To the Editor,
There has been an increasing incidence of brain and leptomeningeal metastases noted in patients with human epidermal growth factor receptor-2 (HER-2) positive breast cancer, especially after the addition of intravenous trastuzumab to systemic therapy has become the standard of care. The exact reason for this increase is unknown, but it may be the result of the aggressive nature of HER-2 positive breast cancer and the increased overall survival seen with the addition of trastuzumab to cytotoxic chemotherapy [Citation1,Citation2].
As prolonged survival continues to be demonstrated with intravenous trastuzumab, new therapeutic techniques need to be studied to deal with progressive disease. Although intrathecal (IT) chemotherapy is recommended for some patients with leptomeningeal metastases [Citation3], the reported outcomes are variable with IT methotrexate [Citation4–6]. The administration of IT trastuzumab has been reported for the treatment of patients with central nervous system (CNS) metastases from HER-2 positive breast cancer [Citation2,Citation7–14]. We describe the successful treatment of leptomeningeal metastases secondary to HER-2 positive metastatic breast cancer in an adult patient using a combination of IT trastuzumab and methotrexate that resulted in rapid and sustained improvement.
Case
In June 2006, a 31-year-old female was diagnosed with stage IV (T4d N2 M1), estrogen receptor positive, progesterone receptor negative, HER-2 positive infiltrating ductal carcinoma that was metastatic to liver and bone. After trastuzumab-containing systemic chemotherapy, workup revealed a complete response in the breast and liver with a significant response in the bony lesions. The patient underwent a modified radical mastectomy with a complete pathological response, followed by radiation therapy. The patient continued to receive trastuzumab every three weeks.
Metastatic disease was diagnosed in June 2009 when magnetic resonance imaging (MRI) of the brain revealed a mass in the left frontal lobe with midline shift. The patient underwent surgical resection followed by gamma knife stereotactic radiosurgery. Trastuzumab was discontinued and the patient was started on capecitabine and lapatinib. A recurrence was found in February 2010 and the patient underwent a second surgical resection followed by additional gamma knife stereotactic radiosurgery.
A follow-up brain MRI in January 2011 identified new metastases and the patient underwent a third craniotomy and gamma knife radiosurgery followed by whole brain radiation. One month later, the patient’s gait became unsteady and she was unable to stand. An MRI study showed diffuse thickening and the presence of contrast enhancement involving the leptomeninges of the entire spine as well as cauda equina nerve roots; findings consistent with cerebrospinal fluid (CSF) seeding. Also noted was an abnormal increase of T2 signal involving the spinal cord from C4-C5 and T1-T2, which could be related to the invasion of the dorsal column of the spinal cord from the adjacent leptomeningeal metastases.
IT therapy with trastuzumab 20 mg and methotrexate 6 mg was initiated in February 2011. All doses were administered via an Ommaya reservoir. Each medication was prepared with preservative-free solutions. Due to the lack of preservatives and unknown time of chemical stability, both agents were prepared as close as possible to the time of administration.
Doses of both trastuzumab and methotrexate were originally administered two times per week. After three weeks, MRI showed a decrease in leptomeningeal disease (). At this time, the patient was able to stand with assistance during physical therapy. A second MRI study in April 2011 showed further improvement and the IT therapy schedule was changed to once weekly. The patient received a total of 17 doses of trastuzumab and 16 doses of methotrexate between February and May 2011. IT therapy was well tolerated, with no apparent side effects.
Figure 1. (a) Pre-treatment axial T1 weighted post-contrast MR image of the brain. The arrow points to enhancement caused by tumor deposits. (b) Post-treatment axial T1 weighted post-contrast MR image of the brain – indicates interval response to treatment.
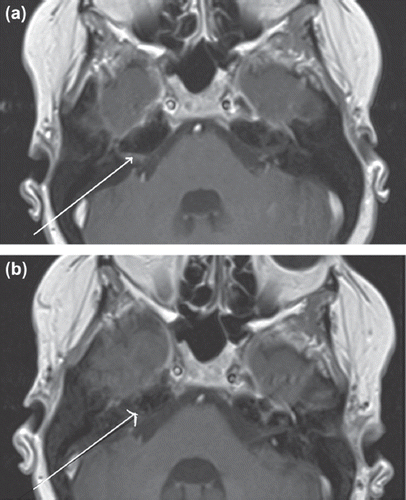
Figure 2. (a) Pre-treatment sagittal T1 weighted post-contrast MR image of the spinal cord – note the diffuse enhancement surrounding the cord as indicated by the arrow. (b) Post-treatment sagittal T1 weighted post-contrast MR image of the spinal cord – improvement is noted as indicated by the arrow.
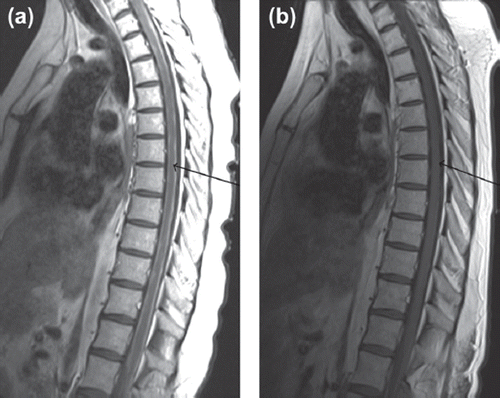
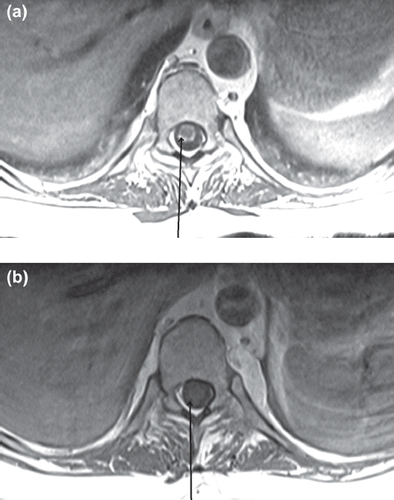
Figure 3. (a) Pre-treatment axial T1 weighted post-contrast MR image of the spinal cord – areas of leptomeningeal involvement are indicated by the arrow and are visible as an area of enhancement that encircles the spinal cord. In (b) improvement is noted and is indicated by the arrow.
The CSF cytology remained negative for malignant cells throughout treatment; however, protein and glucose levels fluctuated. After initial improvement, in May 2011 the patient's lower extremity weakness progressed to paraplegia, and urinary retention requiring self-catheterization resulted. The decision was made to discontinue treatment due to poor quality of life. The patient enrolled into hospice care and expired 24 July 2011.
Discussion
It is estimated that up to 15% of patients with solid tumors experience leptomeningeal metastases, the largest proportion of which are breast cancer patients [Citation1]. Despite this, the treatment of leptomeningeal metastases from breast cancer is not standardized. Radiation therapy may provide symptomatic benefit and is recommended for those patients who can tolerate it, and IT chemotherapy be considered for those patients with normal CSF flow [Citation3].
IT methotrexate is commonly used to treat leptomeningeal metastases, but results of studies have been inconsistent and disappointing. A common dosing schedule is 10 mg administered twice per week, followed by weekly dosing after clinical improvement is noted [Citation4–6].
Several case reports have demonstrated the benefits of IT trastuzumab in the treatment of leptomeningeal metastases secondary to HER-2 positive metastatic breast cancer [Citation2,Citation7–14]. Favorable effects from IT trastuzumab have included a decrease in the presence of tumor cells and relief from clinical symptoms [Citation2,Citation9,Citation11]. In all reports, IT trastuzumab was very well tolerated [Citation2,Citation7–14].
The specific combination of IT trastuzumab with IT methotrexate has been reported in three other cases (), although the optimal dose and method of administration have not been determined [Citation7,Citation9,Citation13]. In the three reports, responses were seen regardless of the administration method (lumbar puncture or use of an Ommaya reservoir) or dose [Citation7,Citation9,Citation13]. The trastuzumab doses ranged from 10 mg [Citation7] to 50 mg per dose [Citation13], and the methotrexate doses were between 10 mg [Citation13] and 15 mg [Citation7]. Our patient received what could be considered an average dose of trastuzumab (20 mg) and slightly lower dose of methotrexate (6 mg) as compared to those previously reported. Additionally, the most effective dosing frequency is unknown. Weekly administration of IT trastuzumab is the most commonly described frequency, but the time between doses varies from as little as three days to as much as three weeks in reports [Citation9,Citation13]. When IT methotrexate was used concurrently, it was given approximately weekly or twice per week [Citation7,Citation9,Citation13], although only one case reports utilizing a planned dosing schedule of the combination [Citation9]. To our knowledge, our report is the first to describe a planned long-term twice weekly administration schedule of both agents. As with the patient described in this case, patients in the previously reported cases received systemic therapy in addition to IT trastuzumab and methotrexate [Citation7,Citation9,Citation13]. Furthermore, additional CNS-active therapy was utilized in two cases [Citation7,Citation13]. The contribution of other intrathecal or systemic antineoplastics is unable to be determined.
Table I. Summary of intrathecal trastuzumab plus methotrexate case reports in breast cancer [Citation7,Citation9,Citation13].
In conclusion, treatment with intrathecal trastuzumab plus methotrexate led to rapid clinical and radiographic improvement as well as prolonged survival in our patient with metastatic HER-2 positive breast cancer. As our experience is consistent with previous reports, we feel that this combination is a safe and active therapeutic option that is worthy of more systematic research. Future studies should be aimed at identifying the most effective doses and frequencies of administration, as well as the role of additional CNS or systemic antineoplastics.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lombardi G, Zustovich F, Farina P, Della Puppa A, Manara R, Cecchin D, . Neoplastic meningitis from solid tumors: New diagnostic and therapeutic approaches. Oncologist 2011;16:1175–88.
- Stemmler HJ, Schmitt M, Harbeck N, Willems A, Bernhard H, Lassig D, . Application of intrathecal trastuzumab (HerceptinTM) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep 2006;15:1373–7.
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. v.1.2012. Accessed 2012 Mar 7. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, . A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5: 3394–402.
- Boogerd W, van den Bent MJ, Koehler PJ, Heimans JJ, van der Sande JJ, Aaronson NK, . The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: A randomized study. Eur J Cancer 2004;40:2726–33.
- Rudnicka H, Niwinska A, Murawska M. Breast cancer leptomeningeal metastasis – the role of multimodality treatment. J Neurooncol 2007;84:57–62.
- Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer 2001;2:235 (letter).
- Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol 2006;7:778–80.
- Stemmler HJ, Mengele K, Schmitt M, Harbeck N, Laessig D, Hermann KA, . Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer: A case report. Anticancer Drugs 2008;19:832–6.
- Shojima K, Suzuki E, Saito K, Sekine D, Kitagawa T, Aruga S, . Application of intrathecal trastuzumab for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. J Clin Oncol 2008;26(Suppl);1138 (abstract).
- Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol 2008;19:1978–80 (letter).
- Colozza M, Minenza E, Gori S, Fenocchio D, Paolucci C, Aristei C, . Extended survival of a HER-2-positive metastatic breast cancer patient with brain metastases also treated with intrathecal trastuzumab. Cancer Chemother Pharmacol 2009;63:1157–9.
- Ferrario C, Davidson A, Bouganim N, Aloyz R, Panasci LC. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol 2009;20:792–5 (letter).
- Allison DL, Glantz M, Werner TL, Kirkegaard SL, Murdock K, Jensen R. Intra-CSF trastuzumab in patients with neoplastic meningitis from breast cancer or primary brain tumors. J Clin Oncol 2009;27(Suppl):2066 (abstract).