Abstract
Purpose. A clinical feasibility study using a removable prostate stent as fiducial for image-guided radiotherapy (IGRT) of localized prostate cancer (PC). Material and methods. The study included patients with local or locally advanced PC. The clinical target volume (CTV) was outlined on magnetic resonance (MR) images co-registered to planning computer tomography (CT) images. Daily online IGRT was delivered using the stent as fiducial. Risk of migration was estimated using multiple MR. Acute urinary toxicity was scored using the international prostate symptom score (IPSS). Late gastro-intestinal (GI) and genito-urinary (GU) toxicity was scored using the Radio Therapy Oncology Group (RTOG) score, biochemical failure (BF) was defined as an elevation of prostate specific antigen (PSA) above nadir plus 2 ng/ml after radiotherapy. Results. One hundred men were enrolled in the study. Ninety completed radiotherapy with the stent as fiducial. No migration of the stent was seen, but three cases of dislocation of the stent to the bladder were observed. Acute urinary toxicity based on IPSS was comparable to toxicity in patients who had gold markers (GM) as fiducials. Removal of the stent was associated with a high frequency of urinary retention. Late GI and GU toxicity and BF were comparable to those of other studies, but longer observation time is needed. Conclusions. This study reports the first clinical results of using a prostate stent as fiducial. No migration of the stent observed. Dislocation of the stent to the urinary bladder was observed in three cases, requiring removal of the stent and insertion of a new fiducial. Acute toxicity during radiotherapy evaluated from IPSS was comparable to toxicity in patients with GM. Removal of the stent was associated with a high frequency of post procedural urinary retention. Late toxicity and BF were comparable to those of other studies, though longer observation time is needed.
It has been shown that three dimensional (3D) conformal radiotherapy or intensity modulated radiotherapy (IMRT) techniques must be used if radiation doses are to be escalated beyond conventional dose levels of 70 Gy in prostate cancer (PC), without increasing morbidity [Citation1]. The use of fiducials in the prostate and image guided radiotherapy (IGRT) makes it possible to reduce margins around the Clinical Target Volume (CTV), which may allow further dose escalation [Citation2]. Implanted gold markers (GM) are the standard for fiducials in IGRT of prostate cancer [Citation3]. An alternative method based on insertion of a commercial nickel-titanium (Ni-Ti) prostate stent, Memokath™, as fiducial for IGRT has been suggested [Citation4]. A previous study demonstrated that the use of the Memokath™ was acceptable only when the urethral part of prostate (UP) exceeded 40 mm. Subsequently, a new version of the Ni-Ti prostate stent, Memocore™, was designed for patients with a UP less than 40 mm [Citation5]. The present paper describes the results from the first clinical feasibility study using a prostate stent as fiducial for co-registration of planning computer tomography (CT) and magnetic resonance imaging (MR) and subsequent IGRT treatment. This new method is evaluated in terms risk of dislocation to the bladder, risk of migration in the prostate, acute and late complications and patient outcome in terms of freedom from biochemical relapse. The study was in accordance with the standards of the Helsinki Declaration of 1975, as revised in 2000. The study was approved by the ethical committee and the Danish Medicine Agency (DMA). All participating patients gave oral and signed informed consents.
Material and methods
Patients
Patients were included in the study from March 2007 until May 2009. Patient flow is shown in . Inclusion criteria were histological verified local or locally advanced prostate cancer and referral to the Department of Oncology for curatively intended radiotherapy. Exclusion criteria were the presence of any urological implant, including stent, penile prosthesis, artificial sphincter or previously surgical treatment for prostate or urethral obstruction, urethral strictures or presence of bladder calculi or tumors.
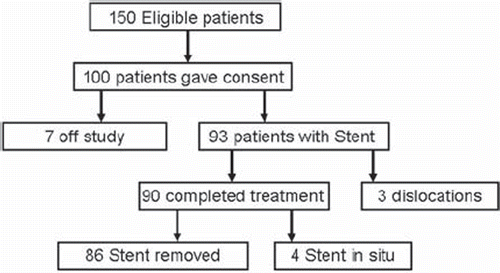
Figure 1. Patient flow diagram. A total of 150 eligible patients, fulfilling the inclusion criteria, were screened. One hundred accepted participation and gave informed consent. Seven patients went off study. Reasons for patients to go off-study were a) two patients refused to accept further waiting time for a second stent after failed insertion of the first stent, b) in three patients previously unknown stricture in the urethra was discovered during insertion, c) one patient had the stent removed as urine outlet was obstructed (unrecognized TURP patient), d) one patient experienced an iatrogenic displacement during an unplanned cystoscopy. In the remaining 93 patients a Memocore™ were inserted in 61 patients and a Memokath™ in 32. The stent migrated in three patients.
Another group of consecutive patients with PC, who had identical radiotherapy, but had GM as fiducial was included in the period from June 2009 until May 2010. In contrast to the group of patients with stents, the group of patients with GM had no MR co-registration, i.e. CTV was outlined on planning CT only. This second group of patients was included in order to compare acute urinary toxicity between two groups of patients, who had either the stent or GM as fiducial.
Insertion and removal
Lengths of the stents were individually adapted using information from diagnostic MR scan. The stent was inserted via the urethra 2–3 weeks prior to radiotherapy. Insertion was done using a Foley Catheter and x-ray fluoroscopy. The stent was mounted on an insertion sheath which was mounted over the Foley Catheter. Once inserted the balloon of the Foley catheter was inflated with x-ray contrast media and the catheter was gently retracted until the balloon touched the base of the prostate. Once in the correct position in the prostate, 50 ml of 60°C water was flushed over the stent to expand the lower part. Subsequently the catheter and insertion kit was removed. Three months after end of radiotherapy, the stent was removed from the prostate using a flexible scope. The stent was then flushed with 5°C cold water until the metal became soft. A grasping forceps inserted trough the scope was used to pull the stent out as an approximately 1 meter long soft metal wire. A comprehensive description of insertion method and treatment details has been given previously [Citation5].
Stent
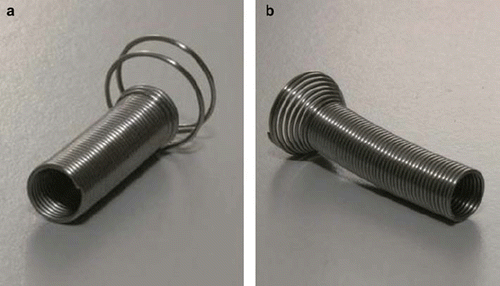
Stents with two different designs of anchoring collar were used. The choice of type was depending on size of the prostate, i.e. length of UP. For patients with small prostates, i.e. an UP less than 40 mm, a Memocore™ stent ( left panel) was inserted. For patients with a large prostate, i.e. an UP larger than 40 mm, the Memokath™ ( right panel) was inserted.
Figure 2. The memory shape Nickel-Titanium (Ni-Ti) prostate stents used as fiducial in the study. Both stents are shown in their expanded form. Both stents are made from 0.65 mm diameter Ni-Ti wire. The length of the stent is adapted to the individual length of the prostate part of urethra prior to insertion. a) The Memocore™ stent used in case the prostate part of urethra less than or equal to 40 mm. b) The Memokath™ stent used in case the prostate part of urethra of more than 40 mm.
Treatment details
The clinical target volume (CTV) was the prostate gland, or prostate gland plus proximal third of seminal vesicles in case of seminal vessel invasion. The CTV was outlined on CT using the co-registered MR images. Image set (volumes) were locally co-registered using the three manually defined points on the stent in both image modalities, a point at each end of the stent and one in the middle. In order to restrict rotation around the central axis of the stent, it was necessary to introduce an external helping point for this was chosen the most anterior point of the prostate in CT and as well as MR. This external point was then adjusted in the anterior-posterior direction of the CT image until the optimal co-registration of the stent was obtained. An isotropic CTV to PTV margin of 5 mm was used. Radiotherapy was given using a five field conformal plan (gantry angles at 9, 90, 140, 220 and 270 degrees). A CTV mean dose of 78 Gy in 39 daily sessions was prescribed. Constraints to organs at risk were: Urinary bladder less than 40% of volume to receive more than 50 Gy; rectum less than 25%, 40% and 50% of volume to receive more than 70 Gy, 60 Gy and 50 Gy respectively and dose to the posterior part of rectum to receive less than 65 Gy; any volume of maximum 2 cm3 should not receive more than 75 Gy. The Brainlab ExacTrac™ system was used for daily online image guided positioning matching the stent with a digital reconstructed radiogram (DRR) from the planning CT.
Risk of dislocation
Dislocation, i.e. the stent moving to the bladder, will give rise to large rotations. Large rotations make matching impossible, and the dislocation will thus be discovered in the daily online check, which was performed during the whole treatment period using kV images from the IGRT system. In case of dislocation the treatment was interrupted and the dislocated stent was removed and a new fiducial inserted. Patients were rescanned, re-planned and treatment continued.
Risk of migration
Migration, i.e. the stent moving within the prostate, was checked using multiple MR scan. Stent position in pre-radiotherapy MR scan was compared to position in a second MR scan done halfway through the radiotherapy sessions. Difference cap volumes were defined and calculated as voxels in MRpre and not in MRmid and voxels in MRmid and not in MRpre. Assuming the prostate to be a sphere and a partial stent migration of 2 mm within the prostate, a corresponding theoretical value for the size of these two differential cap volumes were calculated. In case of any suspicion of migration the patient was rescanned and re-planned. In that case a control MR scan was furthermore added one week later.
Acute adverse effects
Acute urinary toxicity was evaluated using the validated IPSS patient questionnaire. Patients were to fill in the questionnaire before stent insertion (Baseline), one to two weeks after insertion, halfway through radiotherapy, at the end of radiotherapy, three months after the end of radiotherapy (only stent patients), and one month after removal of stent.
Late adverse effects
Late effects were evaluated using the Radio Therapy Oncology Group (RTOG) late morbidity score for gastro-intestinal (GI) and genito-urinary (GU) toxicities [Citation6]. Patients were scored at scheduled visits 3, 6, 12, 24 and 36 months after the end of radiotherapy.
Biochemical relapse
A biochemical failure (BF) was defined as a rise of serum PSA to a value exceeding postradiotherapy PSA nadir plus 2 ng/ml. This is considered the standard definition for biochemical failure after external beam radiotherapy EBRT with or without adjuvant hormone therapy (AHT) [Citation7].
The study was monitored. Monitors visited the investigator periodically to verify the adherence to the protocol, maintenance of study-related source records, and the completeness and accuracy of all CRF entries compared to source data.
Results
The study included 100 patients as shown in the patient flow diagram in . Demographics of the 90 patients completing treatment with the stent are given in . Demographics in the consecutive group of patients with gold markers were not statistically different from the stent group considering age, T-stage and PSA (data not shown). Gleason and risk score was not comparable due to a change in method of calculating the Gleason score. This change was introduced at the beginning of 2009, i.e. for most of the time when the patient group with gold markers were recruited.
Table I. Demographics for patients treated with the stent (n = 90).
Stent Insertion
All stents were inserted by the same oncologist. A learning curve was demonstrated since problems with insertion were limited to the first 25 patients. In four patients the first insertion failed and a second attempt was needed. In nine patients the stent was positioned too low which resulted in urinary incontinence and discomfort and the stent had to be removed. Reinsertion of a new stent or GM was necessary.
Risk of dislocation
Stent dislocation to the bladder occurred in three patients. One was observed at the time of planning CT. Erroneously this patient with a short UP of 30 mm had a Memokath™ inserted instead of a Memocore™ ,which may explain the dislocation. The second patient presented with a dislocation at the first radiotherapy session. Dislocation was observed on the kV images before treatment was given. The third patient presented with a sudden dislocation at the 20th radiotherapy session. All three patients had the stent removed and chose to have GM inserted as new fiducial.
Risk of migration
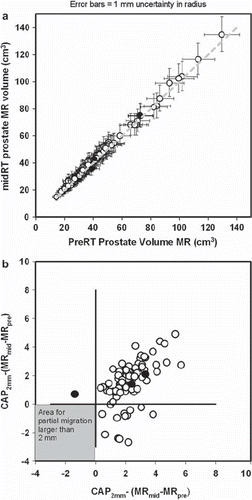
Migration of the stent within the prostate gland was measured comparing pre- and mid-treatment MR scan. The prostate gland was outlined on both MR scans co-registered using manually defined points on the stent in both image sets. Prostate pre-treatment volume outlined on MR (MRpre) was plotted against mid-treatment MR prostate volume (MRmid) as shown in . This figure demonstrates that no detectable change in prostate volume did occur from pre- to mid-treatment Linear regression on the data in give intercept and slope that do not differ statistically from the line of identity (Y = X) and a correlation coefficient of 0.997. Difference between actual cap and theoretical cap volumes were calculated and plotted in , demonstrating that, if any partial migrations occurred, it must have been less than 2 mm, i.e. below measuring accuracy.
Figure 3. Plot of prostate volumes outlined on pre-radiotherapy and mid-radiotherapy MR scans. Black dots represent the three patients in whom a potential partial migration was estimated comparing sagittal MR images from the two MR scans. Other patients are represented by a white dot. a) Mid-treatment MR prostate volume (MRmid) plotted against the prostate pre-treatment volume outlined on MR (MRpre). Data are from the 90 patients in whom volumes were available in both MR scans. Error bars represents change in volume if the radius of the prostate is changed plus or minus one millimetre. b) Mismatch in MR volumes in co-registered MR volumes were defined as MRpre-MRmid and MRmid - MRpre. A corresponding theoretical difference (CAP2mm) was calculated assuming that the prostate is a sphere and the stent has partial migrated 2 mm between MR scans. Difference between the CAP2mm volume and the two mismatch volumes are plotted in Any true migration of equal to or more than 2 mm should give a dot in the grey area.
Stent removal
Stent was removed prematurely, i.e. before three months after end of radiotherapy, in 11 patients. Early removal was due to 1) dislocation in three (two stent removed before start of radiotherapy (RT) and one during RT), 2) urinary retention during radiotherapy in three (removed after end of RT) and 3) incontinence in one and pain/discomfort in four patients (removed after end of RT). The stent was not removed after radiotherapy in four patients due to: 1) one patient died from non-study related reasons before the stent could be removed, 2) One patient refused removal due to biochemical failure and 3) two patients experienced improvement in urinary function and wished to keep the stent.
Acute adverse effect
shows frequencies of acute toxicities related to insertion and removal of prostate stent. Toxicities are compared in the table with data from the literature on acute toxicities [Citation8–12] related to insertion of gold markers or to biopsy of prostate. Rectal bleeding was not observed using the stent. Haematuria seems more frequent in the use of GM or biopsy. Incontinence and retention is seen both using the stent and biopsy. Acute toxicity in this study was dominated by the presence of urinary retentions, six being related to insertion of stent and 14 being related to removal of stent. Retention occurred within the first one to two days after insertion or removal and was treated using a catheter-a-demeure for a few days until resolved.
Table II. Acute toxicities related to insertion and removal of fiducials in the prostate, i.e. gold markers or prostate stent.
Acute urinary toxicity was also scored using the IPSS questionnaires. Approximately two third of patients returned all questionnaires. Results from the patients who had the stent as fiducial are shown in , this table also includes the group of patients with GM from our department receiving the same radiotherapy treatment regime as the stent patients. These results are compared with results from the literature [Citation13–15], results from the literature also include patients treated with radioactive iodine seed brachytherapy (BT). For patients receiving stent as fiducial a small, but significant increase in the IPSS score is seen shortly after insertion of the stent. The increased IPSS score at the end of radiotherapy treatment, however seems to be the same for GM and stent patients, but apparently lower than peak increase in IPSS score in BT. Comparing IPSS score before insertion and after removal of stent was proven not statistically different using a Wilcoxon paired test (p = 0.39).
Table III. Acute urinary toxicities estimated using the International Prostate Symptom Score (IPSS) patient questionnaire.
Late adverse effects
Median observation time is 20 months (range 8–32 months). Three patients have been lost for follow-up. Until present time the highest toxicity score is a grade 2 in two patients (one patient with a GI RTOG grade 2, one patient with a GU RTOG grade 2). Two year actuarial GU toxicity was 8% with a standard error of 8% and for GI toxicity 2% with a standard error of 1%.
Urethral stricture was observed in two patients: one patient at the time of removal of the stent and one patient six months after radiotherapy. These two patients were among the first six enrolled in the study. The reason for the strictures is unknown, but it may be suspected that strictures were caused by mechanical damage either during insertion or removal of stent.
Biochemical (PSA) relapse
Two year actuarial rate of freedom from biochemical failure is 90% with a standard error of 5%.
Discussion
This paper reports the early results from the first clinical study using a prostate stent as fiducial for IGRT of prostate cancer. Using the prostate stent as fiducial is an alternative to GM. Use of the stent may have the following advantages a) large 3D object located on the central axis of the prostate gland, which improves detection of prostate gland rotation, b) allow for local co-registration of planning CT and MR, c) fewer artefacts in CT images compared to GM, due to lower effective atomic number in Ni-Ti compared to gold, d) good contrast in both kilo voltage (kV) and mega voltage (MV) images, e) insertion method based on urethral catheter, i.e. trough a natural body orifice instead of implantation, f) may be done in the Department of Oncology, which may simplify logistics, g) finally the stent may be removed after use. Even though insertion of the stent is simple, a learning curve must be considered when introducing the method. One drawback of the current insertion method was lack of visualization of the external sphincter, which may cause an improper or too low placement of stent by untrained doctors.
Delineation of CTV on CT has demonstrated an overestimation of 34% compared to CTV delineation on MRI, corresponding to an almost 5 mm larger CTV radius on CT compared with MRI [Citation16]. Local co-registration of CT and MR using GM has been performed by others [Citation17], this technique however requires the use of endorectal coils, suppression of peristalsis and subsequent use of an endorectal balloon to mimic deformation of the prostate inflicted by the endorectal coil. Using the prostate stent as fiducial, no coils are needed for local co-registration of CT and T2 weighted MR. New alloy gold markers have been shown to give MR image artefacts using a special MR gradient recalled echo (GRE) sequence without endorectal coils [Citation18]. These artefacts may be used to co-register CT and MR. GRE images do not necessarily present prostate anatomy as well as T2 weighted sequences. The signal from the stent in MR images allow artificial digital reconstructed radiograms (DRR) from MR images and consequently dose planning and subsequent radiotherapy treatment based on MR images only.
Some papers have reported migration of marker [Citation3], and a recent report has demonstrated that migration may influence the daily matching quality [Citation19]. No migration of the stent within the prostate was observed. The detection of migration in this study was based on the observation that the prostate volume did not change. Others has observed a change in prostate volume during radiotherapy treatment [Citation18]. This is however not necessarily in conflict with this study, as the change in volume [Citation18] seems to appear after fraction no. 20, which was the session where the second MR study was performed in this study. In the present study a small risk of a sudden stent dislocation to the bladder was observed. Sudden dislocations do not lead to erroneous treatment of the patient, but requires removal of the dislocated stent and insertion of a new fiducial. This would compare to patients having an extra GM implantation, because one or more markers were missing.
Insertion of either GM or prostate stent as fiducial in the prostate does have acute adverse effects. A moderate risk of urinary retention during stent insertion has been reported [Citation12]. and the authors speculated that retention may be a result of transient oedema or sphincter spasm induced by the insertion. A high risk of urinary retention, requiring medical intervention, was observed post procedural in this study. This negatively impacted the use of a stent in this study, compared to other studies using GM. Thus using the current technique it is recommended to insert a catheter for three to five days after removal of the stent. Administration of an alfa-1-receptor antagonist; a non-steroidal anti-inflammatory drug, may also be indicated before removal.
Acute urinary toxicity was in focus in the study due to the insertion of an intra prostate device, and consequently the IPSS patient assessed score was used. Recording change in IPSS score from baseline during and after treatment seemed to establish that acute toxicity from radiotherapy is comparable to GM and lower than what is seen using brachy-therapy. A small increase in IPSS was observed in the first weeks after insertion of the stent compared to GM, but this difference levelled of as radiotherapy progressed. One possible bias could be that patients with GM may have larger CTV outlined on CT only, as compared to patients with stent. Consequently GM patients may have had a lower acute toxicity during treatment if outlined on MR as well.
The fact that patient IPSS score returned to baseline (IPSS before insertion of stent) one month after removal of stent, documented that the stent did not incur any late damage to patient's urinary function.
The late toxicities reported using 78 Gy vary considerably and are dependent on the radiotherapy techniques used. Large randomized studies report GI toxicity from 17–38% and GU toxicity from 11–39% at five year observation time [Citation20–23]. These studies have a long observation time and radiation techniques have improved including the use of fiducials since the studies were initiated. Later non-randomized studies using IMRT and IGRT and doses in the range 79–81 Gy have reported late GI and GU toxicities of 6–13% and 14–20% respectively [Citation24,Citation25]. Late GU and GI toxicities in this study seem to be comparable or even lower than these more recent results. This may be due to a smaller part of the rectum being irradiated, when MR is used to delineate the prostate. Longer follow-up period is however needed to conclude a lower toxicity.
Outcome in terms of five year freedom from BF varies with risk. For radiation doses in the range from 74 to 79 Gray freedom from BF of 81–95%, 79–86% and 53–66% are reported for low, intermediate and high risk patients respectively [Citation20–23]. Recently it has been reported that use of implanted markers, as opposed to bony structures for patient positioning, unexpectedly could give a significant worse outcome in terms of increased BF if small CTV-PTV margins (3 mm) are used [Citation26]. But this was not seen in this study, even with a high percentage of patients in the high risk group (), consequently the freedom from BF reported in this study appears favorable. But longer observation time is needed to conclude.
Benefit from use of a prostate stent as fiducial as in this study is ambiguous due to a higher acute toxicity from the use of stent and possibly lower late toxicities. Currently the prostate stent is in clinical use and a new prospective study of acute toxicities is ongoing in our department. Furthermore we are conducting a retrospective study comparing late toxicity and outcome in patients treated with either GM or stent as fiducials. Both toxicity and outcome will be crucial for an overall evaluation of the stent techniques, and should be tested in further studies.
Conclusion
This study reports the first clinical results of using a prostate stent as fiducial in IGRT. No migration of the stent within the prostate was observed. Dislocation of the stent to the urinary bladder was observed in three cases, requiring removal of the stent and insertion of a new fiducial. Acute toxicity during radiotherapy evaluated from IPSS was comparable to those of patients with GM. But removal of the stent was associated with a high frequency of post procedural urinary retention. Late toxicity and BF were comparable to other studies, but longer observation time is needed. Further studies and more data is needed to document benefit from this new method over other fiducials especially the use of GM.
Acknowledgements
Pnnmedical A/S (Kvistgaard, Denmark) donated all Memocore™ and Memokath™ stents. The study received foundation from “The IGRT project fund” at Aalborg Hospital and from CIRRO, the Lundbeck Foundation Centre for Interventional Research in Radiation Oncology. Niels Christian Langkilde, Department of Urology is thanked for valuable comments to the manuscript.
Declaration of interest: The authors report no conflict of interest. The author alone are responsible for the content and writing of the paper.
References
- Michalski JM, Purdy JA, Winter K, Roach M, III, Vijayakumar S, Sandler HM, . Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys 2000;46:391–402.
- Zhang M, Moiseenko V, Liu M, Craig T. Internal fiducial markers can assist dose escalation in treatment of prostate cancer: Result of organ motion simulations. Phys Med Biol 2006;51:269–85.
- Moman MR, van der Heide UA, Kotte AN, van Moorselaar RJ, Bol GH, Franken SP, . Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol Epub 2010 Mar 22.
- Carl J, Lund B, Larsen EH, Nielsen J. Feasibility study using a Ni-Ti stent and electronic portal imaging to localize the prostate during radiotherapy. Radiother Oncol 2006;78:199–206.
- Carl J, Nielsen J, Holmberg M, Hojkjaer LE, Fabrin K, Fisker RV. A new fiducial marker for image-guided radiotherapy of prostate cancer: Clinical experience. Acta Oncol 2008;47:1358–66.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
- Roach M, III, Hanks G, Thames H, Jr., Schellhammer P, Shipley WU, Sokol GH, . Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74.
- Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, . Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: Results of a prospective European prostate cancer detection study. J Urol 2001;166:856–60.
- Igdem S, Akpinar H, Alco G, Agacayak F, Turkan S, Okkan S. Implantation of fiducial markers for image guidance in prostate radiotherapy: Patient-reported toxicity. Br J Radiol 2009;82:941–5.
- Langenhuijsen JF, van Lin EN, Kiemeney LA, van d, V, McColl GM, Visser AG, . Ultrasound-guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: Complication rate and risk factors. Int J Radiat Oncol Biol Phys 2007;69:671–6.
- Henry AM, Wilkinson C, Wylie JP, Logue JP, Price P, Khoo VS. Trans-perineal implantation of radio-opaque treatment verification markers into the prostate: An assessment of procedure related morbidity, patient acceptability and accuracy. Radiother Oncol 2004;73:57–9.
- Perry MJ, Roodhouse AJ, Gidlow AB, Spicer TG, Ellis BW. Thermo-expandable intraprostatic stents in bladder outlet obstruction: An 8-year study. BJU Int 2002;90:216–23.
- Thomas C, Keyes M, Liu M, Moravan V. Segmental urethral dosimetry and urinary toxicity in patients with no urinary symptoms before permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2008;72:447–55.
- Cesaretti JA, Stone NN, Stock RG. Urinary symptom flare following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys 2003;56:1085–92.
- Marchand V, Bourdin S, Charbonnel C, Rio E, Munos C, Campion L, . No impairment of quality of life 18 months after high-dose intensity-modulated radiotherapy for localized prostate cancer: A prospective study. Int J Radiat Oncol Biol Phys Epub 2009 Oct 30.
- Sannazzari GL, Ragona R, Ruo Redda MG, Giglioli FR, Isolato G, Guarneri A. CT-MRI image fusion for delineation of volumes in three-dimensional conformal radiation therapy in the treatment of localized prostate cancer. Br J Radiol 2002;75:603–7.
- Huisman HJ, Futterer JJ, van Lin EN, Welmers A, Scheenen TW, van Dalen JA, . Prostate cancer: Precision of integrating functional MR imaging with radiation therapy treatment by using fiducial gold markers. Radiology 2005;236:311–7.
- Nichol AM, Brock KK, Lockwood GA, Moseley DJ, Rosewall T, Warde PR, . A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys 2007;67:48–56.
- Delouya G, Carrier JF, Beliveau-Nadeau D, Donath D, Taussky D. Migration of intraprostatic fiducial markers and its influence on the matching quality in external beam radiation therapy for prostate cancer. Radiother Oncol Epub 2010 Apr 6.
- Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, . Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87.
- Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, . Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74.
- Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, . Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990–6.
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr., Miller DW, Adams JA, . Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005;294:1233–9.
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, . Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
- Martin JM, Bayley A, Bristow R, Chung P, Gospodarowicz M, Menard C, . Image guided dose escalated prostate radiotherapy: Still room to improve. Radiat Oncol 2009;4:50.
- Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: Increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys 2009;74:388–91.