Abstract
Background. Volumetric modulated arc therapy (VMAT) for treatment of non-small cell lung cancer (NSCLC) patients potentially changes the risk of radiation-induced pneumonitis (RP) compared to intensity modulated radiation therapy (IMRT) if the dose to the healthy lung is changed significantly. In this study, clinical IMRT plans were used as starting point for VMAT optimization and differences in risk estimates of RP between the two plan types were evaluated. Material and methods. Fifteen NSCLC patients prescribed 66 Gy in 2 Gy fractions were planned with IMRT and subsequently with single arc VMAT. Dose metrics were evaluated for target and lung together with population averaged dose volume histograms. The risk of RP was calculated using normal tissue complication probability (NTCP) models. Finally, applicability of the plans was tested through delivery on an Elekta accelerator. Results. When changing from IMRT to VMAT only modest differences were observed in the dose to the lung and target volume. On average, fractions of lung irradiated to doses between 18 Gy and 48 Gy were statistically significant reduced using VMAT compared to IMRT. For the fraction of lung receiving more than 20 Gy the reduction was 1.2% percentage points: (range –0.6 –2.6%). The evaluated toxicity were smaller with VMAT compared to IMRT, however only modest differences were observed in the NTCP values. The plans were delivered without any problems. The average beam on time with VMAT was 83 s. This was a reduction of 141 s (ranging from 37 s to 216 s) compared to IMRT. Conclusions. Using IMRT as reference for the VMAT optimization it was possible to implement VMAT in the clinic with no increase in estimated risk of RP. Thus, toxicity is not expected to be a hindrance to using VMAT and will profit from the shorter delivery time with VMAT compared to IMRT.
The introduction of volumetric modulated arc therapy (VMAT) for external beam radiotherapy has increased the delivery efficiency, especially in regards to treatment time, compared to intensity modulated radiation therapy (IMRT) [Citation1]. VMAT plans are delivered continuously during gantry rotation which might change the dose distribution in the healthy tissue from “a lot to a little” towards “a little to a lot”, compared to IMRT , as previously reported in radiotherapy of head and neck cancer [Citation2]. This may not be a problem if the organs surrounding the target tolerate large low dose volumes. However, in radiotherapy of lung cancer large volumes of healthy lung tissue irradiated to low doses is a concern [Citation3]. Animal studies have shown that the volume of healthy lung irradiated to small doses could be the dominant factor for radiation induced lung toxicity [Citation4]. Several clinical studies have shown a relation between fraction of lung volume irradiated to low dose and incidence of pneumonitis [Citation5–8]. Furthermore, a recent study indicates that increased low dose volumes might lead to a higher risk of pneumonitis when combined with chemotherapy [Citation9,Citation10].
In six lung patients VMAT plans generated using a non-commercial VMAT segmentation algorithm have been compared to clinical IMRT plans in a previous planning study [Citation11]. No differences were observed between IMRT and VMAT evaluating single value dose metrics for dose to target and healthy lung for this limited number of patients. Furthermore, a recent study reported acceptable acute toxicity rates for NSCLC patients treated with VMAT [Citation12], but these results were not compared to a similar cohort treated with IMRT.
In the present study, clinical implementations of IMRT and VMAT using commercial available systems were evaluated for a larger number of patients. The purpose of this study was to evaluate whether a clinical implementation of VMAT alters the dose to the healthy lung significantly compared to IMRT plans in such a way that increased lung toxicity should be a concern. Therefore, differences in risk of toxicity were assessed not only using single value dose metrics but also by evaluation of the population mean dose volume histograms (DVHs) and estimates of the risk for pneumonitis calculated using normal tissue complication probability (NTCP) models.
Material and methods
Planning and optimization
Fifteen consecutive non-small cell lung cancer (NSCLC) patients treated in 2011 at Odense University Hospital were included in this planning study. In are listed the patient specifics. The patients were treated supine with their arms elevated and immobilized using a Vacfix vacuum bag with a thermo plastic cover. Treatment planning was based on the mid ventilation phase of a 4D kV-CT scan with slice thickness of 2.5 mm [Citation14] and performed using Pinnacle3® 9.0. The gross tumor volume (GTV) was delineated based on the planning-CT and FDG-PET scans. The planning target volume (PTV) was generated using margins to the GTV in the mediastinum and lung of 10 mm and 15 mm, respectively. The patients were prescribed 66 Gy in 33 fractions to the PTV. Treatment plans were optimized according to our current clinical procedure using the following clinical objectives for plan approval listed in order of importance: maximal dose to spinal cord less than 45 Gy, fraction of residual lung (defined as both lungs excluding the GTV) receiving more than 20 Gy constrained to less than 40% and reduced if possible without compromising the target coverage, i.e. the PTV should receive 95% of the prescribed dose. The heart was not considered as a dose-limiting organ according to a previous study [Citation15]. However, high doses in the heart were avoided if possible due to the possible interaction between dose to the heart and adverse effects in the lung [Citation16]. IMRT and VMAT plans were created for an Elekta Synergy accelerator (MLCi2 leaf bank, 2 × 40 leaves) using 6 MV. For step-and-shoot IMRT plans five to seven co-planer treatment fields were distributed depended on the patient geometry and position of the target volume to avoid unnecessary irradiation of the contra lateral lung. Thus, beam angles were individualized and not evenly spaced. The median length of the arcs spanned by the IMRT fields was 215° (ranging from 150° to 310°). The IMRT optimization process was performed using the Pinnacle3® DMPO segmentation algorithm that sequenced a median number of 58 control points per plan to obtain the required dose modulation.
Table I. Patient specifics.
Due to the patient heterogeneity, use of standardized optimization parameters for all patients would lead to non-optimal balance between dose to target and minimal dose to residual lung. Thus, some planning iterations are needed to find the best optimization parameters for the individual patient. Initial plan iterations are faster with IMRT compared to VMAT, due to large differences in degrees of freedom between the two techniques. Therefore, in our clinical procedure, the VMAT plan was generated, subsequently to the IMRT planning, using the optimization parameters from the IMRT optimization process as starting point. The VMAT plans, generated using the Pinnacle SmartArc algorithm [Citation17], consisted of single arcs with a 2° separation of the control points and median arc length of 216° (ranging from 178° to 358°). The VMAT arcs were in general similar to the arcs spanned by the IMRT fields. However, because the gantry cannot pass 180° (posterior direction) 360° arcs were used for patients with posterior tumor positions. For one patient this restriction was circumvented by use of two 90° arcs (the minimal VMAT arc length in Pinnacle3® 9.0) to reduce irradiation of the anterior part of the lungs (see ). In the optimization process, the maximal delivery time was set to 400 s in order not to limit the modulation depth. The Collapsed Cone dose engine with density correction was used for the final dose calculation [Citation18–20]. For all plans the optimized dose distributions were normalized to give a mean PTV dose of 66 Gy. The IMRT and VMAT plans were approved for treatment by oncologists specialized in radiotherapy of lung cancer. Dose and planning data were transferred to MATLAB for further data analysis using in-house develop routines as well as subroutines of the CERR package [Citation21].
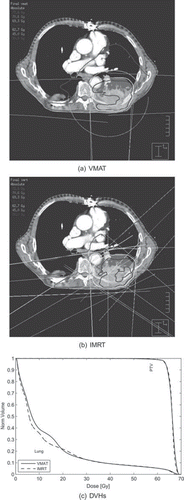
Figure 1. Field setups, dose distributions (a and b) and relevant Dose Volume Histograms (DVHs) (c) of the Volumetric Modulated Arc Therapy (VMAT) and Intensity Modulated Radiation Therapy (IMRT) plans for one patient. The shaded area indicates the PTV. The arc of the VMAT plan is separated in two 90° arcs to avoid using anterior fields for treatment of a target volume with posterior location. The VMAT and IMRT plan provide similar target coverage, while the low dose region (< 20 Gy) is increased with VMAT compared to IMRT for this particular patient.
To validate that all plans were deliverable and to assess the difference in the delivery time between VMAT and IMRT the plans were delivered at an Elekta accelerator, using the Integrity™ R1.1 treatment control system which facilitates continuously variable dose rate [Citation22], while the time from first to last beam-on was measured for each plan. In this process, to maximize the delivery efficiency, the treatments were scheduled as an automatic field sequence with minimal movement of gantry between the individual fields in the record and verify system (MOSAIQ 2.20.05B1, Elekta).
Toxicity models
Two NTCP models were used to calculate the expected risk of radiation induced pneumonitis (grade ≥ 2, steroids needed); the Lyman-Kutcher-Burman (LKB) model and a critical volume model, both using three parameters [Citation6]. The model parameters used in the LKB model were: a volume exponent n, the dose for 50% complication probability TD50 and a steepness parameter m with values of n = 1, TD50 = 31.4 Gy, m = 0.45, respectively, according to recent QUANTEC publication [Citation3]. By choosing n = 1 the LKB model is reduced to a model based on the mean lung dose. The parameters of the critical volume model were: D50 the local threshold dose for subunit tissue damage, rvD the fraction of damage volume for a 50% complication probability and m a steepness parameter. The critical volume model parameters were selected to D50 = 13 Gy, rvD = 77% and m = 0.44, according to data analysis published by Seppenwolde et al. [Citation6]. Doses in the residual lung tissue were corrected for fractionation using α/β ratio of 3 Gy, prior to NTCP calculations, in accordance with the study by Seppenwolde et al. [Citation6]. The α/ β corrected local threshold dose of 13 Gy used in the critical volume model corresponds to a physical dose of 18.3 Gy delivered in 33 fractions, which is close to the 20 Gy used as a threshold dose at many institutions. In the QUANTEC cohort, some of the studies use physical doses. Therefore, the complication probability calculations were repeated for the QUANTEC model using physical doses. All dose metrics as well as DVHs reported in this paper are given as physical doses.
Statistics
Non-parametric Wilcoxon signed rank test was used as a paired test of differences between the planning techniques. Two-tailed p-values less than 0.05 were considered statistically significant. The population mean DVHs were calculated for the residual lung and PTV as the average volume fraction for given dose levels. To illustrate dose regions in which significant differences exist between the DVHs of the two techniques two-sided Wilcoxon signed rank test was calculated for all dose levels of the DVHs [Citation2].
Results
The planning strategy made it possible to create IMRT and VMAT plans that were approved for clinical use for all included cases.
displays IMRT and VMAT field setups, dose distributions and relevant DVHs for one patient. For this particular patient the target coverage is very similar using the two techniques, while the healthy lung DVH of the VMAT plan presented larger volume fractions in the low dose regions compared to IMRT as expected according to the hypothesis.
displays the population mean DVHs. On average the dose to the residual lung for the IMRT and VMAT plans are quite similar. However, small but statistically differences exist between the two plan types. On average, in the large range of intermediate dose values between 18 Gy and 48 Gy the VMAT plan presents statistically significant lower values compared to IMRT. The opposite is the case in the high dose range 66–68 Gy where IMRT is statistically lower than VMAT caused by a lower dose to the part of the PTV in the residual lung for the IMRT plans compared to VMAT. This is also seen in the population mean DVHs of the PTV shown in . In mean values and ranges of dose metrics for PTV and residual lung together with calculated NTCP values are shown. On average, it was possible to reduce the V20 statistically significantly with VMAT compared to IMRT without compromising the target coverage, while no significant difference was observed in the MLD and thus not in the LKB NTCP either using physical doses nor doses corrected for fractionation. However, a significant difference in NTCP was found using the critical volume model predicting the NTCP to be slightly decreased with VMAT compared to IMRT.
Figure 2. Population mean Dose Volume Histograms (DVHs) of residual lung (a) and PTV (b) for Volumetric Modulated Arc Therapy (VMAT) (solid line) and Intensity Modulated Radiation Therapy (IMRT) plans (dashed line). For each dose level p-value of Wilcoxon signed rank test is shown (dotted line) to indicate dose regions in which difference exists between IMRT and VMAT.
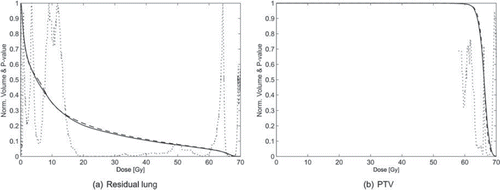
Table II. Dose metrices and toxicity calculations.
It was unproblematic to deliver the plans on the Elekta Synergy accelerator. On average, the beam on time was reduced significantly from 223 s with IMRT to only 83 s with VMAT, i.e. an average reduction of 140 s ranging from 36 s to 216 s.
Discussion
In the present study, the optimization parameters of clinical approved IMRT plans were used as starting point for VMAT optimization. This strategy combined with VMAT arc lengths similar to the arc lengths spanned by the IMRT fields resulted in VMAT plans with dose distributions in residual lung and target volume very similar to those obtained with IMRT. Furthermore, the low dose volume was not increased with VMAT compared to IMRT, contrary to the “little to a lot” hypothesis.
On average, the used planning strategy made it possible to decrease V20 statistically significant with VMAT compared to the reference IMRT plans. This difference in V20 could be related to the use of this dose value as optimization parameter combined with the use of the IMRT plan as reference. However, on average in the intermediate dose range (18 – 48 Gy) the VMAT plans performed statistically significant better than the IMRT plans with a lower mean DVH. This could be due to coupling to the V20 value, but it might also be due to differences in planning technique and the increased degrees of freedom presented by VMAT. With IMRT, decisions such as choice of field angels are left to the planner, with VMAT the dose per entrance angle is optimized by the algorithm, which potentially results in more optimal plans.
The change in V20 from IMRT to VMAT is also reflected in the significant difference in NTCP values calculated using the critical volume model and α/ β corrected doses. Due to slightly larger high dose volumes in the residual lung (close to or within the PTV) with VMAT compared to IMRT no significant difference was found neither for MLD nor for the NTCP values calculated using LKB due to the relation between MLD and the used LKB model. However, for none of the evaluated toxicity metrics the VMAT plans performed worse than the reference IMRT plans.
The absolute uncertainties of the NTCP models are likely larger than any differences observed in the current study. The absolute NTCP values depend on the cohort of patients used to calculate the model parameters, the clinical scoring system and the calculated dose. Small changes in any of these can influence the absolute NTCP values as seen in in which different models do not predict the same NTCP value. Thus, the absolute values of the predicted NTCP for the patients in the current study may have a large uncertainty. However, the conclusions in the study is not based on the absolute NTCP value but the relative differences between NTCP values within a given NTCP model, which is much more robust to the above mentioned uncertainties. As an example, in use of correction for fractionation in the LKB model changes the absolute NTCP values significant while the difference between the IMRT and VMAT calculations is almost unchanged.
The parameters used for NTCP calculations in both models are based on three-dimensional (3D) conformal radiation therapy data, and are not validated for the dose distribution of neither IMRT nor VMAT plans. Thus, more clinical outcome data on patients treated with IMRT/VMAT are needed [Citation3]. Another issue with the currently used models is the reduction from 3D dose information to a single value like the MLD, rdV and equivalent uniform dose. In this process, the spatial distribution of the dose is discarded. It is also a main problem that a large inter-patient toxicity variation is present which diminish the validity of patient specific NTCP values. Both of these effects potentially could be reduced by use of imaging modalities, which have the potential of providing 3D data to predict the expected patient specific toxicity before or during treatment. It would thereby be possible to adapt the treatment based on a patient specific balance between tumour control and toxicity [Citation23].
Studies comparing different clinical setups are important in order to verify that a change of plan classes, i.e. change of dose distribution in the healthy tissue, does not alter or enhance expected toxicity. The weakness of such studies lies in the possible choice of suboptimal reference plans which will favor the evaluated technique. Small changes in the reference or evaluated technique, e.g. changing the number of IMRT fields or VMAT arcs, could probably modify the result [Citation24,Citation25]. One approach for a general comparison of IMRT and VMAT could be made using Paretro front optimization [Citation26]. However, the number of possible parameters to evaluate increases the complexity of such analysis. In the present study a clinical implementation of VMAT is evaluated using clinical IMRT plans as reference. The study does not prove the superiority of VMAT compared to IMRT but verifies that it is possible to create VMAT plans with similar or lower toxicity estimates compared to the IMRT plans used in our clinic, if the IMRT plans are used as reference during the VMAT optimization.
In general, the observed differences between the obtained IMRT and VMAT dose distributions and the related NTCP values were very small and most likely of limited clinical relevance especially given the uncertainty of the absolute NTCP value when the uncertainties in delivery and NTCP models are considered. The similarity of the population mean DVHs indicates that for some patients the IMRT dose distribution may be preferred compared to VMAT when only evaluating the plan quality. However, using VMAT it is possible to profit by the most noticeable benefit with VMAT compared to IMRT, namely the 63% average reduction in delivery time. The short VMAT delivery time (83 s on average) reduces the risk of intra-fractional motions, which potentially enables reduction in treatment margin and thereby the treated volume. Furthermore, previous studies have shown that the fast delivery of VMAT is performed without compromising the accuracy [Citation2,Citation11,Citation22].
Conclusions
New planning techniques, such as VMAT, potentially change the dose to the healthy tissue and alter the toxicity compared to conventional used plans. In this study, clinical acceptable IMRT plans were used as starting point for VMAT optimization. This planning strategy made it possible to create VMAT plans with a statistically significant reduction in the fraction of lung receiving more than 20 Gy compared to the IMRT plans, without compromising the dose to the target volume. However, on average the observed differences in the dose distribution are small and most likely of limited clinical relevance. On average, this implementation of VMAT did not enhance the low dose regions compared to IMRT, and based on NTCP calculations the VMAT plans are not expected to increase the risk of radiation induced pneumonitis. Using the presented method VMAT have been implemented in the clinic, which makes it possible to take advantage of the very short delivery time with VMAT compared to IMRT.
Acknowledgements
This work is supported by The Lundbeck Foundation Center for Interventional Research in Radiation Oncology (CIRRO) and The Danish Council for Strategic Research. AB acknowledges Ph.D. funding from Elekta.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008;35:310–7.
- Bertelsen A, Hansen CR, Johansen J, Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol 2010;95:142–8.
- Marks LB, Bentzen SM, Deasy JO, Kong F-MS, Bradley JD, Vogelius IS, . Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70–6.
- Semenenko VA, Molthen RC, Li C, Morrow NV, Li R, Ghosh SN, . Irradiation of varying volumes of rat lung to same mean lung dose: A little to a lot or a lot to a little? Int J Radiat Oncol Biol Phys 2008;71:838–47.
- Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, . Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999; 45:323–9.
- Seppenwoolde Y, Lebesque JV, de Jaeger K, Belderbos JSA, Boersma LJ, Schilstra C, . Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys 2003;55:724–35.
- Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu C-S, Mohan R, . Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006;66:1399–407.
- Yom SS, Liao Z, Liu HH, Tucker SL, Hu C-S, Wei X, . Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007;68: 94–102.
- Vogelius IS, Westerly DC, Cannon GM, Mackie TR, Mehta MP, Sugie C, . Intensity-modulated radiotherapy might increase pneumonitis risk relative to three- dimensional conformal radiotherapy in patients receiving combined chemotherapy and radiotherapy: A modeling study of dose dumping. Int J Radiat Oncol Biol Phys 2011; 80:893–9.
- Vogelius IR, Westerly DC, Aznar MC, Cannon GM, Korreman SS, Mackie TR, . Estimated radiation pneumonitis risk after photon versus proton therapy alone or combined with chemotherapy for lung cancer. Acta Oncol 2011;50:772–6.
- Rao M, Yang W, Chen F, Sheng K, Ye J, Mehta V, . Comparison of elekta VMAT with helical tomotherapy and fixed field IMRT: Plan quality, delivery efficiency and accuracy. Med Phys 2010;37:1350–9.
- Scorsetti M, Navarria P, Mancosu P, Alongi F, Castiglioni S, Cavina R, . Large volume unresectable locally advanced non-small cell lung cancer: Acute toxicity and initial outcome results with rapid arc. Radiat Oncol 2010;5:94.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, . The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2:706–14.
- Gottlieb KL, Hansen CR, Hansen O, Westberg J, Brink C. Investigation of respiration induced intra- and inter- fractional tumour motion using a standard cone beam CT. Acta Oncol 2010;49:1192–8.
- Schytte T, Hansen O, Stolberg-Rohr T, Brink C. Cardiac toxicity and radiation dose to the heart in definitive treated non-small cell lung cancer. Acta Oncol 2010;49:1058–60.
- van Luijk P, Faber H, Meertens H, Schippers JM, Langendijk JA, Brandenburg S, . The impact of heart irradiation on dose-volume effects in the rat lung. Int J Radiat Oncol Biol Phys 2007;69:552–9.
- Bzdusek K, Friberger H, Eriksson K, Hårdemark B, Robinson D, Kaus M. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys 2009;36:2328–39.
- Knöös T, Wieslander E, Cozzi L, Brink C, Fogliata A, Albers D, . Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys Med Biol 2006;51:5785–807.
- Fogliata A, Vanetti E, Albers D, Brink C, Clivio A, Knöös T, . On the dosimetric behaviour of photon dose calculation algorithms in the presence of simple geometric heterogeneities: Comparison with Monte Carlo calculations. Phys Med Biol 2007;52:1363–85.
- Nielsen TB, Wieslander E, Fogliata A, Nielsen M, Hansen O, Brink C. Influence of dose calculation algorithms on the predicted dose distributions and NTCP values for NSCLC patients. Med Phys 2011;38:2412–8.
- Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys 2003;30: 979–85.
- Bertelsen A, Lorenzen EL, Brink C. Validation of a new control system for elekta accelerators facilitating continuously variable dose rate. Med Phys 2011;38:4802.
- Bertelsen A, Schytte T, Bentzen SM, Hansen O, Nielsen M, Brink C. Radiation dose response of normal lung assessed by cone beam CT – a potential tool for biologically adaptive radiation therapy. Radiother Oncol 2011;100:351–5.
- Guckenberger M, Richter A, Krieger T, Wilbert J, Baier K, Flentje M. Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Radiother Oncol 2009;93:259–65.
- Bortfeld T, Webb S. Single-arc IMRT? Phys Med Biol 2009;54:9–20.
- Ottosson RO, Engstrom PE, Sjöström D, Behrens CF, Karlsson A, Knöös T, . The feasibility of using pareto fronts for comparison of treatment planning systems and delivery techniques. Acta Oncol 2009;48:233–7.
