Abstract
Background. The histopathological classification and staging system for uterine sarcoma (US) were revised in 2003 and 2009, respectively. However, there is currently no consensus on the significance of various prognostic factors. Therefore the available clinicopathological data on US are summarized in this review. Methods. Articles on uterine sarcoma published in English from 1970 to 2011 were identified systematically by computer-based searches in Medline and the Cochrane Library. Results. Prognosis of US is poor, with a five-year survival rate as low as 30%. The most common histological types are leiomyosarcoma (LMS, 63%), endometrial stromal sarcoma (ESS, 21%), adenosarcoma (6%), undifferentiated sarcoma (5%) and other types (5%). Carcinosarcoma is a mixed tumor, which is today regarded as a subset of endometrial carcinoma. Disease stage is the most important prognostic factor for all types of US. However, the prognosis of stage I LMS is also significantly related to tumor size and mitotic index (MI), and stage I ESS is related to MI and tumor cell necrosis (TCN). In adenosarcoma, TCN is the only significant histopathological prognostic factor. Information on the use of preoperative imaging for staging purposes is lacking. Total hysterectomy is the cornerstone of US treatment. The ovary can be preserved in premenopausal women with early-stage LMS and ESS, and routine lymphadenectomy is not necessary unless enlarged lymph nodes are present. As tumor-free resection margins at primary surgery are the most important prognostic factor for survival, sarcoma surgery should be centralized. Adjuvant treatment has changed from radiation therapy to chemotherapy over the last decades, without any change in survival. Conclusion. There are differences in survival between histological types of US. LMS and ESS can be divided into different prognostic groups and should be treated separately.
Uterine sarcomas are rare neoplasms of mesenchymal origin. According to the World Health Organization (WHO) 2003 classification, they consist of two main groups: mesenchymal tumors and mixed epithelial and mesenchymal tumors [Citation1]. The pure mesenchymal tumors can be further classified into endometrial stromal sarcoma (ESS), leiomyosarcoma (LMS) – including the epithelioid and myxoid variants – and undifferentiated endometrial/uterine sarcoma (UUS) according to the cell of origin.
Mixed tumors include carcinosarcoma and adenosarcoma, and are composed of a mixture of epithelial and mesenchymal components. Carcinosarcoma, along with the malignant Müllerian mixed tumor, malignant mesodermal mixed tumor and metaplastic carcinoma, is still classified as a mixed tumor. However, today it is regarded as a subset of endometrial carcinoma, and as such should be excluded from studies of uterine sarcoma [Citation2]. Despite this, carcinosarcoma is still included in most retrospective studies of uterine sarcoma, as well as in the WHO 2003 classification [Citation1,Citation3].
The purpose of the present article was to review the available clinicopathological and treatment data on uterine sarcoma. Earlier reviews on this subject have been performed by d’Angelo and Prat [Citation3] and Toro et al. [Citation4].
Methods
The scientific literature published from 1970 to 2011 on uterine sarcomas was identified systematically by computer-based searches in Medline and a supplementary search in the Cochrane Library. Only studies published in English were included. The resulting studies went through a three-phase examination/elimination process. In phase one article keywords, titles, and abstracts were examined; articles which had the word “uterine sarcoma” in the title or abstract went to phase two. Phase two involved the examination of full-text articles. Phase three consisted of a critical examination of the selected studies and classified their scientific quality and validity of the results, by type of study, as high-, moderate- and low-grade. Only studies classified as high-grade were included in the present review [Citation5–10].
Incidence and etiology
Uterine sarcoma accounts for 3–5% of all corpus uteri malignancies [Citation4,Citation11,Citation12]. Population-based estimates of uterine sarcoma incidence vary between 1.55 and 1.95 per 100,000 women per year [Citation4,Citation11,Citation12]. In the US, about 2400 new cases of uterine sarcoma were reported in 2003, representing less than 10% of all new diagnoses of uterine cancer in the country, and 7% of all reported soft tissue sarcomas [Citation4]. The incidence of uterine sarcoma in black women in the US is twice as high as that in white women [Citation4].
Nordal and Thoresen [Citation12] reported on the incidence and distribution of uterine sarcomas in Norway between 1956 and 1992 using the 1994 WHO classification. LMS was the most frequent histological type, being diagnosed in 41% of study women, followed by carcinosarcoma in 35%, and ESS in 16%. Recently, Abeler et al. [Citation11] published a new population-based study, including a histopathological review of all uterine sarcomas registered in Norway from 1970 to 2000 using the 2003 WHO classification. After carcinosarcoma was excluded, the following relative frequency of the different histological types was: LMS 63%, ESS 21%, UUS 6%, adenosarcoma 6% and other types 5%.
The development of the basic understanding of uterine sarcoma has been slow. The majority of cases are felt to be sporadic, with no specific etiology, and most have complex karyotypes [Citation13]. However, specific chromosomal translocations have been identified in an increasing number of uterine sarcomas, resulting in fusion genes that are constitutive and involve activation of transcription factors. ESS has specific somatic mutations that have been discovered by cytogenic-, fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR) analyses. In the database of chromosome aberrations in cancer by Mitelman [Citation14] the cytogenetic features t(7;17) (P15;q11), t(6;7) (P21, P15) and t(6,10) (P21;P11), and molecular genetic features: (JAZF1/ SUZ 12), (JAZ F1/PH F1) and (EPC1/PH F1 fusion gene) of ESS [Citation15] are recorded. LMS also has karyotypic aberrations, but without any tumor-specific feature [Citation13,Citation14].
Genetic factors have been suggested to play a role, as incidence is twice as high among black women compared to white women [Citation4]. This race-specific incidence pattern is reversed in endometrial carcinoma, in which the incidence among white women is three times higher than that among black women [Citation4]. Brooks et al. [Citation16] showed that differences in the incidence of uterine sarcoma between blacks and whites were limited to LMS. In contrast, Silverberg [Citation17] found no racial difference in patients with LMS. Brooks et al. [Citation16] also showed that 54% of whites and 45% of blacks presented with early-stage disease (p = 0.001). Overall 50% of whites underwent surgery, and 21% underwent radiation therapy in addition to surgery, compared with 53% and 18%, respectively, of blacks. They also found that the five-year relative survival of white women was significantly better than that of black women (53% vs. 42%, p = 0.001).
Little is known regarding the etiology of uterine sarcoma. Association between prior pelvic irradiation and LMS and carcinosarcoma has been reported, but included only a few patients with a history of pelvic irradiation. Mark et al. [Citation18] reviewed the literature and estimated the risk of postirradiated (median dose of 55 Gy) LMS and ESS to range from 0.003% to 0.8% following a latency period of 3–30 years. LMS and ESS also tend to present at an advanced stage and have an extremely poor prognosis. Oral contraceptives increase the risk of LMS and unopposed estrogen increases the risk of ESS; the latter risk factor is similar for endometrial carcinoma [Citation19]. Obesity, hypertension and diabetes are factors associated with endometrial carcinoma, but are also found among women with carcinosarcoma and ESS. These findings indicate that unopposed estrogen plays a role in the etiology of uterine sarcoma. An excess incidence of LMS and UUS has been reported in Tamoxifen users [Citation20,Citation21], and Thomas [Citation19] found that women who had smoked cigarettes had a reduced risk of LMS and ESS.
Clinical presentation
Uterine sarcoma exhibits the typical clinical features of similar tumors at other sites: a firm, fleshy growth with areas of hemorrhage and necrosis. Postmenopausal or abnormal vaginal bleeding is common. These tumors grow in an exophytic pattern within the endometrial cavity, and bleeding and uterine cramping are thus common as the uterus attempts to expel the globular mass [Citation22]. In both LMS and uterine leiomyoma, uterine enlargement is a universal finding. The frequency of LMS in patients with clinical myomas is less than 1%, but increases with age to more than 1% in the 6th decade of life [Citation22]. Unfortunately, at present there is no non-invasive procedure that can safely differentiate between them. Therefore growing myomas in postmenopausal women who are not taking hormone replacement therapy should be suspected for sarcoma, even though the frequency of LMS in this situation is low [Citation22]. Preoperative curettage was diagnostic in 70% of ESS patients, but only 30% of LMS patients [Citation11,Citation23]. This demonstrates the lack of good preoperative diagnostic criteria for malignancy in uterine sarcoma patients, although vaginal ultrasound and magnetic resonance imaging (MRI) may reveal myometrial involvement [Citation24].
All uterine sarcomas have a propensity for hematogenous dissemination, most often to the lungs. Other sites include the liver, bone and brain. Women with distant metastasis at the time of diagnosis have symptoms and findings that correspond to the location(s) of their disease. The presenting symptoms in the study by Nordal are listed in [Citation23]. The tumor marker CA-125 is found to be elevated in uterine sarcoma, especially in LMS patients with extra-uterine spread, which is similar to findings in uterine adenocarcinoma [Citation25]. Other markers have not been consistently useful. When a diagnosis of uterine sarcoma is known or suspected, pretreatment evaluation should include a thorough patient history and physical examination, as well as curettage. Transvaginal ultrasound is the standard imaging technique, but MRI of the pelvis optimizes image evaluation of invasion into adjacent structures of the pelvis. However, MRI can only differentiate uterine sarcomas from endometrial cancer if combined with findings of irregular tumor margins and marginal nodular lesions, which might not be possible in all cases [Citation24,Citation26]. Evaluation of extra-pelvic spread should be performed by computed tomography (CT) of the chest, abdomen and pelvis [Citation26]. A few studies have shown that positron emission tomography (PET)-CT can be useful to differentiate between LMS and leiomyoma, but this remains to be proven [Citation27,Citation28].
Table I. Presenting symptoms.
Because preoperative histological diagnosis of a uterine tumor suspected to be LMS is difficult to obtain, many gynecologists prefer to operate on women with valid symptoms of LMS [Citation29].
Age distribution and total survival
Patients with ESS were the youngest and those with adenosarcoma were the oldest. The median age was 50.7 years for ESS, 56.6 years for LMS, 58.8 years for UUS and 65.7 years for adenosarcoma. The prognosis was relatively good for ESS, but poor for the other types. The five-year total survival by type of uterine sarcoma for patients with a tumor localized to the uterus was: ESS 84%, LMS 51%, adenosarcoma 76%, UUS 57%, and other types 43% [Citation11] (). Uterine sarcomas accounted for 26% of all deaths from uterine malignancies in the population-based Norwegian survey. One important reason for the low cure rate, even in localized uterine sarcoma, was the frequent occurrence of distant metastases, especially to the lungs [Citation11,Citation12,Citation29,Citation30].
Figure 1. Crude survival related to tumor type.From Abeler et al. [Citation11] with permission.
![Figure 1. Crude survival related to tumor type.From Abeler et al. [Citation11] with permission.](/cms/asset/ce108d74-fe25-4748-bfb6-cd7de4099b12/ionc_a_689111_f0001_b.jpg)
Staging
The previous staging criteria applied to uterine sarcoma was a modified version of the staging for endometrial cancer (FIGO 1988). The new FIGO staging for uterine sarcoma was approved and subsequently published in early 2009 [Citation31,Citation32]. For LMS and ESS, stage I can be subdivided according to tumor size, while myometrial invasion is included in stage I adenosarcoma. Carcinosarcoma continues to be staged in the same manner as endometrial carcinoma (see ) [Citation32]. These new staging classifications will serve as a basis for future improvements that will better incorporate and stratify prognostic variables, as they are identified, with the goal of improving the ability to project survival or predict recurrence.
Table II. Staging for uterine sarcomas (leiomyosarcomas, endometrial stromal sarcomas, adenosarcomas, and carcinosarcomas).
Prognostic factors by tumor type
LMS
LMS is defined as a malignant neoplasm composed of cells with smooth muscle differentiation. These tumors frequently exhibit marked cellular atypia, high mitotic index (MI) and tumor cell necrosis (TCN).
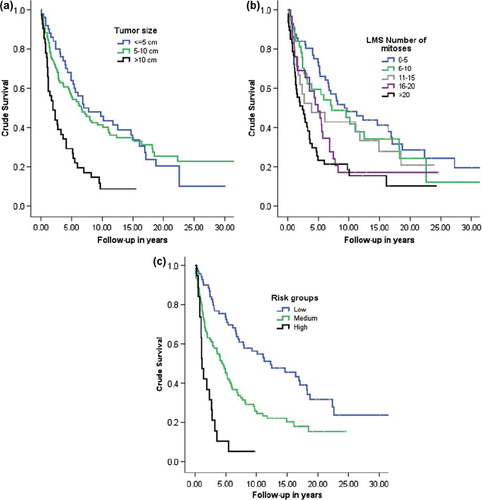
Lack of residual tumor following primary surgery is the main prognostic factor for patients with LMS. Five-year crude survival was 51% for patients with stage I LMS, 25% for those with stage II LMS and 32% for all patients combined. All LMS patients with distant metastasis died within five years [Citation11,Citation29,Citation30,Citation33,Citation34]. Tumor size was the second most important independent prognostic factor for survival. When the tumor diameter was less than 5 cm, the overall survival was 86%, compared to 18% when the tumor diameter was larger than 10 cm [Citation29,Citation35]. MI above 10 mitosis per 10 HpF, in LMS conferred an increase in hazard ratio (HR) of about 2.5-fold. Combining these two risk factors made it possible to further classify patients into three risk groups: a low-risk group (tumor diameter ≤ 10 cm and MI ≤ 10), a medium-risk group (either tumor diameter > 10 cm or MI ≥ 10), and a high-risk group (tumor diameter > 10 cm and MI > 10), which had a 5.3-fold increased risk of death [Citation11]. Grading of LMS is controversial and there is no general grading system [Citation1]. and c show the crude survival for LMS localized to the uterus, by tumor size, number of MI per 10 HpF, and risk group as defined above [Citation11].
ESS
ESS is by definition a hormone-sensitive low-grade tumor with indolent growth, composed of cells resembling those of proliferative phase endometrial stroma [Citation36,Citation37]. There is little cellular atypia, mitosis, or myometrial and vascular space infiltration. TCN may occur in rare cases.
Nordal et al. [Citation23] showed that tumor-free resection margins after primary surgery were the main prognostic factor for ESS. Patients with stage I ESS had a five-year and 10-year crude survival of 84% and 77%. The values were 62% and 49% for those with stage II ESS, and the five-year crude survival of all patients was 69%. After tumor-free resection margins, the most important prognostic factors were grade of malignancy, tumor diameter and menopausal status. It is therefore important that the pathologist take sections from the parametrium to uncover any extra-uterine growth. When the tumor diameter increased from 5 cm to more than 10 cm, the five-year cancer-related survival decreased from 89% to 33% [Citation23,Citation33,Citation37].
ESS was formerly classified into two groups: low-grade ESS with good differentiation (< 10 MI per HpF) and high-grade ESS with poor differentiation (> 10 MI per HpF). In the 2003 WHO classification [Citation1], high-grade ESS is classified as UUS. However, UUS displays much more aggressive behavior than low-grade ESS even for patients in stage I [Citation37–39]. Consequently, low-grade ESS and UUS represent two different clinical tumor types and should be treated as such. In the recent population-based study in Norway by Abeler et al. [Citation11] 83 ESS cases were found in which prognosis was clearly related to MI and TCN. The five- and 10-year crude survival was 88% and 84%, and 57% and 25% for patients with a MI < 5 and > 10, respectively. Patients with no TCN had a highly significantly better five-year crude survival than patients with TCN, 96% versus 69% (p = 0.002). In a multivariate analysis these two factors showed independent prognostic significance (MI, RH = 4.1 and TCN, RH = 3.5) [Citation11]. By combining these two factors, Abeler et al. [Citation11] identified three prognostic groups: a low-risk group with MI ≤ 10 and no TCN, a medium-risk group with either MI > 10 or TCN and a high-risk group with MI > 10 and TCN, which had a 15-fold increased risk of death. Five-year crude survival in ESS by MI, TCN and risk group as described above is shown in and c [Citation11].
Figure 3. Crude survival in ESS. (a) Number of mitoses. (b) Tumor necrosis. (c) ESS risk groups.
From Abeler et al. [Citation11] with permission.
![Figure 3. Crude survival in ESS. (a) Number of mitoses. (b) Tumor necrosis. (c) ESS risk groups.From Abeler et al. [Citation11] with permission.](/cms/asset/7ffcc9dc-a1e4-4ade-96bb-ec528ee24b13/ionc_a_689111_f0003_b.jpg)
UUS
UUS is defined as a high-grade malignant tumor of mesenchymal origin that bears no resemblance to endometrial stroma and shows no evidence of smooth muscle or any other differentiation. These tumors frequently display pleomorphic cells with a high MI [Citation3,Citation11,Citation39].
Patients with stage I UUS had a five-year crude survival of 57%; for all stages combined this figure was 37% (), and all patients with higher than stage I died within five years. Abeler et al. [Citation11] showed that vascular invasion was the only statistically significant factor in the prognosis of UUS, with a five-year crude survival of 83% and 17% in the absence and presence of vascular invasion, respectively (p = 0.02). Localized recurrences and distant metastases were also associated with high mortality [Citation3].
Adenosarcoma
Uterine adenosarcoma accounts for 5.5–9.0% of all uterine sarcomas and is defined as a biphasic neoplasm containing a benign epithelial component and a sarcomatous mesenchymal homologous or heterologous component. In the homologous tumors the non-epithelial component contains only elements normally found in the uterus, as seen in ESS, LMS and sarcomas of non-specific supporting tissues (fibrous vessels, lymphatics). In the heterologous tumors, elements foreign to the uterus, such as bone, cartilage and striated muscle, are also seen [Citation3,Citation11,Citation39].
Sarcomatous overgrowth occurs in 10% of adenosarcomas and is defined as having 25% of the tumor show high-grade differentiation that is severe atypia and many mitoses [Citation40].
Adenosarcoma rarely exhibits an extra-uterine location, but when dissemination occurs it tends to involve the ovaries, pelvic tissues or intestinal serosa [Citation3]. Patients with stage I adenosarcoma had a five- and 10-year crude survival of 76% and 61%, respectively. For all stages combined, the five- and 10-year crude survivals were 72% and 58%, respectively (). Vaginal or pelvic recurrences are estimated to occur in about 25–30% of adenosarcoma cases five years after surgical treatment. Multivariate analysis showed that TCN was the strongest histopathological prognostic factor for adenosarcoma (HR = 5.7, p = 0.014). The five- and 10-year crude survivals were 92% and 72% when TCN was absent, compared to 43% and 29% when present [Citation11]. Patients with adenosarcomas with sarcomatous overgrowth have a prognosis similar to that of women with carcinosarcomas, with more than 50% mortality [Citation41,Citation42].
Molecular biology
Fox [Citation43] reviewed the value of DNA-ploidy in uterine sarcoma and reported contradictory results. Kildal et al. recently examined the prognostic value of DNA-ploidy in 354 uterine sarcomas in Norway between 1970 and 2000, and concluded that DNA-ploidy might be useful as a prognostic factor in patients with LMS and adenosarcoma [Citation44].
Evaluation of p16, Ki-67 and Bcl-2 have been used in LMS, adenosarcoma and UUS to predict outcome [Citation30,Citation45–49]. However, none have shown any prognostic independence.
Nordal et al. [Citation23] studied the prognostic role of p53 protein accumulation (p53) in ESS and LMS using a monoclonal p53 antibody. Nuclear p53 was found in 27% of ESS and in 38% of LMS. A significant correlation was found between p53 and malignancy grade, MI, and DNA-ploidy, but not with FIGO stage. In a multivariate analysis p53 protein expression had no impact on survival of patients with ESS and LMS [Citation50]. p53 protein expression has not been found in adenosarcoma, except for adenosarcoma with sarcomatous overgrowth [Citation39,Citation48,Citation49,Citation51].
Amant et al. [Citation52] studied ErbB-2 (HER-2/neu) gene alterations in LMS, ESS and adenosarcoma. They used the FISH technique, and 10 LMS, 21 ESS, 10 UUS and four adenosarcomas were evaluated. The results showed absence of ErbB-2 overexpression in LMS, ESS and adenosarcoma, whereas the ErbB-2 gene might have a biological role in UUS.
Estrogen and progesterone receptors are expressed in 30–40% of conventional LMS, [Citation49], and are also frequently expressed in ESS [Citation49,Citation53]. Amant et al. reported that in 18 of 20 women with adenosarcoma without sarcomatous overgrowth, the sarcomatous component expressed estrogen and progesterone receptors, whereas loss of estrogen and progesterone receptors is usually seen in adenosarcoma with sarcomatous overgrowth [Citation53].
Treatment
Primary surgery
Patients with preoperative suspected uterine sarcoma should be referred to specialist centers where appropriate surgery can be performed. Surgery correctly performed is imperative and is the most important prognostic factor [Citation54]. The standard treatment for LMS, ESS, UUS, and adenosarcoma is total hysterectomy. In postmenopausal women, SOEB is also recommended [Citation29]. Total hysterectomy is important if uterine sarcoma is suspected, and can be curative if the tumor is confined to the uterus. In the case of a preoperative diagnosis of ESS, radical hysterectomy is also recommended, as this tumor type often involves the parametrium, sometimes only as an intravascular invasion, which is difficult to diagnose preoperatively [Citation29].
Tumor-free resection margins are of utmost importance. It is malpractice to cut through the tumor and it is very important to prevent spillage. If the tumor has broken through the uterine wall to the serosa, all tumors must be removed “en bloc”, without spillage. Laparoscopic removal of known sarcoma by morcellation is not permissible due to the risk of spreading and spillage of tumor cells into the pelvic or abdominal cavity [Citation55]. Additional surgery is important when uterine sarcoma (particularly LMS) is found incidentally after morcellation [Citation56]. About 25% to 75% of patients with early ESS have recurrence in the pelvis and abdomen. These recurrences may be caused by inadvertent tumor morcellation during surgery [Citation57]. Park et al. [Citation58] found that inadvertent tumor morcellation during surgery had adverse effects on the disease-free survival of patients with early ESS. The overall survival of these patients was not affected however, because most patients with recurrent disease were salvaged successfully through additional surgery [Citation58].
Bilateral salpingo-oophorectomy (SOEB) has traditionally been recommended, even in premenopausal women with stage I ESS disease, as ESS is hormone-sensitive, and a much higher recurrence rate was found (50%) among women who kept their ovaries compared to those who did not (4%) [Citation59]. However, recent larger reports indicate that preserving the ovaries may be possible in premenopausal women with stage I ESS if the tumor is radically removed [60–63]. Occult ovarian metastases in women with stage I LMS have been found (range 3.4–3.9%) [Citation61]. However, preservation of ovarian tissues does not increase the risk of recurrence [Citation29] indicating that preservation of the ovaries in premenopausal women may be possible unless these tissues show macroscopic involvement [Citation29]. There is one study showing that SOEB has a negative effect on survival of women with LMS [Citation62].
Resection of lymph nodes is controversial. Some say that it is not necessary in uterine sarcoma surgery unless the lymph nodes are clinically suspicious for metastatic disease [Citation60,Citation63,Citation64], but these lymph node tumors are often diagnosed postoperatively. The incidence of lymph node metastasis in early stages of LMS, ESS and adenosarcoma without sarcomatous overgrowth is very low (0–3.7%, 0–5% and 0–6.5%, respectively [Citation29]). Therefore, lymph node resection is not recommended in early-stage LMS and ESS, but may be done in UUS and adenosarcoma with sarcomatous overgrowth [Citation29]. As lymph node metastasis is most commonly associated with extra-uterine disease, lymphadenectomy should be reserved for patients with clinically suspicious nodes [Citation60,Citation64–66]. Removal of lymph nodes with microscopic disease does not seem to be clinically beneficial [Citation29]. Restaging has been shown to be unnecessary [Citation29,Citation67].
In the study by Abeler et al. [Citation11], a number of cases originally classified as uterine sarcoma, were reclassified during the review, e.g. as cellular leiomyoma, atypical leiomyoma and leiomyomatous tumor of uncertain malignant potential (STUMP). The latter is especially difficult to distinguish from uterine sarcoma, and may be considered by some pathologists as a low-grade LMS. These tumors may be treated more conservatively, by local excision with ample resection margins. For young fertile women who have undergone a myomectomy for low-grade LMS, fertility saving surgery may be acceptable. Conservative surgery must be done only by gynecologic oncologists, and these patients must be followed very carefully.
For patients with inoperable uterine sarcoma, the following options exist: 1) pelvic radiation therapy with or without brachytherapy and chemotherapy; 2) chemotherapy; and 3) hormone therapy (only for ESS).
Surgery at relapse
Preoperative evaluation of the extent of disease is vital in determining the possibility of complete resection. CT of the chest, abdomen and pelvis should be performed in addition to pelvic MRI if the pelvis is implicated [Citation24,Citation26]. However, survival of most patients with recurrent disease is poor. Surgery is the mainstay of treatment for women with localized recurrent disease and complete removal of tumor (residual tumor = 0 cm) can be curative [Citation29,Citation66,Citation67]. In a study from the Mayo Clinic comprising 128 patients with recurrent LMS, secondary cytoreductive surgery prolonged survival in only a select group of patients [Citation68]. Several other studies have also evaluated the feasibility of resection of recurrent LMS [Citation69]. In agreement with the Mayo study, they found a survival benefit only in patients with a disease-free interval of more than six months, with either local or distant recurrence and optimal resection. These factors should be considered when deciding on secondary cytoreductive surgery. Recurrence of ESS can appear long after primary surgery, and is often localized in the lung and/or the pelvis, in which case repeated surgery may be indicated with the intention to prolong survival and cure [Citation29,Citation70]. Women with isolated lung or liver recurrences, regardless of histologic type, can also be good candidates for surgery [Citation29,Citation71–74]. Palliative surgery may be indicated if the patient has bowel obstructions, bleeding or pain. However, patients with wide-spread or bulky unresectable tumors should not go through high-risk debulking surgery [Citation29].
Radiation therapy
The role of radiation therapy in localized disease is controversial. No prospective randomized studies have shown a survival benefit associated with postoperative radiation therapy for patients with uterine sarcoma, and most retrospective studies have shown considerable prognostic imbalances between irradiated and non-irradiated patients. This may be due to the fact that radiation therapy is often given to patients with poor prognosis. Most of these studies also fail to distinguish between the various types of uterine sarcoma in their analyses [Citation3,Citation75]. Despite these limitations, there is some evidence that radiation therapy can be of importance [Citation3,Citation76]. The only published prospective randomized phase III study was performed by the European Organization for Research and Treatment of Cancer, which included surgical stage I and II uterine sarcoma (103 LMS, 91 carcinosarcomas and 28 ESS). Of the 112 subjects in the adjuvant treated cohort (pelvic external beam irradiation), there were 14 relapses (12.5%), versus 24 (21.4%) in the observed group (p = 0.004). This study did not demonstrate a survival benefit for postoperative radiation therapy in LMS and ESS compared to the observed group [Citation77]. Adjuvant treatment has changed from radiation therapy to chemotherapy over the last decades, but this has not been accompanied by any change in survival.
Chemotherapy and hormone therapy
The role of adjuvant chemotherapy is even more poorly defined than radiation therapy for patients with localized disease, but has been considered because of high risk for distant relapse. For stage I and II LMS and UUS with tumor-free resection margins, the following options exist: 1) observation; 2) consider pelvic radiation therapy and/or brachytherapy; and 3) consider chemotherapy [Citation78].
No study so far has been able to demonstrate any benefit related to adjuvant chemotherapy.
Ifosphamid, cisplatin, etoposide, gemcitabine, paclitaxel and doxorubicin have shown modest to minimal response rates in advanced and recurrent LMS: 17%, 3%, 11%, 15%, 9% and 20%, respectively [Citation79]. A combination of hydroxyurea etoposide and dacarbazine has shown a response rate of 18% (response duration 4.1 months survival 9.6 months) without major toxicities [Citation80]. Another combination based on gemcitabine and docetaxel has shown the highest response rate: 53% in 36 unresectable patients [Citation81,Citation82]. Combination chemotherapy yields greater response rates. In 1985 the Gynecologic Oncology Group published phase III studies in recurrent LMS in which combinations of doxorubicin and cyclophosphamide, and doxorubicin and dacarbazine were tested versus single doxorubicin. However, both studies are too small to draw any conclusions [Citation83]. There is currently no evidence to support the use of combination chemotherapy in advanced or recurrent LMS. Other agents under investigation include antiangiogenetic agents such as thalomide, and targeted therapies such as sunitinib [Citation22].
Patients with ESS are often not classified separately in phase II studies of uterine sarcoma, which makes it difficult to identify the specific activity of various agents. The rarity of ESS renders clinical trials difficult. In the end, observation is recommended in radically operated patients with stage I and II ESS. However, as ESS has estrogen and progesterone receptors, hormonal therapy (such as megestrolacetate, MPA, GnRH and aromatase inhibitors) has also been advocated [Citation85], and is recommended for stage III, IV and recurrent ESS [Citation84–86].
UUS is often treated with chemotherapy. The Gynecologic Oncology Group reported a response rate of 33% among 21 patients treated with ifosphamid in a phase II study [Citation87]. Nevertheless, clinical prospective randomized studies are sorely needed. Based on the above results, the following drugs can be recommended as single agents, or in combination: 1) doxorubicin; 2) gemcitabine/docetaxel; 3) single agent dacarbazine, docetaxel, epirubicin, gemcitabine, ifosphamid, liposomal doxorubicin and paclitaxel [Citation78].
Surveillance
The following practice is used at Norwegian Radium Hospital: 1) physical examination every six months for five years (including surveillance of possible hormone therapy); 2) chest x-ray, abdominal/pelvic CT/MRI as clinically indicated, or every six months; 3) annual follow-up over the subsequent five years. If recurrence is suspected and/or detected after routine abdominal CT/MRI, patients should be referred to specialist centers where strict guidelines and protocols are followed [Citation88].
Conclusions and future directions
Uterine sarcoma is a rare, but deadly disease. The prognosis of patients with uterine sarcoma has not changed in the last 20 years, with an overall five-year survival between 17% and 54%. Patients with ESS have a better prognosis than those with other histological types, with a five-year survival of 69%. In multivariate analyses, age, stage, tumor size and parity have been shown to independently influence overall survival. Currently evidence is still lacking about the use of preoperative imaging for staging purposes, and so uterine sarcomas are still surgically staged. Routine lymphadenectomy and SOEB is not necessary, and ovarian tissues can be preserved in stage I premenopausal women unless the ovaries show macroscopic involvement. Primary surgery with tumor-free resection margins, without residual disease and without spillage of tumor cells is the main prognostic factor for the outcome of uterine sarcoma patients. It is of utmost importance that uterine sarcoma surgery be centralized to institutions that have the necessary expertise in radical abdominal sarcoma surgery.
Adjuvant pelvic irradiation can be considered if resection margins in the pelvis are involved. However, introduction of modern radiation therapy and chemotherapy has not influenced the long-term survival of these patients. Uterine sarcoma patients with extra-uterine disease have a poor prognosis and these women should be candidates for new clinical trials.
The uncommon nature of uterine sarcoma must mandate cooperative groups all over the world to join in prospective randomized trials in order to answer the important questions regarding the treatment of the disease. The first priority for the future is to improve our basic understanding of uterine sarcoma, as well as of the different types and treatment modalities. This knowledge is critical in the practice of gynecologic oncology, and we think that the new FIGO classifications will serve as a springboard for future refinements that will incorporate and stratify prognostic variables, with the goal of improving survival and predicting recurrence [Citation89,Citation90]. The future agenda of uterine sarcoma research should focus on the role of therapeutic lymph node resection, the safety of omitting routine SOEB, the prognostic effect of tumor spillage during surgery on survival outcome, improvement of diagnostic modalities which can help to preoperatively differentiate LMS and ESS from leiomyoma, and molecular studies to improve prognostic factors.
Acknowledgements
We thank Ruth Budsberg and Gry Seppola for skillful technical help with the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. Tavassoli FA, Devilee P, editors. Lyon: IARC Press; 2003.
- McCluggage WG. Malignant biphasic uterine tumours: Carcinosarcomas or metaplastic carcinomas? J Clin Pathol 2002;55:321–5.
- D’Angelo E, Prat J. Uterine sarcomas: A review. Gynecol Oncol 2010;116:131–9.
- Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer 2006;119:2922–30.
- SINTEF. Primary treatment of ovarian cancer. SMM report 5/2003. Oslo, Norway: Norwegian Centre for Health Technology Assessment; 2003.
- Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001;323:334–6.
- Liddle J, Williamson M, Irwig L. Method for evaluating research and guideline evidence (MERGE). Sydney, Australia: New South Wales Department of Health; 1996.
- Scottish Intercollegiate Guidelines Network: SIGN 50: A guideline developers’ handbook. Edinburgh, Scotland: SIGN; 2001, 2004.
- Casali P, Licitra L, Tondini C, de Braud F, Bruzzi P, Costa A, . START: A European state-of-the-art on-line instrument for clinical oncologists. Ann Oncol 1999;10:769–73.
- Högberg T, Glimelius B, Nygren P; SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol 2001;40:340–60.
- Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54:355–64.
- Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956–1992: Incidence, survival and mortality. Eur J Cancer 1997;33:907–11.
- Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer 2003;3:685–94.
- Mitelman F, Johansson B, Mertens F,. editors. Mitelman database of chromosome aberrations in cancer 2007.
- Nucci MR, Harburger D, Koontz J, Dal Cin P, Sklar J. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am J Surg Pathol 2007;31:65–70.
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol 2004;93:204–8.
- Silverberg SG. Leiomyosarcoma of the uterus. A clinicopathologic study. Obstet Gynecol 1971;38:613–28.
- Mark RJ, Poen J, Tran LM, Fu YS, Heaps J, Parker RG. Postirradiation sarcoma of the gynecologic tract. A report of 13 cases and a discussion of the risk of radiation-induced gynecologic malignancies. Am J Clin Oncol 1996;19: 59–64.
- Thomas DB. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives: The influence of combined oral contraceptives on risk of neoplasms in developing and developed countries. Contraception 1991;43:695–710.
- Wysowski DK, Honig SF, Beitz J. Uterine sarcoma associated with tamoxifen use. N Engl J Med 2002;346:1832–3.
- Wickerham DL, Fisher B, Wolmark N, Bryant J, Costantino J, Bernstein L, . Association of tamoxifen and uterine sarcoma. J Clin Oncol 2002;20:2758–60.
- Chu CS, Lin LL, Rubin SC. Cancer of the uterine body. DeVita, Hellman, and Rosenberg's cancer: Principles & practice of oncology. 8th ed. DeVita VT, Lawrence TS, Rosenberg SA, editors. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp 1543–63.
- Nordal RR. Uterine sarcomas in Norway 1956–1992: An epidemiological and clinicopathological study. A thesis. Oslo: Faculty of Medicine – University of Oslo; 1998.
- Köhler G, Evert M. Uterine sarkome und mischtumoren. 1st ed. Berlin: De Gruyter; 2009. pp 7–120.
- Patsner B, Mann WJ. Use of serum CA-125 in monitoring patients with uterine sarcoma. A preliminary report. Cancer 1988;62:1355–8.
- Brocker KA, Alt CD, Eichbaum M, Sohn C, Kauczor HU, Hallscheidt P. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT: Part 1. Strahlenther Onkol 2011;187:611–8.
- Chander S, Ergun EL. Positron emission tomographic-computed tomographic imaging of a uterine sarcoma. Clin Nucl Med 2003;28:443–4.
- Umesaki N, Tanaka T, Miyama M, Kawamura N, Ogita S, Kawabe J, . Positron emission tomography with (18)F-fluorodeoxyglucose of uterine sarcoma: A comparison with magnetic resonance imaging and power Doppler imaging. Gynecol Oncol 2001;80:372–7.
- Nam JH. Surgical treatment of uterine sarcoma. Best Pract Res Clin Obstet Gynaecol 2011;25:751–60.
- Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: Experience in Helsinki University Central Hospital 1990–2001. Gynecol Oncol 2008;111:74–81.
- FIGO Committee on gynecologic oncology. FIGO staging for uterine sarcomas. Int J Gynecol Obstet 2009;106:277. Corrigendum to FIGO staging for uterine sarcomas. Int J Gynecol Obstet 2009;104:179.
- Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet 2009;104:177–8.
- Denschlag D, Masoud I, Stanimir G, Gilbert L. Prognostic factors and outcome in women with uterine sarcoma. Eur J Surg Oncol 2007;33:91–5.
- Larson B, Silfverswärd C, Nilsson B, Pettersson F. Prognostic factors in uterine leiomyosarcoma. A clinical and histopathological study of 143 cases. The Radiumhemmet series 1936–1981. Acta Oncol 1990;29:185–91.
- Nordal RN, Kjørstad KE, Stenwig AE, Tropé CG. Leiomyosarcoma (LMS) and endometrial stromal sarcoma (ESS) of the uterus. A survey of patients treated in the Norwegian Radium Hospital 1976–1985. Int J Gynecol Cancer 1993;3: 110–5.
- Evans HL. Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer 1982;50:2170–82.
- Chan JK, Kawar NM, Shin JY, Osann K, Chen LM, Powell CB, . Endometrial stromal sarcoma: A population-based analysis. Br J Cancer 2008;99:1210–5.
- Bodner K, Bodner-Adler B, Obermair A, Windbichler G, Petru E, Mayerhofer S, . Prognostic parameters in endometrial stromal sarcoma: A clinicopathologic study in 31 patients. Gynecol Oncol 2001;81:160–5.
- D’Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol 2009;40:1571–85.
- Clement PB. Müllerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am J Surg Pathol 1989;13:28–38.
- Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Aye LM, Rose GS. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: Comparison of treatment and survival. Gynecol Oncol 2001;83:89–94.
- Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int J Gynecol Pathol 1992;11:75–88.
- Fox H. Ploidy in gynaecological cancers. Histopathology 2005;46:121–9.
- Kildal W, Abeler VM, Kristensen GB, Jenstad M, Thoresen SØ, Danielsen HE. The prognostic value of DNA ploidy in a total population of uterine sarcomas. Ann Oncol 2009;20: 1037–41.
- Atkins KA, Arronte N, Darus CJ, Rice LW. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol 2008;32:98–102.
- Bodner-Adler B, Bodner K, Czerwenka K, Kimberger O, Leodolter S, Mayerhofer K. Expression of p16 protein in patients with uterine smooth muscle tumors: An immunohistochemical analysis. Gynecol Oncol 2005;96:62–6.
- O’Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology 2007;50:851–8.
- Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol 2008;27:326–32.
- Mittal K, Demopoulos RI. MIB-1 (Ki-67), p53, estrogen receptor, and progesterone receptor expression in uterine smooth muscle tumors. Hum Pathol 2001;32:984–7.
- Akhan SE, Yavuz E, Tecer A, Iyibozkurt CA, Topuz S, Tuzlali S, . The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol Oncol 2005;99: 36–42.
- Swisher EM, Gown AM, Skelly M, Ek M, Tamimi HK, Cain JM, . The expression of epidermal growth factor receptor, HER-2/Neu, p53, and Ki-67 antigen in uterine malignant mixed mesodermal tumors and adenosarcoma. Gynecol Oncol 1996;60:81–8.
- Amant F, Vloeberghs V, Woestenborghs H, Debiec-Rychter M, Verbist L, Moerman P, . ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol 2004;95:583–7.
- Amant F, Schurmans K, Steenkiste E, Verbist L, Abeler VM, Tulunay G, . Immunohistochemical determination of estrogen and progesterone receptor positivity in uterine adenosarcoma. Gynecol Oncol 2004;93:680–5.
- Perri T, Korach J, Sadetzki S, Oberman B, Fridman E, Ben-Baruch G. Uterine leiomyosarcoma: Does the primary surgical procedure matter? Int J Gynecol Cancer 2009;19: 257–60.
- Della Badia C, Karini H. Endometrial stromal sarcoma diagnosed after uterine morcellation in laparoscopic supracervical hysterectomy. J Minim Invasive Gynecol 2010;17:791–3.
- Einstein MH, Barakat RR, Chi DS, Sonoda Y, Alektiar KM, Hensley ML, . Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer 2008;18:1065–70.
- Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, . Prognostic factors and treatment outcomes of patients with uterine sarcoma: Analysis of 127 patients at a single institution, 1989–2007. J Cancer Res Clin Oncol 2008;134:1277–87.
- Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. The impact of tumor morcellation during surgery on the outcomes of patients with apparently early low-grade endometrial stromal sarcoma of the uterus. Ann Surg Oncol 2011;18:3453–61.
- Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL Jr. Treatment of endometrial stromal tumors. Gynecol Oncol 1990;36:60–5.
- Amant F, De Knijf A, Van Calster B, Leunen K, Neven P, Berteloot P, . Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br J Cancer 2007;97:1194–9.
- Leitao MM, Sonoda Y, Brennan MF, Barakat RR, Chi DS. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol 2003;91: 209–12.
- Giuntoli RL 2nd, Metzinger DS, DiMarco CS, Cha SS, Sloan JA, Keeney GL, . Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol 2003;89:460–9.
- Li AJ, Giuntoli RL 2nd, Drake R, Byun SY, Rojas F, Barbuto D, . Ovarian preservation in stage I low-grade endometrial stromal sarcomas. Obstet Gynecol 2005;106:1304–8.
- Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008;112:820–30.
- Shah JP, Bryant CS, Kumar S, Ali-Fehmi R, Malone JM Jr, Morris RT. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol 2008;112:1102–8.
- Sutton G, Kavanagh J, Wolfson A, Tornos C. Corpus: Mesenchymal tumors. Principles and practice of gynecologic oncology, 5th ed. Barakat R, Markman M, Randall ME, editors. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2009. pp 733–61.
- Tse KY, Crawford R, Ngan HY. Staging of uterine sarcomas. Best Pract Res Clin Obstet Gynaecol 2011;25:733–49.
- Giuntoli RL 2nd, Garrett-Mayer E, Bristow RE, Gostout BS. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecol Oncol 2007;106:82–8.
- Anraku M, Yokoi K, Nakagawa K, Fujisawa T, Nakajima J, Akiyama H, . Pulmonary metastases from uterine malignancies: Results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107–12.
- McCormack PM, Martini N. The changing role of surgery for pulmonary metastases. Ann Thorac Surg 1979;28:139–45.
- Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:537–43; discussion 543–4.
- Chen H, Pruitt A, Nicol TL, Gorgulu S, Choti MA. Complete hepatic resection of metastases from leiomyosarcoma prolongs survival. J Gastrointest Surg 1998;2:151–5.
- Tepetes K, Tsamandas AC, Ravazoula P, Petsas T, Bonikos DS, Karavias DD. Survival for 5 years after repeat liver resections and multimodality treatment for metastatic intestinal leiomyosarcoma: Report of a case. Surg Today 2002;32: 925–8.
- Lang H, Nussbaum KT, Kaudel P, Frühauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann Surg 2000;231:500–5.
- Livi L, Paiar F, Shah N, Blake P, Villanucci A, Amunni G, . Uterine sarcoma: Twenty-seven years of experience. Int J Radiat Oncol Biol Phys 2003;57:1366–73.
- Knocke TH, Kucera H, Dörfler D, Pokrajac B, Pötter R. Results of postoperative radiotherapy in the treatment of sarcoma of the corpus uteri. Cancer 1998;83:1972–9.
- Reed NS, Mangioni C, Malmström H, Scarfone G, Poveda A, Pecorelli S, . Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 2008;44: 808–18.
- Greer BE, Koh WJ, Abu-Rustum N, Bookman MA, Bristow RE, Campos SM, . Uterine neoplasms. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2009;7:498–531.
- Sutton G, Blessing J, Hanjani P, Kramer P; Gynecologic Oncology Group. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecol Oncol 2005;96:749–52.
- Currie J, Blessing JA, Muss HB, Fowler J, Berman M, Burke TW. Combination chemotherapy with hydroxyurea, dacarbazine (DTIC), and etoposide in the treatment of uterine leiomyosarcoma: A Gynecologic Oncology Group study. Gynecol Oncol 1996;61:27–30.
- Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, . Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol 2002;20:2824–31.
- Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol 2008;109:329–34.
- Muss HB, Bundy B, DiSaia PJ, Homesley HD, Fowler WC Jr, Creasman W, . Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 1985;55:1648–53.
- Spano JP, Soria JC, Kambouchner M, Piperno-Neuman S, Morin F, Morere JF, . Long-term survival of patients given hormonal therapy for metastatic endometrial stromal sarcoma. Med Oncol 2003;20:87–93.
- Pink D, Lindner T, Mrozek A, Kretzschmar A, Thuss-Patience PC, Dörken B, . Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: Single center experience with 10 cases and review of the literature. Gynecol Oncol 2006;101:464–9.
- Hardman MP, Roman JJ, Burnett AF, Santin AD. Metastatic uterine leiomyosarcoma regression using an aromatase inhibitor. Obstet Gynecol 2007;110:518–20.
- Sutton G, Blessing JA, Park R, DiSaia PJ, Rosenshein N. Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: A study of the Gynecologic Oncology Group. Obstet Gynecol 1996;87:747–50.
- Scandinavian Sarcoma Group and Oncologic Center, Lund, Sweden. Recommendations for the diagnosis and treatment of intraabdominal, retroperitoneal and uterine sarcoma. December 2008. Available from: www.ssg-org.net, SSG XVII, Version 2, 1–37.
- Garg G, Shah JP, Liu JR, Bryant CS, Kumar S, Munkarah A, . Validation of tumor size as staging variable in the revised International Federation of Gynecology and Obstetrics stage I leiomyosarcoma: A population-based study. Int J Gynecol Cancer 2010;20:1201–6.
- Carlson JW. Sarcomas of the uterus. Staging of gynecologic malignancies handbook. Society of Gynecologic Oncologists, 3rd edition, January 2010. pp. 41–4.