Abstract
Background. International comparisons have indicated low colorectal cancer (CRC) survival in Estonia, compared to other European countries. The objective of this paper is to analyse long-term survival as well as staging and treatment patterns of CRC in Estonia. Material and methods. The analysis included all incident cases of CRC diagnosed in Estonia in 1997 (n = 546), identified through the Estonian Cancer Registry and followed up for 10 years after diagnosis. Staging and treatment data were retrospectively collected from medical records. Relative survival rate (RSR) was used to estimate the outcome. Results and conclusion. The 5-year RSR was 51% for colon cancer and 38% for rectal cancer; the corresponding 10-year RSR was 50% and 39%. We observed no excess mortality for early disease. For stages II and III, the survival was markedly higher in colon cancer (5-year RSR 79% and 66%, respectively) compared to rectal cancer (66% and 30%, respectively). Around 30% of cases were diagnosed with distant disease. Among radically operated colon and rectal cancer patients, the 10-year RSR was 90% and 70%, respectively. Most patients with available pathological information had one to four lymph nodes examined. Survival has notably improved for colon cancer, but not for rectal cancer in Estonia. High proportion of cases with distant metastasis at first diagnosis along with inadequate staging and low proportion of patients treated with curatively intended surgery and appropriate chemotherapy and radiotherapy may have contributed to this outcome. Progress could be achieved by earlier diagnosis and implementing higher standards for staging and treatment. These conclusions are likely to be relevant also for other Eastern European countries.
Colorectal cancer (CRC) which affects both men and women is overall the most frequent malignancy in Europe today. In Estonia, a steady increase in the incidence of CRC has been observed during the past decades, with an age standardised (world) incidence rate of 25.4 per 100 000 person-years in 2008 [Citation1]. The incidence is slightly higher than that in Finland (23.8), but considerably lower than in the other Nordic countries. Given the modest incidence of CRC in Estonia, the mortality is relatively high (age standardised mortality rate of 12.3 per 100 000 vs. 8.6 in Finland in 2008) [Citation1], and no clear decline in mortality can be observed as yet [Citation2]. International comparisons have pointed to low but improving survival of patients with CRC in Estonia [Citation3,Citation4]. Rectal cancer survival has been shown to be considerably poorer in Estonia than colon cancer survival, which is not a typical finding for the rest of Europe [Citation3].
The paper aims to analyse long-term survival, staging and treatment patterns in colon and rectum cancers in Estonia, based on the one-year incidence cohort for the whole country. Estonia was a part of the Soviet Union until regaining its independence in 1991, which was followed by transition to an open-market economy and an insurance-based health care system [Citation5]. The study is associated with the EUROCARE High-Resolution Study described in detail elsewhere [Citation6].
Material and methods
The incident cases of CRC (ICD-10 codes C18, C19 and C20) diagnosed in Estonia in 1997, except death certificate only and autopsy cases (n = 16), were included in the analysis. The primary data source was the Estonian Cancer Registry, which is population based and covers the whole country (territory 45 216 km2, population 1.41 million in January 1997). To analyse CRC management patterns in Estonia, retrospective data collection from patients’ medical records was carried out according to the protocol of the EUROCARE High Resolution Study on Colon and Rectum Cancer [Citation6]. The collected data set included characteristics such as sex, diagnostic and staging procedures, morphology, disease staging (TNM) and modes of treatment.
The patients were followed through 2007 using data from the Estonian Population Registry. The vital status of the patients’ as well as the date of death/emigration was ascertained. Additional data retrieved from the Estonian Cancer Registry included date of birth, extent of disease, and diagnosis as reported to the registry.
For the present analyses, all cases were reviewed and the extent of disease was reconstructed into stage groups based on pathological (if available) or clinical TNM: stage I (T1,2 N0 M0); stage II (T3,4, N0 M0), stage III (Tany N1-3 M0, T3,4 Nx M0), stage IV (Tany Nany M1). Stage group “Unknown” includes cases with no information on stage, and those with missing information on at least two fields of the TNM classifications (either M0, T and N missing, or T known, N and M missing). Surgical management was classified according to disease stage and information on residual tumour mentioned in the surgery or in the pathology reports: 1) radical surgery (stage I–III operated cases with primary tumour entirely resected); 2) palliative or incomplete surgery (stage I–III operated cases with residual tumour or stage IV operated cases); 3) no surgery; 4) surgical intent unknown (surgery performed, but not known whether radical, palliative or incomplete; unknown whether surgery performed or not). In addition, we formed a group of patients with a potentially curative resection (radically operated patients plus those stage I–III patients who had surgery that was not specifically mentioned as palliative).
Relative survival estimates, which may be interpreted as cancer-related excess mortality within a cancer patient population, were calculated as the ratio of the observed survival of the cancer patients and the expected survival of the underlying general population. The latter estimate was calculated according to the Ederer II method [Citation7] using national life tables stratified by gender, and single year of age and calendar year, with rates smoothed using three-year moving averages over time. The International Cancer Survival Standard (ICSS) population 1 was used for age-standardising overall site-specific survival estimates [Citation8]. All calculations were carried out using the Stata package (StataCorp LP, Texas USA) [Citation9]. The study was approved by the Tallinn Medical Ethics Committee.
Results
A total of 546 cases of CRC were available for the analysis; 88% of cases were histologically confirmed. Median age at diagnosis was 68 years (range 30 to 94 years). The characteristics of the cohort are presented in . A total of 74% of CRC cases were left-sided, defined as cancers of descending colon, sigmoid, rectosigmoid and rectum. Right sided CRC, defined as cancer of caecum, appendix, ascending colon, hepatic flexure, transverse colon and splenic flexure comprised 26% of all cases. Around 30% of patients were diagnosed with distant metastasis, and 7% were not staged (6% and 8% of colon and rectal cancer cases, respectively).
Table I. Characteristics of the 1997 colorectal cancer cohort in Estonia.
We explored the treatment and diagnostic patterns of colon and rectal cancer patients by stage (). Overall, radical surgery was performed in slightly less than half of the patients. Among radically operated patients, the total number of lymph nodes examined was not reported for about 15%, and the majority of those with available information had one to four nodes examined. Seven patients altogether had more than eight nodes examined.
Table II. Diagnostics and treatment by stage in the 1997 colorectal cancer cohort in Estonia.
Data on preoperative chemotherapy is not shown as only one colon cancer patient and two rectal cancer patients received this kind of treatment.
Overall, 61% of patients were treated at specialised cancer centres (51% of colon and 78% of rectal cancer patients, respectively). Among radically operated patients, 63% of colon cancer and 89% of rectal cancer patients were treated at cancer centres. Perioperative mortality, defined as death within 30 days of surgery, was overall 2.7% among radically operated patients (3.7% vs. 1.6% among colon and rectal cancer patients, respectively). Patients treated at cancer centres had notably lower perioperative mortality than those treated in general hospitals (1.6% vs. 5.6%, respectively).
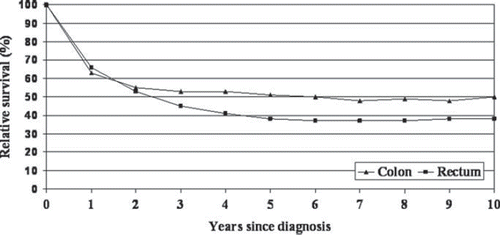
During the first two years after diagnosis the overall survival curves were closely similar for colon and rectum cancers (). Subsequently, the curves diverged and the survival remained markedly higher for colon cancer. The overall relative survival stabilised among colon cancer patients around three to four years after diagnosis compared to around six to seven years among rectal cancer patients. The 5-year relative survival was 51% for colon and 38% for rectal cancer; the corresponding 10-year relative survival rates were 50% and 39% (). While no gender differences were apparent in colon cancer survival, women had lower survival for rectal cancer up to 10 years after diagnosis. Both colon and rectal cancer patients with stage I disease did not experience any excess mortality compared to the general population. The 5- and 10-year survival for stages II and III was markedly higher in colon cancer compared to rectal cancer. To analyse the effect of including/excluding Nx cases (n = 28) with stage III category, we calculated survival rates separately for patients with pathologically determined regional metastases (Tany N1-3 M0). In colon cancer, this group (n = 45) showed excellent prognosis, with the 1-year, 5-year and 10-year survival of 88%, 81% and 77%, respectively; in rectal cancer (n = 35), the corresponding survival rates were 100%, 37% and 35%, respectively. Overall, radically operated colon cancer patients had a 90% survival up to 10 years after diagnosis; among radically operated rectal cancer patients, the respective estimate was around 70%. The 5-year relative survival among all patients who had a potentially curative resection (radically operated patients plus those stage I–III patients who had surgery that was not specifically mentioned as palliative) was 87% for colon cancer (176 patients) and 68% for rectal cancer (105 patients). Among patients treated at cancer centres and general hospitals, the overall 5-year relative survival was 61% and 40% for colon cancer, and 43% and 21% for rectal cancer, respectively.
Table III. Relative survival in the 1997 colorectal cancer cohort in Estonia.
Discussion
In our nationwide 1-year incidence cohort, the majority of deaths occurred during the first years after diagnosis and there was practically no difference between the 5- and 10-year relative survival rates. This finding supports the notion that the excess risk of death, mainly related to advanced disease, applies soon after diagnosis and patients surviving five years after diagnosis do not experience any excess mortality [Citation10]. In cases with early disease, the relative survival even exceeded 100% during long-term follow-up, which is a well known phenomenon also described elsewhere [Citation11].
The study had several limitations. First, the survival estimates are based on one year incidence in Estonia and the relatively small number of cases caused fluctuations in survival estimates, particularly in stage-specific analyses. Also, the small number of cases did not allow for a more thorough analysis of survival differences by stage and treatment as well as the multivariate modelling of the effect of different factors on survival. Due to incomplete staging information we had to make several assumptions when classifying cases. Cases with tumour stage T3,4 that were determined to have no distant metastasis, but without nodal information, were classified as stage III in this analysis. It is possible that some of these cases did not have lymph node metastasis and should have been classified as stage II; this may have caused some overestimation of both stage II and stage III survival. Our finding of the 5-year survival of 81% among stage III pathologically confirmed node-positive patients suggests, however, that this is a highly selected group with well treatable disease, and not likely to reflect the prognosis of all stage III patients. Some studies [Citation12] have assumed N0 status in cases with no pathological information on lymph nodes. In the Estonian setting, however, this would clearly not be appropriate. At the same time, given the small number of lymph nodes examined in most patients, some misclassification of true node-positive disease to node-negative disease is also possible.
Survival in rectal cancer has been considerably lower than survival in colon cancer in Estonia, whereas particularly low survival in rectal cancer in Estonia has been observed for women. These are not typical findings for the rest of Europe [Citation3,Citation13]. When comparing survival rates calculated for the patients diagnosed in 1997 and the results reported by the EUROCARE-3 Study (1990–1994; relative survival for colon cancer 38% and 37% among men and women, respectively; for rectal cancer 33% and 28% among men and women, respectively) [Citation3], a marked improvement in Estonia can be seen for colon cancer, while improvement remained only modest for rectal cancer. Estonian data were not included in the EUROCARE-4 Study [Citation13], but for colon cancer, the 5-year relative survival of patients diagnosed in 1997 (51%) has nearly approached the European estimate presented for the diagnostic period 1995−1999 (55%); for rectal cancer the 5-year survival in Estonia remained notably below the European rate (38% vs. 53%) and was similar to that in Poland (39%) and Czech Republic (40%) [Citation13]. In contrast to Eastern Europe, a marked improvement was seen in initially low survival for rectal cancer in the UK (England, Scotland, Wales) from EUROCARE-3 to EUROCARE-4 [Citation3,Citation13].
International comparisons of stage-specific survival in CRC are quite complicated as routinely collected staging information and classification principles vary by cancer registries [Citation14]. In the present study, we have used the so-called “high-resolution” approach, which needed extra efforts for obtaining TNM staging details from the original clinical records.
About one third of CRC patients in Estonia were diagnosed with stage IV disease, which showed poor survival. According to the results of the EUROCARE High Resolution Study, the percentage of CRC cases diagnosed with distant metastases or unresected cases with stage not available varied from 25% to 37% in Europe in 1996−1998, and were the highest in Eastern Europe (Poland, Slovakia, Estonia) and Spain [Citation6]. A high proportion of patients presenting with advanced disease corresponded to low overall survival in these countries [Citation3,Citation13].
Both in colon cancer and rectal cancer, early detection greatly improved the chance for favourable outcome. Survival of the patients diagnosed with stage I disease was excellent, and also for stage II, the 5-year survival exceeded 80% in colon cancer and approached 70% in rectal cancer; our results are very similar to those presented by the Finnish Cancer Registry for the period 1985–1994 [Citation15]. While the survival of stage III colon cancer patients was well comparable to that seen in Finland, the survival estimate of stage III rectal cancer patients in Estonia (30%) appeared to be significantly lower than that in Finland [Citation15]. In a study comparing rectal cancer 5-year relative survival in the Nordic countries and Scotland, the country-specific age-standardised estimates for stage III ranged from 41% to 67% among men and 43% to 60% among women [Citation16].
Information about lymph node status is shown to be highly important for accurate staging and subsequent treatment choice [Citation17]. Recent studies have demonstrated a positive correlation between the survival of CRC patients and the number of morphologically studied lymph nodes, although the mechanisms underlying this association are unknown [Citation18]. In our cohort, the median number of examined nodes was very small and only for 2% of radically operated patients, 12 or more nodes were studied, suggesting inadequate thoroughness of pathological staging. Patients in this study were diagnosed in 1997, when the recommendation to retrieve at least 12 lymph nodes was not yet widespread in Estonia. There have been reports from other countries that show smaller lymph node retrieval numbers in the 1990s [Citation19,Citation20]. The situation appears to be improving in Estonia – according to unpublished data from one cancer centre, Tartu University Clinic of Haematology and Oncology, the average number of morphologically examined lymph nodes among radically operated patients was 11 in 2009, and 12 or more nodes were examined in 43% of the patients.
The first national guidelines for diagnosing and treating malignant tumours were agreed upon in Estonia in 1997 and gradually introduced into practice thereafter. The recommended treatment for colon cancer includes radical surgery for stages I and II, and radical surgery with adjuvant chemotherapy for stage III. For rectal cancer, the recommended treatment includes radical surgery for stage I patients, while neoadjuvant radiation and chemotherapy prior to radical surgery and subsequent adjuvant chemotherapy are recommended for stages II and III. The proportion of surgically resected patients has been shown to be positively correlated with 5-year survival [Citation14]. Our data on colon cancer are in accordance with these findings as all patient groups with high proportion of radical surgery had good survival, even node-positive patients. The 5-year survival of 81% among pathologically confirmed node-positive stage III colon cancer patients in our cohort, along with the 90% survival of all radically operated colon cancer patients, is a clear indication of effective colon cancer surgery.
In contrast, in rectal cancer, the survival for stage III patients was poor, which may be related to surgical techniques used at that time (the concept of total mesorectal excision was not applied in Estonia in 1997) as well as inadequate use of neoadjuvant and adjuvant therapies. The low survival for stage III rectal cancer could be a contributor to markedly divergent overall relative survival curves seen in our study for rectal cancer and colon cancer. It has been suggested that the recent favourable trends in rectal cancer survival seen in many countries reflect improvements in surgical techniques and the widespread use of preoperative radiation [Citation21,Citation22]. There is strong evidence that preoperative radiotherapy reduces rectal cancer local failure rates and increases survival [Citation23].
The use of advanced surgical procedures requires specialist skills and experience that is facilitated by the concentration of rectal cancer surgery to fewer surgeons in cancer centres [Citation21,Citation22,Citation24]. Survival improvements achieved in Sweden and Scotland provide strong support for treatment centralisation [Citation16,Citation21]. While there are two specialised cancer centres in Estonia, the patients in this cohort were treated at a variety of hospitals across the country. Both for colon and rectal cancers, the overall 5-year relative survival was significantly higher for patients treated at cancer centres than at general hospitals. The small number of cases did not allow us to assess the effects of the type of treating hospital on survival, accounting for disease stage and patient characteristics. However, it is probably safe to assume that the experience of surgeons performing radical excision is limited at hospitals that perform only a few operations annually. Also, perioperative mortality among radically operated patients was many times higher in general hospitals compared to specialised cancer centres, which, in part, may reflect higher proportion of emergency surgeries.
In conclusion, our population-based survival analysis in the 1997 CRC incidence cohort demonstrated that, compared to earlier estimates, the outcome has notably improved for colon cancer, but not for rectal cancer in Estonia. High survival was observed for patients diagnosed with localised disease, and also among patients with pathologically diagnosed node positive disease (especially colon cancer), suggesting that these patient groups have generally received adequate treatment. Prognosis remained poor for advanced cases and for insufficiently staged cases. Limited access to modern diagnostic and treatment methods yet in the late 1990s, but also low quality of pathological examination along with high proportion of patients with distant metastasis at diagnosis may be responsible for slow progress, particularly in rectal cancer. Up-to-date population-based analyses of patient survival and clinical characteristics are needed to see whether the application of new diagnostic and treatment standards as well as improved diagnostic and therapeutic modalities have contributed to better prognosis of CRC patients in Estonia.
Further increases in survival, but also declines in mortality of CRC in Estonia could be achieved by earlier diagnosis, improved quality of staging as well as increasing the proportion of patients treated with curatively intended surgery and appropriate radiotherapy and chemotherapy. These conclusions can be important also for other Eastern European countries, which are similar to Estonia in terms of their health care transition.
Acknowledgements
The authors thank Dr. Margit Mägi and Mrs. Pille Härmaorg from the Estonian Cancer Registry for providing the data and technical help. This work was supported by the Estonian Ministry of Education and Research [target funding SF0940026s07].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]. International Agency for Research on Cancer; Lyon, France, 2010. [cited on 2011 Feb 14]. Available from: http://globocan.iarc.fr.
- World Health Organization. World Health Organization, mortality database. [cited on 2011 Feb 14]. Available from: http://www.who.int/whosis/whosis.
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, . EUROCARE-3: Survival of cancer patients diagnosed 1990–94 – results and commentary. Ann Oncol 2003;14(Suppl 5):v61–118.
- Gondos A, Bray F, Hakulinen T, Brenner H. Trends in cancer survival in 11 European populations from 1990 to 2009: A model-based analysis. Ann Oncol 2009;20:564–73.
- Aareleid T, Brenner H. Trends in cancer patient survival in Estonia before and after the transition from a Soviet republic to an open-market economy. Int J Cancer 2002;102: 45–50.
- Gatta G, Zigon G, Aareleid T, Ardanaz E, Bielska Lasota M, Calceran J, . Patterns of care for European colorectal cancer patients diagnosed 1996–1998: A EUROCARE high resolution study. Acta Oncol 2010;49:776–83.
- Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda, MD: End Results Evaluation Section, National Cancer Institute; 1959.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- Janssen-Heijnen ML, Houterman S, Lemmens VE, Brenner H, Steyerberg EW, Coebergh JW. Prognosis for long-term survivors of cancer. Ann Oncol 2007;18:1408–13.
- Hakulinen T. On long-term relative survival rates. J Chronic Dis 1977;30:431–43.
- Threlfall T, Wittorff J, Boutdara P, Heyworth J, Katris P, Sheiner H, . Collection of population-based cancer staging information in Western Australia – a feasibility study. Popul Health Metr 2005;3:9.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, . EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Gatta G, Capocaccia R, Sant M, Bell CM, Coebergh JW, Damhuis RA, . Understanding variations in survival for colorectal cancer in Europe: A EUROCARE high resolution study. Gut 2000;47:533–8.
- Dickman PW, Hakulinen T, Luostarinen T, Pukkala E, Sankila R, Söderman B, . Survival of cancer patients in Finland 1955–1994. Acta Oncol 1999;38(Suppl 12):1–103.
- Folkesson J, Engholm G, Ehrnrooth E, Kejs A-M, Pahlman L, Harling H, . Rectal cancer survival in the Nordic countries and Scotland. Int J Cancer 2009;125:2406–12.
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J Natl Cancer Inst 2007;99: 433–41.
- Ricciardi R, Baxter NN. Association versus causation versus quality improvement: Setting benchmarks for lymph node evaluation in colon cancer. J Natl Cancer Inst 2007;99: 414–5.
- Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daudé H, . Lymph node harvest reporting in patients with carcinoma of the large bowel. A French population-based study. Cancer 1998;82:1482–6.
- Wright FC, Law CHL, Last L, Khalifa M, Arnaout A, Naseer Z, . Lymph node retrieval and assessment in stage II colorectal cancer: A population-based study. Ann Surg Oncol 2003;10:903–9.
- Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol 2005;31:845–53.
- Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med 2006;260:103–17.
- Glimelius B, Gronberg H, Jarhult J, Wallgren A, Cavallin-Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003;42:476–92.
- Smedh K, Olsson L, Johansson H, Aberg C, Andersson M. Reduction of postoperative morbidity and mortality in patients with rectal cancer following the introduction of a colorectal unit. Br J Surg 2001;88:273–7.