To the Editor,
Ondansetron is a serotonin (5-hydroxitriptamine, 5-HT) receptor antagonist which is widely used as anti-emetic agent in the prophylactic treatment of chemotherapy-induced nausea and vomiting (CINV) in children with acute lymphoblastic leukemia (ALL) [Citation1–3]. The leukemia cell line REH was the first B-cell precursor (BCP) ALL cell line which could be established in the laboratory for research purposes, and was derived from the peripheral blood of a 15-year-old girl with ALL at first relapse [Citation4]. In this BCP-ALL cell line, it was found the t(12;21)(p13;q22) translocation, producing the TEL:ETV6-AML1 chimeric fusion gene, which is the most common genetic abnormality in childhood ALL [Citation5]. At present, the cell line REH represent one of the best characterized and described BCP-ALL cell lines, and may be considered as one of the most representative cell lines for in vitro studies in childhood ALL [Citation4,Citation5]. Since it had previously been reported that 5-HT is able to increase the cell proliferation of human T-lymphoblastic leukemia cells in vitro [Citation6], it was of further interest to analyze the possibility that the presence of ondansetron, as a 5-HT-receptor-3 (5-HT3) antagonist, would also be able to induce any kind of changes in the proliferation of human BCP-ALL cells.
In all the performed experiments, we used the BCP-ALL cell line REH [Citation4]. REH cells in the logarithmic growth phase were cultured in 24-well culture-plates at cell concentrations of 1–2 × 104 cells/ml (1 ml/well) in RPMI-1640 Medium (Biochrom, Berlin, Germany). The analyzed REH cells were incubated for 72 h at 37°C in a humidified atmosphere of 5% CO2 in air. Cell proliferation of viable REH cells was analyzed by using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Mannheim, Germany). 5-HT was purchased from Sigma-Aldrich, Taufkirchen (Germany), and ondansetron (Zofran®) from Glaxo Wellcome GmbH, Munich (Germany). All experiments were performed at least three times in double series of triplicates and the results were considered statistically significant for p<0.05 by using the two-sided unpaired Student's t-test.
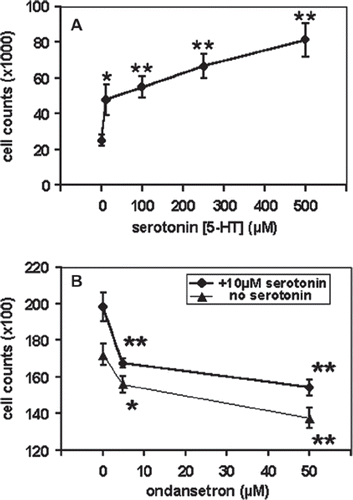
Because it had previously been described that 10−7–10−5 M 5-HT significantly increases the cell proliferation in the human T-lymphoblastic leukemia cell line CCRF-CEM [Citation6], we first analyzed whether this effect would also be observable in the BCP-ALL cell line REH. Indeed, when 5-HT was applied to the corresponding cell culture wells at concentrations of 10−6–5 × 10−4 M, significant proliferation increases (56–226%) were observed in REH-cells after 72 h incubation (p=0.0169 – p=0.0023, respectively; ). Interestingly, the presence of 5–50 × 10−6 M ondansetron, which is comparable to the concentrations achieved by current clinical dosing, significantly reduced the proliferation of viable REH cells by 10–20% (p =0.048 and p=0.005, respectively; ). This antiproliferative effect could also be observable in the presence of 10−5 M 5-HT (means: −15 and −22%; p=0.005 and p=0.002, respectively; ).
Figure 1. Effects of serotonin (5-HT, Panel A) and ondansetron (Panel B), on cell proliferation in the BCP-ALL cell line REH, after 72 h incubation at 37°C. Values are means and standard error of the means (SEM). All experiments were performed at least three times in double series of triplicates. Stars indicate statistical significance when compared either to the controls with no 5-HT (Panel A), or to those with no ondansetron (Panel B), by using the two-sided unpaired Student's t-test (one star: 0.01< p<0.05, and two stars: p<0.01).
Antiproliferative properties of ondansetron have also been tested in different solid tumor cell lines. Thus, in the human malignant glioma cell line Mg251, mostly known as U-251 MG, it has previously been reported, that ondansetron fully inhibits EMP-induced cell volume increases by retaining an high cellular potassium-efflux, which is directly related to a reduction of cell growth and proliferation, as well as to an increased apoptosis rate [Citation7]. However, in neuroendocrine tumor (NET) cell lines, ondansetron did not show any antiproliferative effect, neither in the NET gastrointestinal cell line KRJ-I, nor in the NET bronchopulmonary cell lines NCI-H720 / 727 [Citation8].
To our knowledge, this is the first report about an antiproliferative effect of a 5-HT3-antagonist in a BCP-ALL cell line. This finding may represent further beneficial effects of the anti-emetic agent ondansetron as a putative anti-leukemic compound, and further support its actual wide use in the current therapy of CINV in ALL patients.
Acknowledgements
The authors are grateful to A. Zelmer for excellent technical assistance and to S. Wellmann for helpful comments and discussions. J. Prada and S. Shalapour were both partially supported by “Deutsche Krebshilfe e.V.”, Bonn, Germany.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Holdsworth MT, Raisch DW, Winter SS, Chavez CM. Assessment of the emetogenic potential of intrathecal chemotherapy and response to prophylactic treatment with ondansetron. Support Care Cancer 1998;6:132–8.
- Parker RI, Prakash D, Mahan RA, Giugliano DM, Atlas MP. Randomized, double-blind, crossover, placebo-controlled trial of intravenous ondansetron for the prevention of intrathecal chemotherapy-induced vomiting in children. J Pediatr Hematol Oncol 2001;23:578–81.
- Jaing TH, Tsay PK, Hung IJ, Yang CP, Hu WY. Single-dose oral granisetron versus multidose intravenous ondansetron for moderately emetogenic cyclophosphamide-based chemotherapy in pediatric outpatients with acute lymphoblastic leukemia. Pediatr Hematol Oncol 2004;21:227–35.
- Matsuo Y, Drexler HG. Establishment and characterization of human B-cell precursor leukemia cell lines. Leuk Res 1998;22:567–9.
- Tsuzuki S, Karnan S, Horibe K, Matsumoto K, Kato K, Inukai T, . Genetic abnormalities involved in t(12;21) TEL-AML1 acute lymphoblastic leukemia: Analysis by means of array-based comparative genomic hybridization. Cancer Sci 2007;98:698–706.
- Sibella-Argüelles C. The proliferation of human T lymphoblastic cells induced by 5-HT1B receptors activation is regulated by 5-HT-moduline. Comptes rendus de l'Académie des sciences. Série III, Sciences de la vie. C R Acad Sci III 2001;324:365–72.
- Behnam-Motlagh P, Sandström PE, Henriksson R, Grankvist K. Ondansetron but not granisetron affect cell volume regulation and potassium ion transport of glioma cells treated with estramustine phosphate. J Cancer Res Clin Oncol 2002;128:449–55.
- Drozdov I, Kidd M, Gustafsson BI, Svejda B, Joseph R, Pfragner R, . Autoregulatory effects of serotonin on proliferation and signaling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer 2009;115: 4934–45.