Abstract
Introduction. Hypoxia induced radioresistance has been acknowledged for decades. One of the indirect evidences of the influence of hypoxia on radiation response comes from the observations of a correlation between tumor control and hemoglobin level. This review examines the clinical data on the prognostic and predictive role of hemoglobin level and hemoglobin manipulation in radiotherapy of squamous cell carcinomas of the head and neck, a tumor type where hypoxic radioresistance have been previously documented. The influence of hemoglobin concentration on tumor oxygenation and outcome. The aim is to evaluate the existing literature for information of the influence of hemoglobin concentration and hemoglobin modifications on tumor oxygenation and outcome in head and neck squamous cell cancer patients. The data from several randomized trials show that while most studies have confirmed the prognostic value of hemoglobin, increasing the hemoglobin level through transfusion or erythropoietin stimulation did not result in improved outcome for patients with low initial hemoglobin levels. Clinical studies showed that smoking reduced the oxygen carrying capacity of the blood through formation of carboxyhemoglobin, and lead to poorer response to radiotherapy in smokers compared to non-smokers. Smoking also increased the risk of the development of secondary cancers. Conclusion and future perspectives. In conclusion, low hemoglobin is a significant negative prognostic factor for radiotherapy of head and neck cancer. Correction of pre-treatment low hemoglobin by blood transfusion and/or erythropoietin stimulating agents does, however, not improve the outcome. Smoking leads to a decrease in effective hemoglobin and poorer treatment outcome. Smoking should be avoided in order to improve the therapeutic efficacy of radiotherapy and development of other smoking-related diseases and/or secondary cancers.
Hypoxia induced radioresistance has been acknowledged for decades [Citation1]. Some of the significant evidence for the influence of hypoxia on radiation response comes indirectly from the relationship between tumor control and hemoglobin level [Citation2–9].
Head and neck squamous cell carcinomas (HNSCC) are cancers of the oral cavity, pharynx (nasopharynx, oropharynx and hypopharynx) and larynx. HNSCC are predominantly loco-regional diseases (i.e. disease located to the primary tumor site and loco-regional lymph nodes). Worldwide in 2008, head and neck cancer was the seventh leading cause of cancer death [Citation10]. The overall five-year survival is poor, being on average 50%. Tobacco use is the predominant risk factor in the carcinogenesis of HNSCC, but increased alcohol consumption is also a risk factor for the development of HNSCC when combined with smoking; recently infection with human papilloma virus (HPV) has also been recognized as a risk factor [Citation11–13].
In HNSCC the treatment modalities are mainly radiotherapy and surgery, directed towards avoiding loco-regional failure. Primary radiotherapy is used for cancers located in phanynx, larynx and some oral carcinomas. Head and neck cancer can be cured by radiotherapy, but the optimum treatment strategy is unclear. There is clinical evidence indicating that hypoxia may be a critical factor when treating carcinomas of the head and neck with radiotherapy. However, the extent of hypoxia has been found to be heterogenic in tumors of the same size, site and histopathology [Citation14]. In Denmark six-fraction-weekly radiotherapy is the standard treatment in combination with the hypoxic radiosensitizer nimorazole [Citation15–17].
Hemoglobin is the oxygen carrying molecule. It is a protein consisting of four subunits, each with a heme moiety attached to a polypeptide chain. Heme consists of a porphyrin and one ferrous atom, each capable of reversely binding a single oxygen molecule. The structure of hemoglobin determines its affinity for oxygen and by shifting the relationship of the polypeptide chains the molecule is capable of either capturing or releasing oxygen. Once the uptake of oxygen is initiated, structural changes facilitate additional uptake. This is the basis for the characteristic sigmoid shape of the oxygen-hemoglobin dissociation curve (). The position of the curve is influenced by 2,3-diphosphoglycerate (2,3-DPG), carbon dioxide (CO2), carbon monoxide (CO), pH and temperature [Citation18]. A smaller quantity of the oxygen transported in the blood is in physical solution in plasma and intracellular fluid. This amount can be influenced by the oxygen content in the inspired air and atmospheric pressure, and can be increased by a factor of 20 with 100% oxygen at 3 atmospheres of pressure [Citation19].
Figure 1. Oxygen dissociation curve (calculated by Kelman's equation [Citation18]).
![Figure 1. Oxygen dissociation curve (calculated by Kelman's equation [Citation18]).](/cms/asset/743c8fb9-d852-4715-8a59-b75a8bb1536c/ionc_a_653438_f0001_b.gif)
Values generally accepted as normal hemoglobin levels are 12–15 g/dl or 7.5–9.5 mmol/l for women and 13–16 g/dl or 8–10 mmol/l for men [Citation18]. The definition of anemia varies with different studies. The WHO definition of anemia uses 12 g/dl for females and 13 g/dl in males as cut off values, and below these values anemia is often graded by numbers or as mild, moderate and severe [Citation20–23]. Anemia is frequently seen in cancer patients and is a complication often underestimated and untreated, since the primary focus often is treatment of the cancer itself [Citation22,Citation24]. A low hemoglobin value in a cancer patient can, however, by itself indicate a poor general condition of the patient. The etiology of cancer related anemia is often multifactorial, including effects of the disease process itself (e.g. bleeding) or its treatment, whether it is chemoor radiotherapy. Factors associated with anemia are disorders of iron metabolism, reduced number of erythroid progenitor cells, increased levels of inflammatory cytokines, extracorpuscular hemolysis, catabolism of patients with tumor burden and relative deficiency of erythropoietin [Citation25,Citation26]. Anemia caused by cancer is usually normochromic and normocytic, with a low reticulocyte count [Citation27]. An association with poor outcome, morbidity, fatigue and poor quality of life is also often seen in anemic cancer patients [Citation28–30]. Additionally, in iron deficiency anemia an impairment of the immune system has been observed, probably due to a decrease in B- and T-cell function [Citation31].
The aim of the present paper is to provide a summary of the influence of hemoglobin concentration on tumor oxygenation and outcome of radiotherapy in HNSCC, as well as the results of radiotherapy when modifying hemoglobin level with transfusion and erythropoietin stimulating agents.
The effect of high hemoglobin level in clinical trials using radiotherapy
The first evidence of the existence of the oxygen enhancement ratio was demonstrated as early as 1909, when Gotwald Schwarz introduced the concept of “Kompressionsanämie”; that skin with reduced blood flow due to compression had decreased response to radiation. Initially the reduced response in the presence of local anemia was thought to be caused by changes in metabolism in the anemic tissue or reduced backscatter resulting from a decrease in hemoglobin-bound atoms within the irradiated area. It took more than 40 years before Gray and coworkers in the mid 1950s postulated that oxygen deficiency was the major source of radiation resistance. Later Thomlinson and Gray suggested that areas within tumors containing viable tumor cells could be oxygen deficient (hypoxic). Clinically their results facilitated the use of hyperbaric oxygen [Citation4,Citation18,Citation32,Citation33].
Since then, several clinical and experimental studies and reviews on the influence of hemoglobin and radiosensitivity have been published [Citation2–4, Citation18,Citation33–35]. In general, it has been shown that patients with high hemoglobin level have a better probability of tumor control than patients with low hemoglobin levels. Low hemoglobin does not necessarily mean anemia, but can include patients in the low normal range [Citation34]. A literature search from 1998 of studies analyzing the prognostic relationship between hemoglobin concentration and local control, revealed 39 studies (14 482 patients) showing a relationship, and only 12 studies (2790 patients) that did not [Citation18].
One of the early studies examining the effect of hemoglobin level on radiotherapy was performed by Evans and Bergsjö in 1965 where they evaluated cancers of the cervix treated with transfusion before irradiation. They showed that anemic patients had a significantly lower survival rate and higher incidence of persistent local cancer and found it likely that the prognosis for these patients could be improved by transfusion [Citation36]. Several studies in uterine cervix carcinoma have confirmed this effect of high hemoglobin level for both loco-regional control and survival [Citation18,Citation37–40]. In a retrospective trial in uterine cervix cancers there was a correlation between low hemoglobin level and decreased loco-regional control and poor survival, this correlation was observed whether the hemoglobin level was measured at admission, before or during radiotherapy [Citation37]. In cancers of the uterine cervix, Thomas et al. [Citation38,Citation39] also showed impaired survival in patients with low baseline hemoglobin level and a reduced survival was reported in patients with high hemoglobin level which decreased during radiotherapy. Overall, a high hemoglobin level prior to and during treatment has been associated with good prognosis in patients treated with radiotherapy for cancers of the uterine cervix, head and neck, bronchus, esophagus and bladder, with studies primarily being available in squamous cell cancers of the head and neck and uterine cervix [Citation2,Citation18,Citation37–42].
In HNSCC one of the early evaluations of patients in the DAHANCA database showed that in irradiated larynx and pharynx cancers, patients in the higher range of normal hemoglobin level had a better loco-regional control and survival. The hemoglobin level at which the benefit in local control became stable was used to establish the high and low hemoglobin group definition (below 13 g/dl in females and 14.5 g/dl in males) used in the DAHANCA studies that followed (). The difference in locoregional control was, however mainly present in pharyngeal and supraglottic laryngeal cancers [Citation43]. A benefit of high hemoglobin in glottis cancers was, however, established by van Act et al. They also showed in a multivariate analysis that it was only the hemoglobin value at the end of treatment that carried significantly prognostic information [Citation44]. Lee et al. confirmed the importance of hemoglobin level in a large trial including 451 advanced head and neck cancers. They found that low hemoglobin level measured before the second week of radiotherapy was associated with failure in both loco-regional control and survival [Citation45]. The benefit of high hemoglobin level has been confirmed in publications of the subrandomization to transfusion in both the DAHANCA 5 study and in the combined analyses of the DAHANCA 5 and 7 randomized trials [Citation6,Citation8]. In the DAHANCA 5 analysis high hemoglobin patients had a significantly better outcome compared to low non-transfused patients in all outcome measurements in the univariate analysis (loco-regional control (HR 0.71; 0.51–0.97; p = 0.03), disease-specific (HR 0.66; CI 0.47–0.92; p = 0.01) and overall survival (HR 0.69; CI 0.51–0.92; p = 0.01) [Citation6]. In the combined analyses of the DAHANCA 5 and 7 trials the high hemoglobin patients showed statistical significant better outcomes in univariate analysis compared to all the low patients [Citation8].
Figure 2. Loco-regional control and hemoglobin concentration (redrawn with permission from Overgaard et al. [Citation34]).
![Figure 2. Loco-regional control and hemoglobin concentration (redrawn with permission from Overgaard et al. [Citation34]).](/cms/asset/b8100f03-a9e5-41e4-8c01-cf7abc2f508c/ionc_a_653438_f0002_b.gif)
The optimal time of measurement of hemoglobin level as a prognostic factor has never been established [Citation21]; several studies use pretreatment hemoglobin measurement [Citation46], whereas other studies have shown prognostic relevance of hemoglobin values at the end of treatment [Citation44,Citation47] or hemoglobin levels during treatment [Citation38]. It is, however, an important discussion considering possible ways of dealing with the low hemoglobin level. Transfusion to patients prior to radiotherapy would be able to correct pretreatment hemoglobin levels, but if the decrease during treatment is considered to be more important, then treatment with erythropoietin stimulating agents might be the answer.
In anemic HNSCC patients the relative risk of death has been shown to be increased by 75% (CI 37–123%); the adjusted hazard rate ratio (HHR) (results from studies using Cox proportional hazards model) was found to be 1.75 (95% CI 1.37–2.23) and the unadjusted HRR 2.35. This study also looked at median survival time for other cancers as well, and for all locations the median survival was longer for patients without anemia compared with anemic patients [Citation25].
Relationship between hemoglobin level and oxygenation of tumors
Several reports have examined the connection between hemoglobin level and oxygenation in solid tumors [Citation46,Citation48]. In cancers of the uterine cervix, Vaupel showed that at very low and high hemoglobin levels, a low tumor pO2 was found, whereas the highest pO2 values are seen at hemoglobin values around 12 g/dl [Citation49,Citation50]. Fyles et al. demonstrated that anemia correlated with the low oxygenation status of tumors, but that compensatory mechanisms are likely to reduce the impact, implying complex mechanisms [Citation48]. In squamous cell carcinomas of the head and neck a number of trials have been conducted to examine the relationship between hemoglobin level and tumor oxygenation [Citation51–53]. The relationship between low median partial pressure of oxygen (pO2) at low and high hemoglobin levels, with maximum oxygen levels at a hemoglobin level around 13–14 g/dl has been shown [Citation54]. The most convincing evidence of a correlation between tumor oxygenation and hemoglobin level in head and neck cancer seems to be the multicenter analysis including 397 patients by Nordsmark et al. [Citation55]. This analysis which also included a fair number of patients from the Becker study [Citation54], compared pretreatment tumor pO2 and pretreatment hemoglobin and showed a quadratic regression correlation between hemoglobin level and median pO2 measurements.
In conclusion, pretreatment tumor oxygenation status is a prognostic factor for outcome after radiotherapy [Citation55,Citation56], but the link between the oxygenation status and hemoglobin level is not linear. There is a trend that very low hemoglobin values and very high hemoglobin values correlate with low tumor pO2, but the numbers of patients studied at these data points are limited. This is not too peculiar as numerous parameters influence hypoxia (e.g. tumor metabolism, diffusion/perfusion factors, blood flow, oxygenation, vascular network and hemoglobin), hence, the lack of a clear correlation is not surprising [Citation21].
Acute and chronic hypoxia
Hypoxia has been shown to exist in most solid animal tumor models [Citation9] and most human cancer types [Citation57,Citation58]. The presence of hypoxic areas within a tumor are classically explained by the cord structure model of tumors, in which, the diffusion distance for oxygen and nutrients taper off continuously from the arterial to the venous end of microvessels and is typically around 150 μm in the arterial end. Cells beyond this distance are chronic hypoxic (diffusion limited hypoxia), as opposed to oxygenated cells. Acute hypoxia can result from temporary occlusions in tumor blood vessels (perfusion limited hypoxia). Acute hypoxia has been given many names such as intermittent, transient, cyclic, repetitive, fluctuating, short-term or perfusion limited hypoxia [Citation1,Citation32,Citation58,Citation59].
The classic definitions of acute and chronic hypoxia have been challenged recently [Citation60]. It is stated that the classic definition of hypoxia widely discounts for the multiple pathogenic processes involved, and refines hypoxic subtypes by their pathophysiologic consequences with relevance to the clinical setting. The examples related to hemoglobin level and oxygen carrying capacity of the blood are categorized under hypoxemic chronic hypoxia, but the subdivision and challenge of classic definitions shows how several other mechanisms are involved in explaining the complexity of hypoxia and hypoxia induced radioresistance [Citation60].
In chronic hypoxia, a modified model of the oxygen availability theories introduced by Hirst [Citation18,Citation35,Citation61–63] is shown in . A cord of hypoxic cells is present due to limited diffusion. If retransfused, the radius of the tumor cord will be within the diffusion distance of oxygen, resulting in an increased number of cells receiving sufficient oxygen supply for improvement of radiosensitivity, but also increased proliferation. An idea that should be considered when treating patients with radiotherapy, transfusions and/or erythropoietic proteins, correction of anemia should not be done unless a sufficient blockage of tumor cell proliferation is established by the radiotherapy treatment [Citation18]. Although it has been argued that hypoxia may select for tumor cells with an aggressive phenotype [Citation50], such tumor aggressiveness has not been demonstrated in squamous cell carcinomas of the head and neck.
Figure 3. Tumor adaption to red cell blood transfusion by growth (adapted from Hirst et al. [Citation60]).
![Figure 3. Tumor adaption to red cell blood transfusion by growth (adapted from Hirst et al. [Citation60]).](/cms/asset/46f543af-b09b-4262-86e0-f04885525bec/ionc_a_653438_f0003_b.jpg)
Transfusion
Anemia is often corrected with red blood cell transfusions and the benefit of high hemoglobin levels in radiotherapy naturally lead to the question of whether transfusions could lead to improved oxygenation and subsequent response to radiotherapy. Pioneer work was done by Evans and Bergsjö as previously described and in addition to the better survival in high hemoglobin patients, they also showed that transfusion led to an increased oxygen tension in the tumor [Citation36]. In a small randomized trial Bush et al. showed that a correction of anemia by transfusion could decrease the recurrence and increase the cure rate, consistent with tumor hypoxia being greater in anemic patients. It was concluded that patients with a hemoglobin level during treatment below 12 g/dl had a significantly higher recurrence rate and lower cure rate [Citation64]. Fyles et al. revised the data and when based on the intention-to-treat principle, transfusion did not result in the initially described therapeutic gain [Citation48]. However, at the time of the original Bush study, it was the only randomized trial of that kind, and it supported the theory that transfusion would be beneficial in increasing the amount of oxygen applied to the tissue, assuming blood flow is unaltered, and thereby improving the response to radiotherapy. This was supported by other non-randomized studies in cancer of the uterine cervix showing an importance of hemoglobin level and effect of transfusion [Citation37–39,Citation65]. Of significance was a large multicenter study by Grogan et al. [Citation38] including a total of 605 patients with carcinoma of the cervix. They reported that blood transfusion appeared to overcome the negative prognostic effects of low pretreatment hemoglobin levels. Interestingly, at specific hemoglobin levels, patients who were transfused and maintained at that hemoglobin level had a survival rate that was not significantly different from patients who were at that level spontaneously.
However, cancer of the uterine cervix may be a suboptimal study population since they often suffer from bleeding from the tumor. Bleeding could be a sign of more advanced disease, poorer performance status, and worse overall condition of the patient. In contrast bleeding is rarely seen in head and neck cancer.
Sealy examined 72 patients with advanced head and neck or cervical cancer in the 1980s. Anemia was induced by venesection and maintained for 72-hours, hemoglobin then returned to normal by retransfusion and followed by radiotherapy under hyperbaric oxygen conditions. Interesting this intervention showed tumor shrinkage after induction of anemia and prior to radiotherapy. However, the overall survival was not improved for the head and neck cancer patients, but further studies were encouraged [Citation66].
In the DAHANCA 5 and 7 protocols including a total of almost 1200 head and neck cancer patients, low hemoglobin patients were subrandomized to transfusion or no transfusion [Citation6,Citation8]. These studies showed that transfusions to patients with hemoglobin level below 13 g/dl in females and 14.5 g/dl in males, raised the hemoglobin levels and brought these patients into the high hemoglobin group (). However, there was no benefit of transfusion on radiotherapy outcome in loco-regional control (HR 1.06; 0.82–1.38; p = 0.7), disease-specific (HR 1.27; CI 0.97–1.65; p = 0.1) or overall survival (HR 1.24; CI 0.99–1.54; p = 0.08) (). Even when accomplishing the wanted effect of transfusion by bringing the patients up to the same start hemoglobin levels as the high hemoglobin patients, there was still a continuous fall in hemoglobin level during treatment. The patients primarily received transfusion(s) prior to the start of radiotherapy. The studies were designed to keep the patients above the hemoglobin levels mentioned, and if the patients fell below the limit, transfusion was repeated. However, the decrease in hemoglobin level during radiotherapy did not fall below the cut off value, and facilitated repeated transfusion in very few patients. The transfusion regimen may, thereby not supply sufficient oxygenation to overcome the hypoxic radioresistance during treatment. Another explanation for the lack of benefit on outcome of transfusion may be the timing between transfusion and irradiation. The tumors may have adapted by compensatory mechanisms to the increased oxygen supply by growth, hence a correction without sufficient blockage of tumor cell proliferation by irradiation ().
Figure 4. Hemoglobin level during treatment in patients treated with radiotherapy and randomized to ESA, transfusion, no treatment high or low Hb level (combined data from DAHANCA 5, 7 and 10).
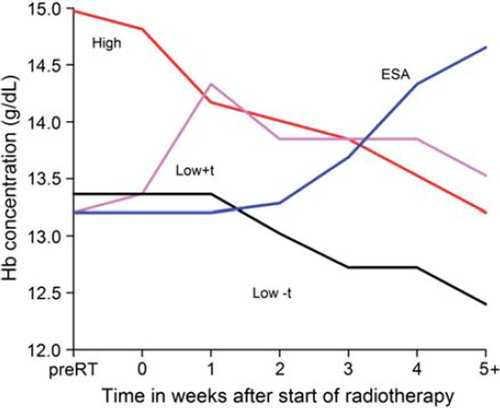
Figure 5. Survival outcomes of 1166 patients in the DAHANCA 5 & 7 trials randomized to transfusion during radiotherapy (by Hoff et al. [Citation8]).
![Figure 5. Survival outcomes of 1166 patients in the DAHANCA 5 & 7 trials randomized to transfusion during radiotherapy (by Hoff et al. [Citation8]).](/cms/asset/35bf3115-7b93-405d-bbaf-159e6fd3c410/ionc_a_653438_f0005_b.gif)
The lack of benefit of transfusion may also be due to immune regulatory effects [Citation8,Citation67–71]. It has been shown that blood transfusions can cause immune suppression in recipients. This syndrome is referred to as transfusion-associated immunomodulation and has been observed when treating patients with renal transplants to induce graft survival, but may also relate to the poor survival in transfused cancer patients [Citation67,Citation70]. In animal models it has been shown that the tumor growth promoting effect might be due to the presence of donor leukocytes in the transfused blood, and that this can be improved by pre-storage leukodepletion, but not by post-storage depletion of the blood [Citation67]. A Danish study reported decreased long-term survival in colorectal cancer patients receiving blood transfusion after elective surgery [Citation72]. Cause specific mortality was examined in these patients and showed that the patients had an increased risk of death from cardiovascular diseases, but not the cancer disease itself [Citation69]. Two different transfusion regimens were also compared, and on the basis of this the decreased survival in the transfused patients could not be explained by immune suppression [Citation72]. In another study involving colorectal cancer, blood transfusion was shown to be a univariate prognostic negative factor, but when adjusting with well-established prognostic factors in a multivariate prognostic model the negative effect of transfusions disappeared [Citation68]. Hence, the theory of transfusion induced cancer recurrence or cancer related death has not been supported by clinical data, at least in patients with colorectal cancer [Citation70].
Non-hemoglobin bound oxygen content alterations
Oxygen delivered to tissues can be administered by oxygen in physical solution in plasma and intracellular fluid, and not only by oxygen bound to hemoglobin. This phenomenon was used clinically by allowing the patient to inhale, high-oxygen-content gases under normobaric or hyperbaric conditions.
Normobaric high-oxygen-content inhalations have been used in experimental animal tumors [Citation73–75] and evaluated in a few clinical trials tested either alone [Citation76,Citation77] or in combination with carbon dioxide (carbogen) when treating head and neck cancer patients with radiotherapy [Citation78,Citation79]. A meta-analysis showed a total effect of these trials in loco-regional failure (OR: 0.73, p = 0.05) [Citation33].
Hyperbaric oxygen was tested in head and neck cancer patients using hyperbaric oxygen chambers during radiotherapy [Citation80–89]. The meta-analysis showed a significant benefit in loco-regional control of hyperbaric oxygen treatment (LRF; OR 0.46, p < 0.001 and OS; OR 0.73, p = 0.09). The treatment is, today, not used in the clinical setting, mainly due to its complex nature [Citation33]. However, the use of hyperbaric oxygen serves as proof of principle, that the diffusion limited hypoxia can be overcome and result in improved tumor control as shown in the Sealy trial [Citation66] where transfusion and hyperbaric oxygen treatment was combined.
Artificial blood substitutes or perflurochemical emulsions are small particles capable of carrying more oxygen than hemoglobin [Citation90]. In animal studies these agents have been shown to decrease the proportion of hypoxic cells in solid tumors and enhance the effectiveness of radiotherapy, but most effectively when the simultaneous inspired oxygen levels were close to 100% [Citation19,Citation90–94].
In severely anemic patients the perflurochemical agent, Fluosol-DA, was able to carry a small amount of oxygen when the patients was breathing normal air (functioning primarily as volume expander), but when breathing pure oxygen the oxygen carrying capacity was increased dramatically and oxygen delivery was increased [Citation95]. In head and neck cancers a phase I/II study using fluosol-DA in combination with inspiration of 100% oxygen before and during treatment showed favorable one-year survival rates without serious complications [Citation96,Citation97]. However, to our knowledge, no further clinical trials have been published and the compounds are not used in the clinic today.
Erythropoietin stimulation agents
Erythropoietin is a hormone secreted from the kidneys in response to tissue hypoxia and a low serum level is often seen in anemic cancer patients [Citation26,Citation27]. Because of the risk of the immunosuppression associated with transfusions, as well as the risk of acute and chronic transfusion reactions, the use of recombinant human erythropoietin (rh-EPO) was introduced as a good alternative to blood transfusions [Citation98]. In the treatment of cancer patients, three preparations are used; epoetin alpha, epoetin beta and darbepoetin alpha; they all have similar efficacy and will be considered together and referred to as erythropoietin stimulating agents (ESAs) [Citation99].
Animal studies have shown that anemia can be corrected by injection with ESAs and in addition to the correction of anemia; consequently improve tumor radiosensitivity [Citation100–103]. In clinical phase I/II trials serial treatment with recombinant human erythropoietin demonstrated a significant increase in hemoglobin level in the erythropoietin treated groups compared with controls [Citation104–107]. Patients in the DAHANCA 10 trial [Citation108,Citation109] show a similar effect with a continuous increase in hemoglobin level during treatment with radiotherapy in the patients treated with ESA (). The critical question is whether a continuous increase in hemoglobin level during treatment will improve the therapeutic outcome. A number of clinical trials have addressed the question [Citation108–116] and the topic has been reviewed [Citation99,Citation117–121].
In patients with HNSCC treated with radiotherapy the role of ESA's have been investigated in four clinical trials; the DAHANCA 10 trial [Citation108,Citation109], the RTOG 99 - 03 trial [Citation116], the Enhance trial [Citation115,Citation122] and the EPO-GBR-7 trial [Citation114], all together including a total of 1306 patients. The Enhance trial was published in 2003 and revealed that patients responded well to ESA treatment with an increase in hemoglobin level as expected, but that local tumor control and overall survival was decreased in the group of patients receiving ESA compared to the control group [Citation115]. The RTOG 99 - 03 trial also showed an increase in hemoglobin, but no significant difference in outcomes was detected between the treatment groups. Hoskin et al. published the EPO-GBR-7 trial in 2009 and the results neither positively nor negatively affected survival, tumor outcome, anemia or fatigue in patients [Citation114]. The DAHANCA 10 trial was stopped after an interim analysis, and the outcome showed significantly reduced loco-regional control (RR 1.66, 95% CI 1.16–2.37, p = 0.004) and decreased survival (RR 1.45, 95% CI 1.02–2.06, p = 0.04) in the ESA treatment group [Citation108,Citation109]. A Cochrane meta-analysis of the head and neck cancer trials show that the ESA treated patients did significantly worse in overall survival [Citation121].
Clinical trials in non-small-cell lung cancer [Citation110] and cervical cancer [Citation111] using radiation have shown the same trend as the HNSCC trials, and both studies were closed prematurely due to potential concerns for patient safety. A review examining the mortality in all ESA treated cancer patients including 53 trials and 13 933 patients showed worsened overall survival [Citation119].
The answer to why treatment with ESA worsens the prognosis for cancer patients has been discussed since the publication of the first unexpected results. The thought of a functional erythropoietin receptor on cancer cells was raised and a decreased locoregional progression-free survival in patients with unresected tumors and above median levels of the EPO-receptor has been shown [Citation123], although the initial antibody used to detect the EPO-receptor in the ENHANCE trial [Citation122] was unspecific and also detected heat shock protein 70 (HSP70) [Citation124–126].
It has been postulated that the hemoglobin level reached in the DAHANCA, ENHANCE, the RTOG 99 - 03 and the EPO-GBR-7 trial has been set too high compared to the optimal hemoglobin level for oxygenation of tumors [Citation127,Citation128], and that this may induce tumor hypoxia by decreased microcirculation. However, preclinical data supporting such relationship between hemoglobin level and oxygenation of tumors has not been substantiated in clinical studies.
Another possible explanation to the decreased survival in the ESA treated patients may be an increased risk of thromboembolic events. The GOG trial in cervical cancer was stopped prematurely due to potential concerns for thromboembolic events in the ESA treatment arm [Citation111]. Risk factors using erythropoietic proteins show an approximately 1.6-fold increase in thromboembolic events; however this might be related to factors including target hemoglobin level and the rate by which hemoglobin was raised [Citation127]. Papers outlying the complex mechanisms between coagulation pathways, angiogenesis, tumor progression, inflammatory response, erythropoiesis and ESA effects have been published recently, and show that much research is needed to clarify the understanding of these complicated interactions [Citation99,Citation129,Citation130].
In March 2007 the FDA issued a black box warning about the potential tumor promoting effects and risk of thromboembolic events when using ESA's in cancer patients [Citation128]. The European Medicines Agency also took action on trial results and in June 2008 they stated that “transfusion should be the preferred method of correcting anaemia in patients suffering cancer and that use of epoetins should be based on an informed assessment of the benefits against the risks on individual basis, taking into account the type and stage of tumor, the degree of anaemia, the patient's life-expectancy, the environment in which the patient is being treated and patient preference” [Citation131].
Smoking
Hemoglobin alone cannot reflect the true oxygen status of the tumor, partly due to variations in tumor blood flow and because the oxygen unloading capacity of the blood may widely differ, e.g. due to smoking [Citation5,Citation58]. The physiological effect of smoking on oxygenation of tumors may constitute a problem in radiotherapy. In smokers, the delivery of oxygen to tissues is influenced by the formation of carboxyhemoglobin (COHb), the binding of carbon monoxide (CO) to hemoglobin. Carboxyhemoglobin levels in non-smokers are usually 1–2%, whereas smokers can have values as high as 15–20%, but in general about 4–6%. Hemoglobin's affinity for CO is 200–280 its affinity for O2. The affinity for low concentrations of CO can lead to a significant amount of carboxyhemoglobin, especially since CO is also released very slowly with a biological half life of 4–5 hours [Citation132]. The formation of carboxyhemoglobin causes a left shift of the hemoglobin-oxygen dissociation curve (). This will cause a lower pO2 level and less oxygen released to the tissues, when assuming that other factors remain unchanged [Citation19]. Low pO2 values result in a decreased diffusion distance of oxygen and a subsequent raise in the fraction of hypoxic cells in a tumor. In addition to the formation of carboxyhemoglobin, smoking may influence the amount of oxygen carried to the tissues by a vasoconstrictive effect of nicotine [Citation132,Citation133].
Animal studies have shown that at clinically relevant carboxyhemoglobin levels the oxygen available for tumor cells is about 30–40% of that in normal air-breathing mouse [Citation134]. The formation of carboxyhemoglobin was time- and dose-dependent as increasing levels of tumor hypoxia had a negative impact on local tumor control [Citation134–136]. In measurements using an Eppendorf pO2 electrode a clear relationship between carboxyhemoglobin concentration and tumor pO2 was shown [Citation73].
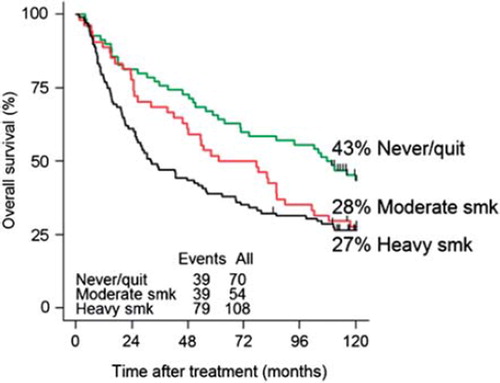
Smoking is a well established etiological factor for the development of several cancer types (e.g. head and neck, lung and bladder) treated with irradiation, and many patients are current or former smokers. The influence of smoking on comorbidity, quality of life, and the development of secondary cancers, and/or smoking related diseases have been well established [Citation137–140]. The influence of smoking on the effect of radiotherapy in HNSCC patients showed that local control, response to treatment and survival among smokers were significantly reduced compared to non-smokers [Citation141–143]. A Danish study of 232 consecutive patients with head and neck cancer treated with radiotherapy was conducted to examine the effect of smoking on oxygenation and effect in outcome. A significant negative impact of smoking when receiving radiation treatment for head and neck cancer was shown in loco-regional control (HR 1.84; CI 1.14–2.96; p = 0.01), disease-specific (HR 1.99; CI 1.13–3.51; p = 0.02) and overall survival (HR 2.01; CI 1.28–3.15; p = 0.002). In loco-regional control the effect could be explained by increasing carboxyhemoglobin concentrations seen in the smoking patients (e.g. a decreased amount of available oxygen to the tumor). The risk of death was found to be increased with each additional pack year of smoking (years of smoking 20 cigarettes per day). The study also showed that several smokers develop secondary cancers over a 10-year period, especially compared to never-smoking patients. The 10-year survival curve comparing non-current-smokers to moderate and heavy smokers is shown in . It is worth noticing that the apparent benefit of moderate smoking seen at five-years disappears with time, and at 10-years moderate and heavy smokers have equal risks of death (p = 0.3), but still significantly different from non-smokers (p = 0.002). These data therefore advocate for smoking cessation in order to improve the therapeutic efficacy of radiotherapy and the development of other smoking-related diseases and/or secondary cancers [Citation144,Citation145].
Figure 6. Ten-year survival curve comparing never smokers and quitters to moderate and heavy smokers (smoking more than 20 cigarettes or 1 pack per day) (smk = smokers).
In uterine cervix cancers a possible correlation between smoking and tumor hypoxia was examined. Pretreatment tumor oxygenation was measured using the Eppendorf electrode and compared to pretreatment smoking status, however, no significant association between smoking and tumor hypoxia was established, nor a connection between smoking and hemoglobin level [Citation146].
One flaw in studies evaluating smoking status may be linked with the reporting of smoking status. In a prospective study, the self-reported smoking status of head and neck cancer patients in a weekly structured interview was compared with measurements of carbon monoxide in the expired air and serum concentration of cotinine. These results revealed that up to 50% of self-reported non-smokers were in fact still actively smoking [Citation147].
Overall, smoking during and after radiotherapy should be avoided in order to improve the therapeutic efficacy of radiotherapy. Although p50 (the position of the oxygen-hemoglobin dissociation curve, ) and COHb may be restored after a 12-hours smoking halt [Citation148], short-term abstinence has never been documented to improve outcome. So, based on the documented benefits, a complete and permanent stop should be advised.
Conclusion and future perspectives
Hypoxia induced radioresistance has been acknowledged for decades and the inverse correlation between hypoxia and tumor control is well established, as is the benefit of high hemoglobin levels on favorable overall survival. The desired effect of correcting low hemoglobin levels with blood transfusion, blood substitutes or ESAs on local control and survival have unfortunately not been established in the clinical setting. All methods showed promising results in preclinical studies, and the intended raise in hemoglobin values was, as expected, experienced in clinical trials. The clinical effect of interventions with transfusion and ESA on outcome was, however, disappointing and not able to improve the efficacy of radiotherapy in patients, and in some cases even showing detrimental effect and a worse prognosis in the intervention groups.
It must be concluded, therefore, that hypoxia is a well established cause of radioresistance, but modification of this cannot be achieved by correcting low hemoglobin levels by the use of transfusion and/or erythropoietin stimulation agents. Theoretically transfusion may be beneficial when used after sufficient blockage of tumor cell proliferation by irradiation, but needs clinical confirmation. The benefit in hypoxia induced radioresistance must therefore be found by modifying the hypoxia by other means than improving the oxygen carrying capacity of the blood in low hemoglobin patients.
A low hemoglobin value in a cancer patient could by itself indicate a poor general condition of the patient, since hypoxia may be an expression of tumor aggressiveness. Work at clarifying the importance of comorbidity in head and neck cancers is currently being done [Citation149].
Tobacco use is a well established etiological cause of HNSCC and many patients have previously been smoking both prior to, during and after radiotherapy, giving them decreased effect of treatment and risk of other smoking-related diseases and development of secondary primary cancers. In the last decade a new subgroup of patients with head and neck cancers have emerged, these are younger, more fit, non-smokers and HPV positive. They have a far better prognosis than the “traditional” head and neck cancer patient [Citation12]. The focus on smoking in this setting has shown that smoking decreases tumor control and survival, and the decrease in local control could be explained by an increase in tumor hypoxia. However, each additional pack year is of importance for the clinical outcome of radiotherapy [Citation13,Citation144,Citation145] and smokers are encouraged to quit smoking. A profile for evaluating the risk of death in head and neck cancer patients has been suggested including HPV-status, pack years above or below 10 and nodal status [Citation13].
The only oxygen modification that has been shown to increase outcome of radiotherapy in the clinical setting has been the use of oxygen breathing under normobaric or hyperbaric pressure. The main hypoxic modification used clinically today is the use of nitromidazoles which in the meta-analysis [Citation33] was also shown to improve outcome in head and neck cancer patients. However, because of the heterogeneity of hypoxia in tumors, it may only be some of the patients that benefit from the hypoxic modifying treatment. Ongoing work in predicting the patients that may benefit from hypoxic modification shows promising future perspectives for individualized treatment strategies using hypoxic modification. In functional imaging 18F-FMISO PET [Citation150,Citation151] has been shown to be able to detect hypoxia and tumor binding of pimonidazole [Citation152] can identify hypoxia. Another approach to predict hypoxia and the effect of cancer treatment may also be the ongoing work of developing a hypoxic gen-classifier profile for head and neck cancers [Citation153,Citation154]. Such gen-classifier has shown promising results in predicting benefit of hypoxic modification in treatment with nimorazole, but has yet to be implemented in the broader clinical setting [Citation153,Citation154].
Acknowledgements
Supported by CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology, the Danish Council for Strategic Research, The Danish Cancer Society and “Fondet til fremme af dansk radiologi”. No conflicts of interest declared by the author.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26:638–48.
- Dische S. Radiotherapy and anaemia – the clinical experience. Radiother Oncol 1991;20(Suppl 1):35–40.
- Overgaard J. Sensitization of hypoxic tumour cells – clinical experience. Int J Radiat Biol 1989;56:801–11.
- Overgaard J. Hypoxic radiosensitization: Adored and ignored. J Clin Oncol 2007;25:4066–74.
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, . A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5 - 85. Radiother Oncol 1998;46:135–46.
- Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, Bentzen J, . The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy – results from the randomized DAHANCA 5 study. Radiother Oncol 2011;98:28–33.
- Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol 1996;6:10–21.
- Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, . Does transfusion improve the outcome for HNSCC patients treated with radiotherapy? – Results from the randomized DAHANCA 5 and 7 trial. Acta Oncol 2011;50:1006–14.
- Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: Experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys 1984;10: 695–712.
- IARC [Internet]. GLOBOCAN 2008. [updated 2008; cited 2011 Aug 30]. Available from: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno = 900#KEY
- Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: A focus on human papillomavirus. J Dent Res 2007;86:104–14.
- Lassen P. The role of human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol 2010;95:371–80.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, . Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35.
- Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31–9.
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, . Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003;362:933–40.
- DAHANCA [Internet]. Retningslinier for strålebehandling af hoved-hals cancer 2004 inkl IMRT. [updated 2004 September 14; cited 2011 Aug 1]. Available from: http://www.dahanca.dk/get_media_file.php?mediaid = 57
- Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: Results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 2005;6:757–64.
- Grau C, Overgaard J. Significance of hemoglobin concentration for treatment outcome. In: Molls M, Vaupel P, editors. Blood perfusion and microenvironment of human tumors, implications for clinical radiooncology. Berlin: Springer; 1998. p. 101–12.
- Lumb AB. Oxygen. In: Lumb AB, editor. Nunn's applied respiratory physiology. 6th ed. Philadelphia: Elsevier, Butterworth, Heinemann; 2005. p. 166–200.
- Beutler E, Waalen J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006;107:1747–50.
- Hu K, Harrison LB. Impact of anemia in patients with head and neck cancer treated with radiation therapy. Curr Treat Options Oncol 2005;6:31–45.
- Calabrich A, Katz A. Management of anemia in cancer patients. Future Oncol 2011;7:507–17.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Erythropoietin or Darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009; CD007303.
- Harrison L, Shasha D, Shiaova L, White C, Ramdeen B, Portenoy R. Prevalence of anemia in cancer patients undergoing radiation therapy. Semin Oncol 2001;28:54–9.
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001;91: 2214–21.
- Bron D, Meuleman N, Mascaux C. Biological basis of anemia. Semin Oncol 2001;28:1–6.
- Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL. Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med 1990;322:1689–92.
- Hedenus M, Adriansson M, San MJ, Kramer MH, Schipperus MR, Juvonen E, . Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: A randomized, double-blind, placebo-controlled study. Br J Haematol 2003;122:394–403.
- Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol 2001;28:7–14.
- Sobrero A, Puglisi F, Guglielmi A, Belvedere O, Aprile G, Ramello M, . Fatigue: A main component of anemia symptomatology. Semin Oncol 2001;28:15–8.
- Gafter U, Kalechman Y, Orlin JB, Levi J, Sredni B. Anemia of uremia is associated with reduced in vitro cytokine secretion: Immunopotentiating activity of red blood cells. Kidney Int 1994;45:224–31.
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955;9:539–49.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – A systematic review and meta-analysis. Radiother Oncol 2011;100: 22–32.
- Overgaard J. The influence of haemoglobin concentration on the response to radiotherapy. Scand J Clin Lab Invest 1988;48:49–53.
- Hirst DG. Anemia: A problem or an opportunity in radiotherapy? Int J Radiat Oncol Biol Phys 1986;12:2009–17.
- Evans JC, Bergsjo P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology 1965;84:709–17.
- Pedersen D, Sogaard H, Overgaard J, Bentzen SM. Prognostic value of pretreatment factors in patients with locally advanced carcinoma of the uterine cervix treated by radiotherapy alone. Acta Oncol 1995;34:787–95.
- Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, . The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999;86:1528–36.
- Thomas G. The effect of hemoglobin level on radiotherapy outcomes: The Canadian experience. Semin Oncol 2001;28:60–5.
- Overgaard J, Bentzen SM, Kolstad P, Kjoerstad K, Davy M, Bertelsen K, . Misonidazole combined with radiotherapy in the treatment of carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1989;16:1069–72.
- Zhao KL, Liu G, Jiang GL, Wang Y, Zhong LJ, Wang Y, . Association of haemoglobin level with morbidity and mortality of patients with locally advanced oesophageal carcinoma undergoing radiotherapy – a secondary analysis of three consecutive clinical phase III trials. Clin Oncol (R Coll Radiol ) 2006;18:621–7.
- Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer 1968;22:258–73.
- Overgaard J, Hansen HS, Jorgensen K, Hjelm HM. Primary radiotherapy of larynx and pharynx carcinoma – an analysis of some factors influencing local control and survival. Int J Radiat Oncol Biol Phys 1986;12:515–21.
- van Acht MJ, Hermans J, Boks DE, Leer JW. The prognostic value of hemoglobin and a decrease in hemoglobin during radiotherapy in laryngeal carcinoma. Radiother Oncol 1992;23:229–35.
- Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, . Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: A secondary analysis of RTOG 85 - 27. Int J Radiat Oncol Biol Phys 1998;42: 1069–75.
- Vaupel P, Mayer A, Hockel M. Impact of hemoglobin levels on tumor oxygenation: The higher, the better? Strahlenther Onkol 2006;182:63–71.
- Tarnawski R, Skladowski K, Maciejewski B. Prognostic value of hemoglobin concentration in radiotherapy for cancer of supraglottic larynx. Int J Radiat Oncol Biol Phys 1997;38:1007–11.
- Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: A review. Radiother Oncol 2000;57:13–9.
- Vaupel P, Thews O, Mayer A, Hockel S, Hockel M. Oxygenation status of gynecologic tumors: What is the optimal hemoglobin level? Strahlenther Onkol 2002;178:727–31.
- Vaupel P. Hypoxia and aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist 2008;13(Suppl 3):21–6.
- Clavo B, Perez JL, Lopez L, Suarez G, Lloret M, Morera J, . Influence of haemoglobin concentration and peripheral muscle pO2 on tumour oxygenation in advanced head and neck tumours. Radiother Oncol 2003;66:71–4.
- Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: Changes during radiotherapy and impact on treatment outcome. Radiother Oncol 1999;53:113–7.
- Stadler P, Becker A, Feldmann HJ, Hansgen G, Dunst J, Wurschmidt F, . Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 1999;44:749–54.
- Becker A, Stadler P, Lavey RS, Hansgen G, Kuhnt T, Lautenschlager C, . Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2000;46:459–66.
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, . Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005;77:18–24.
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol 2004;43:396–403.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res 1989;49:6449–65.
- Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 2001;28:29–35.
- Horsman MR. Measurement of tumor oxygenation. Int J Radiat Oncol Biol Phys 1998;42:701–4.
- Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P. Acute versus chronic hypoxia: Why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys 2011; 80:965–8.
- Hirst DG, Wood PJ. The influence of haemoglobin affinity for oxygen on tumour radiosensitivity. Br J Cancer 1987; 55:487–91.
- Hirst DG, Wood PJ. The adaptive response of mouse tumours to anaemia and retransfusion. Int J Radiat Biol Relat Stud Phys Chem Med 1987;51:597–609.
- Hirst DG, Hirst VK, Joiner B, Prise V, Shaffi KM. Changes in tumour morphology with alterations in oxygen availability: Further evidence for oxygen as a limiting substrate. Br J Cancer 1991;64:54–8.
- Bush RS, Jenkin RD, Allt WE, Beale FA, Bean H, Dembo AJ, . Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl 1978; 3:302–6.
- Kapp KS, Poschauko J, Geyer E, Berghold A, Oechs AC, Petru E, . Evaluation of the effect of routine packed red blood cell transfusion in anemic cervix cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:58–66.
- Sealy R, Jacobs P, Wood L, Levin W, Barry L, Boniaszczuk J, . The treatment of tumors by the induction of anemia and irradiation in hyperbaric oxygen. Cancer 1989;64:646–52.
- Blajchman MA, Bordin JO. The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest 1995;24:311–7.
- Bentzen SM, Balslev I, Pedersen M, Teglbjaerg PS, Hanberg-Sorensen F, Bone J, . Blood transfusion and prognosis in Dukes’ B and C colorectal cancer. Eur J Cancer 1990;26:457–63.
- Mortensen FV, Jensen LS, Sorensen HT, Pedersen L. Cause-specific mortality associated with leukoreduced, buffy coat-depleted, or no blood transfusion after elective surgery for colorectal cancer: A posttrial 15-year follow-up study. Transfusion 2011;51:259–63.
- Blajchman MA. Immunomodulation and blood transfusion. Am J Ther 2002;9:389–95.
- Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet 1996;348:841–5.
- Jensen LS, Puho E, Pedersen L, Mortensen FV, Sorensen HT. Long-term survival after colorectal surgery associated with buffy-coat-poor and leucocyte-depleted blood transfusion: A follow-up study. Lancet 2005;365: 681–2.
- Horsman MR, Khalil AA, Nordsmark M, Grau C, Overgaard J. Relationship between radiobiological hypoxia and direct estimates of tumour oxygenation in a mouse tumour model. Radiother Oncol 1993;28:69–71.
- Horsman MR, Khalil AA, Siemann DW, Grau C, Hill SA, Lynch EM, . Relationship between radiobiological hypoxia in tumors and electrode measurements of tumor oxygenation. Int J Radiat Oncol Biol Phys 1994;29: 439–42.
- Horsman MR, Khalil AA, Nordsmark M, Grau C, Overgaard J. Measurement of pO2 in a murine tumour and its correlation with hypoxic fraction. Adv Exp Med Biol 1994;345:493–500.
- Evans JC, Sanfilippo LJ. Oxygen tension of oral cavity carcinoma. Radiol Clin Biol 1970;39:54–8.
- Evans JC, Cavanaugh PJ. Clinical trial of atmospheric oxygen breathing during radiotherapy for cancer of the oropharynx. Radiol Clin (Basel) 1975;44:210–3.
- Rubin P, Hanley J, Keys HM, Marcial V, Brady L. Carbogen breathing during radiation therapy – the Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys 1979;5:1963–70.
- Mendenhall WM, Morris CG, Amdur RJ, Mendenhall NP, Siemann DW. Radiotherapy alone or combined with carbogen breathing for squamous cell carcinoma of the head and neck: A prospective, randomized trial. Cancer 2005; 104:332–7.
- Van den Brenk HA. Hyperbaric oxygen in radiation therapy. An investigation of dose-effect relationships in tumor response and tissue damage. Am J Roentgenol Radium Ther Nucl Med 1968;102:8–26.
- Tobin DA, Vermund H. A randomized study of hyperbaric oxygen as an adjunct to regularly fractionated radiation therapy for clinical treatment of advanced neoplastic disease. Am J Roentgenol Radium Ther Nucl Med 1971; 111:613–21.
- Chang CH, Conley JJ, Herbert C, Jr. Radiotherapy of advanced carcinoma of the oropharyngeal region under hyperbaric oxygenation. An interim report. Am J Roentgenol Radium Ther Nucl Med 1973;117:509–16.
- Berry GH, Dixon B, Ward AJ. The Leeds results for radiotherapy in HBO for carcinoma of the head and neck. Clin Radiol 1979;30:591–2.
- Sause WT, Plenk HP. Radiation therapy of head and neck tumors: A randomized study of treatment in air vs. treatment in hyperbaric oxygen. Int J Radiat Oncol Biol Phys 1979;5:1833–6.
- Sealy R, Cridland S, Barry L, Norris R. Irradiation with misonidazole and hyperbaric oxygen: Final report on a randomized trial in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 1986;12:1343–6.
- Haffty BG, Hurley R, Peters LJ. Radiation therapy with hyperbaric oxygen at 4 atmospheres pressure in the management of squamous cell carcinoma of the head and neck: Results of a randomized clinical trial. Cancer J Sci Am 1999;5:341–7.
- Henk JM. Late results of a trial of hyperbaric oxygen and radiotherapy in head and neck cancer: A rationale for hypoxic cell sensitizers? Int J Radiat Oncol Biol Phys 1986;12:1339–41.
- Henk JM, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet 1977;2:104–5.
- Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet 1977;2:101–3.
- Rockwell S. Use of a perfluorochemical emulsion to improve oxygenation in a solid tumor. Int J Radiat Oncol Biol Phys 1985;11:97–103.
- Teicher BA, Rose CM. Perfluorochemical emulsions can increase tumor radiosensitivity. Science 1984;223:934–6.
- Teicher BA, Rose CM. Oxygen-carrying perfluorochemical emulsion as an adjuvant to radiation therapy in mice. Cancer Res 1984;44:4285–8.
- Teicher BA, Herman TS, Hopkins RE, Menon K. Effect of oxygen level on the enhancement of tumor response to radiation by perfluorochemical emulsions or a bovine hemoglobin preparation. Int J Radiat Oncol Biol Phys 1991;21:969–74.
- Rockwell S. Perfluorochemical emulsions and radiation therapy. Artif Cells Blood Substit Immobil Biotechnol 1994;22:1097–108.
- Tremper KK, Friedman AE, Levine EM, Lapin R, Camarillo D. The preoperative treatment of severely anemic patients with a perfluorochemical oxygen-transport fluid, Fluosol-DA. N Engl J Med 1982;307:277–83.
- Rose C, Lustig R, McIntosh N, Teicher B. A clinical trial of Fluosol DA 20% in advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1986;12:1325–7.
- Lustig R, McIntosh-Lowe N, Rose C, Haas J, Krasnow S, Spaulding M, . Phase I/II study of Fluosol-DA and 100% oxygen as an adjuvant to radiation in the treatment of advanced squamous cell tumors of the head and neck. Int J Radiat Oncol Biol Phys 1989;16:1587–93.
- Dunst J. The use of epoetin alfa to increase and maintain hemoglobin levels during radiotherapy. Semin Oncol 2001;28:42–8.
- Glaspy JA. Erythropoietin in cancer patients. Annu Rev Med 2009;60:181–92.
- Joiner B, Hirst VK, McKeown SR, McAleer JJ, Hirst DG. The effect of recombinant human erythropoietin treatment on tumour radiosensitivity and cancer-associated anaemia in the mouse. Br J Cancer 1993;68:720–6.
- Kelleher DK, Matthiensen U, Thews O, Vaupel P. Tumor oxygenation in anemic rats: Effects of erythropoietin treatment versus red blood cell transfusion. Acta Oncol 1995; 34:379–84.
- Thews O, Koenig R, Kelleher DK, Kutzner J, Vaupel P. Enhanced radiosensitivity in experimental tumours following erythropoietin treatment of chemotherapy-induced anaemia. Br J Cancer 1998;78:752–6.
- Stuben G, Pottgen C, Knuhmann K, Schmidt K, Stuschke M, Thews O, . Erythropoietin restores the anemia-induced reduction in radiosensitivity of experimental human tumors in nude mice. Int J Radiat Oncol Biol Phys 2003;55:1358–62.
- Lavey RS. Clinical trial experience using erythropoietin during radiation therapy. Strahlenther Onkol 1998; 174(Suppl 4):24–30.
- Dusenbery KE, McGuire WA, Holt PJ, Carson LF, Fowler JM, Twiggs LB, . Erythropoietin increases hemoglobin during radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys 1994;29:1079–84.
- Vijayakumar S, Roach M, III, Wara W, Chan SK, Ewing C, Rubin S, . Effect of subcutaneous recombinant human erythropoietin in cancer patients receiving radiotherapy: Preliminary results of a randomized, open-labeled, phase II trial. Int J Radiat Oncol Biol Phys 1993;26: 721–9.
- Lavey RS, Dempsey WH. Erythropoietin increases hemoglobin in cancer patients during radiation therapy. Int J Radiat Oncol Biol Phys 1993;27:1147–52.
- Overgaard J, Hoff CM, Hansen HS, Specht L, Overgaard M, Grau C, . Randomized study of darbepoetin alfa as modifier of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): Final outcome of the DAHANCA 10 trial. J Clin Oncol 2009;27(Suppl 15s):abstr 6007.
- Overgaard J, Hoff CM, Hansen HS, Specht L, Overgaard M, Grau C, . Randomized study of Aranesp as modifier of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): Final outcome of the DAHANCA 10 trial. Radiother Oncol 2010; 96(Suppl 1):197–8.
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, . Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007;25: 1027–32.
- Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, . Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008;108:317–25.
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, . Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 2005;23: 5960–72.
- Glaser CM, Millesi W, Kornek GV, Lang S, Schull B, Watzinger F, . Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys 2001;50:705–15.
- Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol 2009;27:5751–6.
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, . Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 2003;362:1255–60.
- Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, . Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: A randomized trial of the Radiation Therapy Oncology Group (RTOG99 - 03). Int J Radiat Oncol Biol Phys 2007;69:1008–17.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Erythropoietin or Darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009; CD007303.
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, . Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2006;3: CD003407.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 2009;373: 1532–42.
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, . Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98: 708–14.
- Lambin P, Ramaekers BL, van Mastrigt GA, Van den Ende P, de JJ, De Ruysscher DK, . Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer. Cochrane Database Syst Rev 2009; CD006158.
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, . Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol 2006;24:4708–13.
- Miller CP, Lowe KA, Valliant-Saunders K, Kaiser JF, Mattern D, Urban N, . Evaluating erythropoietin-associated tumor progression using archival tissues from a phase III clinical trial. Stem Cells 2009;27:2353–61.
- Agarwal N, Gordeuk VR, Prchal JT. Are erythropoietin receptors expressed in tumors? Facts and fiction – more careful studies are needed. J Clin Oncol 2007;25:1813–4.
- Jelkmann W, Laugsch M. Problems in identifying functional erythropoietin receptors in cancer tissue. J Clin Oncol 2007;25:1627–8.
- Della RF, Cucciolla V, Borriello A, Oliva A, Perrotta S. Erythropoietin receptors on cancer cells: A still open question. J Clin Oncol 2007;25:1812–3.
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, . EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007;43:258–70.
- Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med 2007;356: 2445–8.
- Barbera L, Thomas G. Erythropoiesis stimulating agents, thrombosis and cancer. Radiother Oncol 2010;95:269–76.
- Spivak JL, Gascon P, Ludwig H. Anemia management in oncology and hematology. Oncologist 2009;14(Suppl 1): 43–56.
- The European Medicines Agency [Internet]. Press Release EMEA recommends a new warning for epoetins for their use in cancer patients. [updated 2008 June 26; cited 2011 Aug 3]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015069.pdf
- Siemann DW, Hill RP, Bush RS. Smoking: The influence of carboxyhemoglobin (HbCO) on tumor oxygenation and response to radiation. Int J Radiat Oncol Biol Phys 1978; 4:657–62.
- Overgaard J, Nielsen JE, Grau C. Effect of carboxyhemoglobin on tumor oxygen unloading capacity in patients with squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1992;22:407–10.
- Grau C, Khalil AA, Nordsmark M, Horsman MR, Overgaard J. The relationship between carbon monoxide breathing, tumour oxygenation and local tumour control in the C3H mammary carcinoma in vivo. Br J Cancer 1994; 69:50–7.
- Grau C, Nordsmark M, Khalil AA, Horsman MR, Overgaard J. Effect of carbon monoxide breathing on hypoxia and radiation response in the SCCVII tumor in vivo. Int J Radiat Oncol Biol Phys 1994;29:449–54.
- Grau C, Horsman MR, Overgaard J. Influence of carboxyhemoglobin level on tumor growth, blood flow, and radiation response in an experimental model. Int J Radiat Oncol Biol Phys 1992;22:421–4.
- Garces YI, Schroeder DR, Nirelli LM, Croghan GA, Croghan IT, Foote RL, . Second primary tumors following tobacco dependence treatments among head and neck cancer patients. Am J Clin Oncol 2007;30:531–9.
- Jensen K, Jensen AB, Grau C. Smoking has a negative impact upon health related quality of life after treatment for head and neck cancer. Oral Oncol 2007;43:187–92.
- Ford MB, Sigurdson AJ, Petrulis ES, Ng CS, Kemp B, Cooksley C, . Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer 2003;98:1457–64.
- van Leeuwen FE, Klokman WJ, Stovall M, Hagenbeek A, van den Belt-Dusebout AW, Noyon R, . Roles of radiotherapy and smoking in lung cancer following Hodgkin's disease. J Natl Cancer Inst 1995;87:1530–7.
- Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, . Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 1993;328:159–63.
- Chen AM, Chen LM, Vaughan A, Farwell DG, Luu Q, Purdy JA, . Head and neck cancer among lifelong never-smokers and ever-smokers: Matched-pair analysis of outcomes after radiation therapy. Am J Clin Oncol 2011; 34:270–5.
- Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, . Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys 2011; 79:414–9.
- Hoff CM, Grau C, Overgaard J. Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma – a prospective study. Radiother Oncol 2011 (in press).
- Hoff CM, Grau C, Overgaard J. A prospective study evaluating the influence of smoking on effective hemoglobin level and outcome in patients with squamous sell carcinoma of the head and neck. Eur J Cancer 2011;47(Suppl 1):544.
- Fyles A, Voduc D, Syed A, Milosevic M, Pintilie M, Hill R. The effect of smoking on tumour oxygenation and treatment outcome in cervical cancer. Clin Oncol (R Coll Radiol) 2002;14:442–6.
- Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status – a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncol 2003;42:154–9.
- Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg 1986;65:1186–8.
- Boeje CR, Dalton SO, Kristensen CA, Andersen E, Johansen J, Andersen LJ, . Comorbidity among 13651 head and neck cancer patients from the DAHANCA-database. Radiother Oncol 2011;98(Suppl 1):3.
- Mortensen LS, Buus S, Nordsmark M, Bentzen L, Munk OL, Keiding S, . Identifying hypoxia in human tumors: A correlation study between 18F-FMISO PET and the Eppendorf oxygen-sensitive electrode. Acta Oncol 2010; 49:934–40.
- Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, . Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006;24: 2098–104.
- Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, . Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 2002;62:7066–74.
- Toustrup K, Sorensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, . Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 2011;71:5923–31.
- Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012;102:122–9.
