Abstract
Background. Sunitinib induces partial responses in 47% of patients with metastatic renal cell carcinoma (mRCC). However, the achievement of complete responses remains scarce and all patients will eventually develop progressive disease. Recombinant interleukin-21 (rIL-21) is a novel cytokine, which is believed to deliver sustained cellular anti-tumor response and the combination of both agents may work synergistically. Material and method. From July 2007 to July 2008 in this phase I trial nine therapy-naive patients with metastatic RCC in five European centers were enrolled. Patients with either good or intermediate risk according to Memorial Sloan-Kettering Cancer Center (MSKCC) were eligible without restrictions to histology subtype nor measurable disease. Patients were treated with increasing doses of rIL-21 administered subcutaneously (s.c.) in combination with sunitinib 50 mg once daily (OD) orally at the ‘4 weeks on/2 weeks off’ schedule. Dose-escalation was applied by a conventional ‘3+3 design’. Planned dose levels (DL) for rIL-21 were 3, 10, 30 and 100 μg/kg s.c. The primary endpoint was to determine the maximum tolerated dose (MTD) and recommended dose (RD). Secondary objectives included pharmacokinetics of sunitinib and rIL-21, and the induction of rIL-21 antibodies. Results. At 10 μg/kg two dose-limiting toxicities (DLT) occurred in four patients, consisting of grade 4 neutropenia and grade 3 thrombocytopenia. The MTD was 3 μg/kg rIL-21 combined with sunitinib 50 mg OD at the ‘4 weeks on/2 weeks off’ schedule. Frequent occurring adverse events were injection site reaction, stomatitis, fatigue and dysgeusia. Conclusions. The combination of sunitinib 50 mg at the ‘4 weeks on/2 weeks off’ schedule and 10 μg/kg IL-21 was not tolerated due to hematological DLTs. The dose level of 3 μg/kg rIL-21 was considered too low to be therapeutically relevant for further evaluation and therefore the study was discontinued.
Sunitinib is a tyrosine kinase inhibitor (TKI), which targets VEGFR1-3, PDGFR α/ß, KIT, RET, CSF 1R and FLT-3. Its distinct clinical activity led to the approval for treatment of metastatic renal cell carcinoma (mRCC). The pivotal trial reported an objective response rate of 47% and a progression free survival (PFS) of 11 months. Overall survival was 26.4 months for sunitinib versus 21.8 months for interferon-alpha (IFN-α), with a significant benefit in patients censored for crossover patients from IFN-α to sunitinib [Citation1]. However, complete remissions remain scarce with sunitinib treatment and were not reported to be durable after cessation of treatment [Citation2].
Immunotherapy has been known to induce complete and durable responses for many years in selected patients with mRCC, potentially delivering cure. High dose interleukin-2 (IL-2) has been reported to induce durable responses in approximately 15% of patients and duration of response was 84 months [Citation3,Citation4]. IL-21 is a four-helix bundle cytokine, most homologous to IL-15 and to a lesser extent to IL-2 and IL-4 [Citation5,Citation6]. IL-21 is a helper cytokine that orchestrates a potent innate and adaptive immune response, which leads to an amplification of the immune response. It has activity on all lymphocyte subsets, dendritic cells and to a lesser extent monocytes [Citation7]. It affects proliferation and differentiation of Natural Killer (NK) cells together with IL-2 and IL-15 [Citation6,Citation8,Citation9] and plays a role in activating CD8+ T cells to become killer cells and B cells to differentiate into potent antibody-producing cells [Citation9–11]. Together with IL-6, IL-21 has a crucial role in the differentiation of CD4+ T cells into T-helper 17 (TH17) cells [Citation12,Citation13]. Furthermore, in contrast to IL-2, IL-21 can reverse the suppressive effects of regulatory T cells (Tregs) on CD8+ T cells, thereby enhancing the host immunity against tumors [Citation14]. The efficacy of IL-2 in mRCC is limited, while the toxicity is high [Citation15]. IL-21was associated with cellular immune response in various tumor models, including RCC and melanoma [Citation6,Citation16].
The combination of a VEGFR inhibitor and immunotherapy may synergistically induce objective and durable responses. This study therefore explores the safety and tolerability of increasing doses of rIL-21 administered s.c. three times a week (TIW) in combination with the registered ‘4 weeks on/2 weeks off’ schedule of sunitinib 50 mg once daily (OD), with the aim to determine the maximum tolerated dose (MTD) and recommended dose (RD) of rIL-21 of this combination.
Patients and methods
Patient selection and study design
Therapy-naive patients with mRCC with adequate organ function were eligible for the study. Histology was unrestricted and measurable disease was not mandatory. Key inclusion criteria consisted of age greater than 18 years, ECOG performance status of 0 or 1, 0-2 risk factors according to Memorial Sloan-Kettering Cancer Center (MSKCC) criteria and mandatory prior nephrectomy. Key exclusion criteria consisted of CNS metastases, chronic infectious disease, prior or active auto-immune disease and cardiovascular events within the last 12 months. All patients gave written informed consent according to Good Clinical Practice (GCP) requirements prior to study inclusion. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by institutional review boards of participating centers. Granulocyte colony-stimulating factor was not allowed. The use of strong inducers or inhibitors of the cytochrome P450 3A4 (CYP3A4) system within two weeks of first dosing were not allowed because of interactions with sunitinib. Weak inducers or inhibitors were only used with caution and preferably changed into comparable drugs without CYP3A4 metabolism.
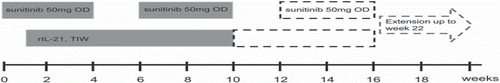
The study was designed as a combined phase I and II trial with a conventional 3+3 dose-escalation in order to evaluate the safety, tolerability and MTD of increasing doses of rIL-21 administered s.c. TIW starting at 3 μg/kg in combination with sunitinib 50 mg OD at the ‘4 weeks on/2 weeks off’ schedule (). Planned dose levels (DL) were 3, 10, 30 and 100 μg/kg. The starting DL was 3 μg/kg. Treatment with sunitinib was initiated one week in advance of rIL-21 to observe the toxicity of sunitinib as a single agent during the first week. The safety evaluation was performed after four weeks of combined treatment of the third patient at each dose level. If a dose limiting toxicity (DLT) was observed in one patient at any DL, three additional patients were allocated to the same DL. The phase II part of the trial was designed as two-arm parallel comparison of sunitinib with or without the addition of rIL-21. Due to the outcome of the phase I part of the trial, the phase II part of the trial was never initiated.
Figure 1. Study design of the phase I part of the study. Sunitinib was initiated 1 week prior to rIL-21 exposure and consisted of 50 mg OD at a ‘4 weeks on and 2 weeks off’ schedule.
Toxicities were continuously evaluated according to Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0. A DLT was defined as an adverse event related to treatment with combined rIL-21 and sunitinib with CTCAE severity grade ≥ 3 with exceptions for grade 3 fever, grade 3 asymptomatic hyperglycemia, grade 3 or 4 hyperuricaemia, grade 4 lymphopenia lasting ≤ seven days and grade 3 neutropenia ≤ seven days. Grade 4 fever with response to paracetamol to grade 3 was not considered a DLT.
MTD was defined as the highest dose-level administered with ≤ one of six patients experiencing a DLT. Patients experiencing sunitinib-related severe toxicity as defined above within the first week of single agent administration had to be replaced.
Pharmacokinetic, pharmacodynamic and pharmacogenomic evaluations
For pharmacokinetic analysis of rIL-21 and sunitinib samples were taken at day 8, i.e. the first day of the combination of sunitinib and IL-21, at baseline, and 30 minutes, 2, 4, 6, 8, 10, 12 and 24 hours after administration. Concentrations of rIL-21 were detected in serum analyzed by a custom enzyme-linked immunoadsorbant assay (ELISA), which was validated for human samples. Concentrations of sunitinib were assessed in plasma by a validated LC/MS/MS assay (York Bioanalytical Solutions) according to GCP. Pharmacokinetic (PK) parameters for rIL-21 and sunitinib were determined where possible using a non-compartimental model. The PK parameters included: Cmax, tmax, AUC, AUC(0-τ), CL/f, t1/2 for terminal elimination and Vz/f.
Serum samples for detection of antibodies specific for rIL-21 were measured by radio immuno assay (RIA). Pharmacodynamic effects of rIL-21 and sunitinib were measured by detection of soluble CD25 as a general marker for immune response, employing a custom ELISA.
Tumor response
A CT-scan was performed after each two cycles of treatment, i.e. each 12 weeks. Response was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 [Citation17]. Measurable disease according to these RECIST criteria was only required in the phase II part of the study.
Statistical methods
Analysis of clinical data was performed using the statistical analyses system (SAS Institute Inc., Cary, NC). Descriptive statistics were implemented. No sample size calculation was performed. PK parameters were estimated by non-compartmental methods and descriptive statistics for clinical data were calculated using SAS release 9.1 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
Between July 2007 and July 2008 a total of nine Caucasian patients with mRCC were treated with rIL-21 in combination with sunitinib in this study. Demographics and clinical characteristics are presented in .
Table I. Patient characteristics.
Dose-escalation and toxicities
Two dose levels of rIL-21 were explored at 3 and 10 μg/kg s.c. TIW. Five patients received rIL-21 at 3 μg/kg, of whom two patients were withdrawn during the first week due to sunitinib-related grade 3 toxicity (hepatic toxicity, anorexia and syncope, respectively); as per protocol those two patients were replaced. Four patients were exposed to 10 μg/kg rIL-21 of whom one patient decided to stop because of grade 2 flue-like toxicity.
At 10 μg/kg rIL-21 two patients developed a DLT, which consisted of grade 4 neutropenia and grade 3 thrombocytopenia. After treatment discontinuation the neutrophils and thrombocytes recovered. Thus, two DLTs were observed in four patients and according to the protocol three subjects could be entered at a lower dose level or a new intermediate dose level could be investigated. However, further exploration was omitted since 3 μg/kg rIL-21 was considered too low to be clinically meaningful.
During the entire trial, 160 adverse events (AE) were reported in all exposed patients (n=9), of which 79 (49%) were related to sunitinib, 20 (12.5%) to rIL-21 and 40 (25%) of the AEs to both sunitinib and rIL-21. The most common AE related to rIL-21 and sunitinib were injection site reaction (n=6), stomatitis (n=5), fatigue (n=4) and dysgeusia (n=4). AEs occurring in more than 30% of patients or severity grade 3/4 adverse events are listed in .
Table II. Drug-related adverse event profile occurring in more than 30% of patients or grade 3-4 severity.
Pharmacokinetics (PK)
The vast majority of serum concentrations of rIL-21 at the 3 μg/kg dose-level remained below lower limit of quantification (LLoQ). At the 10 μg/kg dose level of rIL-21 the mean concentration after 0.5 h of application was 0.96 μg/L.
The mean AUC0-24h, Cmax and t1/2 of sunitinib in combination with 10 μg/kg rIL-21 were determined to be 1135.8 μg.h/L, 64.6 μg/L and 28.0 h, respectively. The PK parameters obtained did not suggest mutual PK interactions for either agent at these dose levels.
Pharmacodynamics (PD)
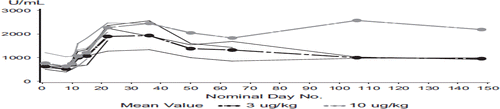
Soluble (s)CD25, as a general marker of immune activation, was assessed at multiple time points after the application of rIL21 at both dose-levels, 3 and 10 μg/kg, respectively. At both dose-levels an increase of sCD25 was detected, which suggests some degree of immune response to the combination of rIL-21 and sunitinib ().
Figure 2. Soluble (s)CD25 after the treatment with rIL-21 for 3 and 10 μg/kg dose-levels. Increase of sCD25 was seen at both dose-levels suggesting activation of immune response.
The development of neutralizing anti-rIL-21 antibodies was reported in three patients at the 3 μg/kg dose-level and in one patient at the 10 μg/kg dose-level.
A total of ten single-nucleotide polymorphism (SNP) and nine haplotypes were observed in the rIL-21 receptor gene, eight SNP and five haplotypes in the granzyme B gene (cytotoxic T-lymphocyte-associated serine esterase 1) and 13 SNP and eight haplotypes in the PRF1 gene were found. Results were not correlated to tumor response in this limited number of patients.
Tumor response
Three patients at the 3 μg/kg dose-level were assessable for tumor response. One patient had a partial response (PR), one a stable disease (SD) and one patient had progressive disease (PD) as best overall response. The only patient who continued treatment at the 10 μg/kg rIL-21 DL had non-measurable disease and was not evaluable for response.
Discussion
The current phase I trial was designed to assess the safety and tolerability of the addition of rIL-21 to sunitinib at the ‘4 weeks on/2 weeks off’ schedule in treatment-naive mRCC patients. Two hematological DLTs were observed at a relatively low dose level of 10 μg/kg, and led to early termination of the trial.
The increase of toxicity shown in our data is supported by other studies, which combine immunotherapy with tyrosine kinase inhibitors (TKI). A phase I trial with sunitinib and IFN-α reported grade 3-4 adverse events in all 25 therapy-naive patients and due to doubts about the suitability for long-term treatment, the combination was not recommended for further investigation [Citation18]. Although a phase I study with sorafenib and IFN-α reported that the combination was well tolerated [Citation19], two phase II studies with sorafenib and IFN-α reported enhanced toxicity [Citation20,Citation21]. One study reported a high need for dose reductions in 65% of the participants and dose interruptions in order to make chronic therapy possible and the second study reported an excessive toxicity rate of 77% of patients experiencing grade 3 or higher toxicity. Objective responses were found in 32% and 19% respectively, with small but significant CR in 5% and 2% respectively [Citation20,Citation21]. The combination of sorafenib 400 mg BID and 30 μg/kg i.v. rIL-21 (d1-5 and 15–19 every six weeks) has been explored in a phase I/II trial [Citation22]. Again, sorafenib was dose-reduced in 59% and discontinued in 19% of the patients, indicating substantial toxicity [Citation22].
In addition to its prime role as an inhibitor of angiogenesis, sunitinib is thought to exert immunomodulatory effects in the host. Reduction of regulatory T-cells (Tregs) and myloid-derived suppressor cells (MDSC) were detected after sunitinib treatment in mice, which may enable synergistic anti-tumor effects when combined with immunotherapeutics [Citation23–25]. Treatment with sunitinib in mRCC patients reduced the number of myeloid-derived suppressor cells (MDSC), which was correlated with a shift towards type-1 immune responses [Citation24,Citation26,Citation27]. VEGFR inhibitors might therefore add both anti-angiogenic as well as immune-modulatory effects to immunotherapy.
The addition of rIL-21 to sunitinib was considered to induce more complete and durable responses. It is not really possible to determine the synergy of the treatment combination in this limited number of patients. The observed PR and SD can be caused by sunitinib alone or by the combination of sunitinib and rIL-21. Due to toxicity, the MTD of rIL-21 was found at the very low 3 μg/kg DL. In phase I-II clinical trials intravenous application of rIL-21 reached MTD at 30 μg/kg using different schedules, which elicited clear signs of tumor responses at the 30 μg/kg dose-level [Citation22,Citation28,Citation29], and suggesting a threshold for tumor response at that dose. The sunitinib ‘4 weeks on/2 weeks off’ schedule was chosen because of its favorable objective response rate compared to the continuous 37.5 mg schedule (47% vs 20%) [Citation30]. Therefore, further investigation of the combination of rIL-21 with sunitinib in a continuous dose schedule was not performed.
Conclusions
The combination of rIL-21 and sunitinib was associated with dose-limiting hematological toxicities on a dose level of 10 μg/kg of rIL-21. The MTD of 3 μg/kg of rIL-21 was not considered therapeutically relevant and led to early trial termination. Our results are supported by other studies, which reveal an increased toxicity for a combination of cytokines and angiogenesis inhibitors.
Acknowledgments
This study was sponsored by Novo Nordisk, which was responsible for the study design, collection and analyses of data. The principal investigator was involved in the study design (CvH). Interpretation of the data, writing and submission of this manuscript was initiated by the involved investigators. This trial has been registered as NCT00617253 at clinicaltrial.gov (http://www.clinicaltrials.gov/ct2/show/NCT00617253).
Declaration of interest: U. Mouritzen and M. W. Brændholt Olsen are employees of NovoNordisk. None of the other authors wish to declare a conflict of interest.
References
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, . Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27: 3584–90.
- Johannsen M, Florcken A, Bex A, Roigas J, Cosentino M, Ficarra V, . Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. Eur Urol 2009;55:1430–8.
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, . High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–16.
- Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Fyfe G. High-dose aldesleukin in renal cell carcinoma: Long-term survival update. Cancer J Sci Am 1997;(Suppl 1): S70–2.
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, . IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007;178:2827–34.
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, . Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000;408(6808):57–63.
- Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: Combination strategies for cancer therapy. Nat Rev Drug Discov 2008;7:231–40.
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol 2004; 172:2048–58.
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, . Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 2005;201: 139–48.
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, . A critical role for IL-21 in regulating immunoglobulin production. Science 2002;298:1630–4.
- Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, . Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007;179:5886–96.
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, . IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967–74.
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, . IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–7.
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, . IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol 2007;178:732–9.
- McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res 2007;13:716s–20s.
- Foster D, Parrish-Novak J, Fox B, Xu W. Cytokine-receptor pairing: accelerating discovery of cytokine function. Nat Rev Drug Discov 2004;3:160–70.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Motzer RJ, Hudes G, Wilding G, Schwartz LH, Hariharan S, Kempin S, . Phase I trial of sunitinib malate plus interferon-alpha for patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2009;7:28–33.
- Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, . Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res 2007;13:1801–9.
- Gollob JA, Rathmell WK, Richmond TM, Marino CB, Miller EK, Grigson G, . Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol 2007;25:3288–95.
- Ryan CW, Goldman BH, Lara PN, Jr., Mack PC, Beer TM, Tangen CM, . Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: A phase II study of the Southwest Oncology Group. J Clin Oncol 2007;25: 3296–301.
- Flaig T, Curti B, Gordon M, Van Veldhuizen P, Miller W, Ernstoff M. Recombinant IL-21 in combination with sorafenib as second- or third-line therapy for metastatic renal cell carcinoma (mRCC): Interim results from a phase 2 study [abstract]. Eur J Cancer Suppl 2008;204.
- Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, . Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 2008;111:5610–20.
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, . The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009;69:2514–22.
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, . Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009;15:2148–57.
- van CH, van d, V, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, . Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res 2008;14:5884–92.
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, . Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 2008;14:6674–82.
- Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: Functions in cancer and autoimmunity. Clin Cancer Res 2007;13:6926–32.
- Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, . Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol 2008;26:2034–9.
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, . Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:4068–75.