:325–35.
- Leuenberger PM, Englert U, Schepens JM. Biological effect of laser-photo-coagulation on the retina (author's transl). Klin Monbl Augenheilkd 1977;170:228–37.
- Diddie KR, Ernest JT. The effect of photocoagulation on the choroidal vasculature and retinal oxygen tension. Am J Ophthalmol 1977;84:62–6.
- Augustin AJ, Scholl S, Kirchhof J. Treatment of neovascular age-related macular degeneration: Current therapies. Clin Ophthalmol 2009;3:175–82.
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103:1796–806.
- Muqit MM, Sanghvi C, McLauchlan R, Delgado C, Young LB, Charles SJ, . Study of clinical applications and safety for Pascal(®) laser photocoagulation in retinal vascular disorders. Acta Ophthalmol 2010.
- Arnarsson A, Stefansson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci 2000;41:877–9.
- Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol 2009;20:200–4.
- Kinyoun JL, Chittum ME, Wells CG. Photocoagulation treatment of radiation retinopathy. Am J Ophthalmol 1988; 105:470–8.
- Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol 1995;79: 144–9.
- Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2005;89:730–8.
- Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology 1998;105:1425–9.
- Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol 1996;114:1097–100.
- Miller JW, Walsh AW, Kramer M, Hasan T, Michaud N, Flotte TJ, . Photodynamic therapy of experimental choroidal neovascularization using lipoprotein-delivered benzoporphyrin. Arch Ophthalmol 1995;113:810–8.
- Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials–TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329–45.
- Bakri SJ, Beer PM. Photodynamic therapy for maculopathy due to radiation retinopathy. Eye (Lond) 2005;19:795–9.
- Lee SC, Song JH, Chung EJ, Kwon OW. Photodynamic therapy of subretinal neovascularization in radiation retinopathy. Eye (Lond) 2004;18:745–6.
- Nakano T, Ohara O, Teraoka H, Arita H. Glucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expression. J Biol Chem 1990;265:12745–8.
- Tano Y, Chandler D, Machemer R. Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol 1980;90:810–6.
- Matsuda S, Gomi F, Oshima Y, Tohyama M, Tano Y. Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress. Invest Ophthalmol Vis Sci 2005;46: 1062–8.
- Shields CL, Demirci H, Dai V, Marr BP, Mashayekhi A, Materin MA, . Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina 2005;25:868–74.
- Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol 2003;121: 1491–3.
- Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 2006;113:1533–8.
- Conti SM, Kertes PJ. The use of intravitreal corticosteroids, evidence-based and otherwise. Curr Opin Ophthalmol 2006; 17:235–44.
- Horgan N, Shields CL, Mashayekhi A, Teixeira LF, Materin MA, O'Regan M, . Periocular triamcinolone for prevention of macular edema after iodine 125 plaque radiotherapy of uveal melanoma. Retina 2008;28:987–95.
- Horgan N, Shields CL, Mashayekhi A, Salazar PF, Materin MA, O'Regan M, . Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: A randomized controlled trial. Ophthalmology 2009;116:1383–90.
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 2000;55:15–35; Discussion 35–6.
- Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 1996;114:964–70.
- Tolentino MJ, McLeod DS, Taomoto M, Otsuji T, Adamis AP, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol 2002;133:373–85.
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, . A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34.
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005;112:1035–47.
- Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: Six-month results of a prospective pilot study. Ophthalmology 2007;114:2190–6.
- Kondo M, Kondo N, Ito Y, Kachi S, Kikuchi M, Yasuma TR, . Intravitreal injection of bevacizumab for macular edema secondary to branch retinal vein occlusion: Results after 12 months and multiple regression analysis. Retina 2009;29: 1242–8.
- Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 2006;26:1006–13.
- Mason JO, 3rd, Albert MA, Jr, Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina 2007;27:903–7.
- Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina 2008;28:964–8.
- Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (avastin) for radiation retinopathy. Arch Ophthalmol 2007;125:751–6.
- Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008;70:974–7.
- Vasquez LM, Somani S, Altomare F, Simpson ER. Intracameral bevacizumab in the treatment of neovascular glaucoma and exudative retinal detachment after brachytherapy in choroidal melanoma. Can J Ophthalmol 2009;44:106–7.
- Ziemssen F, Voelker M, Altpeter E, Bartz-Schmidt KU, Gelisken F. Intravitreal bevacizumab treatment of radiation maculopathy due to brachytherapy in choroidal melanoma. Acta Ophthalmol Scand 2007;85:579–80.
- Solano JM, Bakri SJ, Pulido JS. Regression of radiation-induced macular edema after systemic bevacizumab. Can J Ophthalmol 2007;42:748–9.
- Arriola-Villalobos P, Donate-Lopez J, Calvo-Gonzalez C, Reche-Frutos J, Alejandre-Alba N, Diaz-Valle D. Intravitreal bevacizumab (Avastin) for radiation retinopathy neovascularization. Acta Ophthalmol 2008;86:115–6.
- Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, . Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol 2002;120:338–46.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31.
- Dunavoelgyi R, Zehetmayer M, Simader C, Schmidt-Erfurth U. Rapid improvement of radiation-induced neovascular glaucoma and exudative retinal detachment after a single intravitreal ranibizumab injection. Clin Experiment Ophthalmol 2007;35:878–80.
- Finger PT, Chin KJ. Intravitreous ranibizumab (lucentis) for radiation maculopathy. Arch Ophthalmol 2010;128:249–52.
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006;5: 123–32.
- VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group, Chakravarthy U, Adamis AP, Cunningham ET, Jr, Goldbaum M, Guyer DR, . Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006;113:1508.e1–1508.25.
- Gonzalez VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol 2009;93:1474–8.
- Querques G, Prascina F, Iaculli C, Delle Noci N. Intravitreal pegaptanib sodium (Macugen) for radiation retinopathy following episcleral plaque radiotherapy. Acta Ophthalmol 2008;86:700–1.
- Oguz H, Sobaci G. The use of hyperbaric oxygen therapy in ophthalmology. Surv Ophthalmol 2008;53:112–20.
- Gall N, Leiba H, Handzel R, Pe'er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye (Lond) 2007;21:1010–2.
- Stanford MR. Retinopathy after irradiation and hyperbaric oxygen. J R Soc Med 1984;77:1041–3.
- Chiao TB, Lee AJ. Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann Pharmacother 2005;39:516–22.
- Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: The role of pentoxifylline. Retina 2001;21: 545–7.