Abstract
Re-irradiation using high-precision radiation techniques has been established within the clinical routine for patients with recurrent gliomas. In the present work, we developed a practical prognostic score to predict survival outcome after re-irradiation. Patients and methods. Fractionated stereotactic radiotherapy (FSRT) was applied in 233 patients. Primary histology included glioblastoma (n = 89; 38%), WHO Grade III gliomas (n = 52; 22%) and low-grade glioma (n = 92; 40%). FSRT was applied with a median dose of 36 Gy in 2 Gy single fractions. We evaluated survival after re-irradiation as well as progression-free survival after re-irradiation; prognostic factors analyzed included age, tumor volume at re-irradiation, histology, time between initial radiotherapy and re-irradiation, age and Karnofsky Performance Score. Results. Median survival after FSRT was 8 months for glioblastoma, 20 months for anaplastic gliomas, and 24 months for recurrent low-grade patients. The strongest prognostic factors significantly impacting survival after re-irradiation were histology (p < 0.0001) and age (< 50 vs. ≥ 50, p < 0.0001) at diagnosis and the time between initial radiotherapy and re-irradiation ≤ 12 vs. > 12 months (p < 0.0001). We generated a four-class prognostic score to distinguish patients with excellent (0 points), good (1 point), moderate (2 points) and poor (3–4 points) survival after re-irradiation. The difference in outcome was highly significant (p < 0.0001). Conclusion. We generated a practical prognostic score index based on three clinically relevant factors to predict the benefit of patients from re-irradiation. This score index can be helpful in patient counseling, and for the design of further clinical trials. However, individual treatment decisions may include other patient-related factors not directly influencing outcome.
Over the last years, re-irradiation of recurrent gliomas using high-precision photon techniques has been established within the clinical routine. It has been shown that a second course of radiotherapy can be applied safely and effectively, and it has always been argued that some “subgroups of patients” benefit from this treatment more than others [Citation1,Citation2]. However, to date, the decision on when to indicate a second course of radiotherapy has been made on an individual basis. For example, in our institution, presence of a single contrast-enhancing lesion with a maximum diameter of 4 cm, and a time interval between primary radiotherapy of six months or more were set as criteria for re-irradiation. Other groups argue that the time interval between first and second radiotherapy should be at least nine months, or that even larger lesions or multifocal disease might benefit from a second course of radiotherapy [Citation1–8].
When diagnosing recurrent glioma, the radiation oncologist is faced with a heterogeneous group of patients with respect to initial histology, previous treatments, clinical performance status or size and location of the lesion. Prognostic factors for outcome have been identified to be the extent of neurosurgical resection at initial diagnosis, initial neuropathological classification, age of the patients, size of the lesion as well as overall performance status; this has been shown in several studies reporting on the outcome of radiation therapy for patients with gliomas, also in the recurrent setting [Citation1–8].
In treating patients with recurrent gliomas, potential treatment alternatives, i.e. surgery, radiation or chemotherapy, must be weighted against each other taking into account overall treatment time, potential morbidity, treatment-related side effects as well as the impact on the patients’ outcome, including survival.
To determine a potential benefit of a treatment at recurrence, Park and colleagues defined an NCI prognostic score to help indicate surgical resection in patients with recurrent gliomas, and to chose subgroups of patients that benefit maximally from a second neurosurgical intervention [Citation9]. For re- irradiation, no clear arguments for decision making have been generated in the past. Therefore, in the present work we re-evaluated our very large group of patients with recurrent gliomas treated with fractionated stereotactic radiotherapy (FSRT) for re-irradiation. To our knowledge, it represents the largest group of patients treated with a homogeneous technique at a single institution. Using the identified prognostic factors for outcome, we determined a practical predictive model and a prognostic score index to support decision making for radiation therapy in this recurrent glioma setting.
Patients and methods
Patients
The patient cohort used to generate and validate the prognostic score consisted of 233 patients with recurrent gliomas treated between 1990 and 2010 with FSRT in a single institution. Initial neuropathological diagnosis was WHO Grade II glioma in 92 patients (40%), WHO Grade III glioma in 52 patients (22%), and glioblastoma in 89 patients (38%). Patients’ characteristics are shown in .
Table I. Demographic, clinical and radiographic characteristics of 233 patients with recurrent gliomas treated with FSRT for re-irradiation.
All patients had been treated with radiation therapy during first-line treatment with a median dose of 60 Gy in conventional fractionation. The median time between primary radiotherapy and re-irradiation was 57 months (range 5–204 months) for initially WHO Grade II tumors, 33 months (range 3–144 months) for WHO Grade III gliomas, and 12 months (range 3–72 months) for patients initially diagnosed with glioblastoma.
General indication for re-irradiation was set according to our institutional standards: We treated recurrent Grade III and IV gliomas, as well as WHO Grade II tumors with recurrences and signs of malignization in terms of contrast-enhancing lesions. Patients were required to present with unifocal disease, with a maximum diameter of the contrast- enhancing lesion of 4 cm.
Radiation therapy
Guidelines for re-irradiation have been published in detail previously [Citation1]. For treatment planning, individual mask fixations were manufactured for each patient using Scotch Cast™ material, and examinations for treatment planning were performed in a stereotactic setup assuring an overall accuracy of 1–2 mm [Citation10]. We defined the gross tumor volume (GTV) as the contrast-enhancing lesion on magnetic resonance imaging (MRI), and the clinical target volume (CTV) adding a margin of 0.5–1 cm to the GTV. The planning target volume (PTV) of 1–2 mm was added to account for setup uncertainties. The median PTV was 47 ml (range 3–758 ml).
Re-irradiation was performed as FSRT with a median dose of 36 Gy in 2 Gy daily single fractions, 5 fractions per week, delivered by a 6 MV linear accelerator (Siemens, Erlangen, Germany).
Follow-up and statistical analysis
All patients were seen for regular follow-up visits, initially six weeks after completion of FSRT, thereafter in two to three months intervals or sooner as required clinically. Follow-up examinations included contrast-enhanced imaging, clinical-neurological follow-up as well as additional examinations including amino-acid-PET as needed.
We evaluated survival after re-irradiation calculated from the first day of re-irradiation until the last follow-up (censored observation) or death. Influence of prognostic factors was analyzed using the Kaplan-Meier-method as well as multivariate analysis including age, histology based on WHO grading at recurrence, time between initial radiotherapy and re-irradiation, age and Karnofsky Performance Score, both at re-irradiation. Statistical analyses were performed using the software Statistica 6.1 (StatSoft, Hamburg, Germany).
Prognostic score
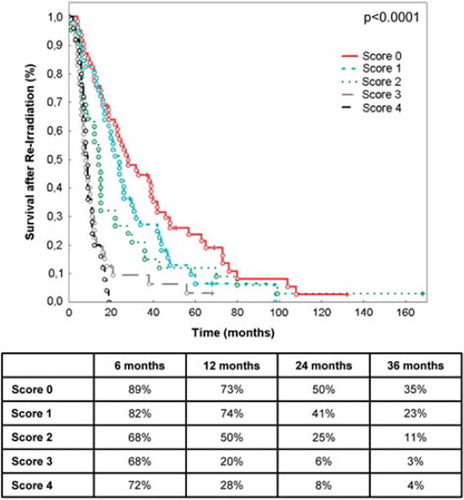
Prognostic factors identified as significantly influencing outcome after re-irradiation were used to generate the prognostic score index. Therefore, using clinical information, all factors were either dichotomized into a favorable and an unfavorable prognostic group or, in the case of histology, trichotomized. Those exhibiting a statistically significant difference in survival outcome of p < 0.05 were combined to a simple predictive model using a composite score index was then generated by giving weights 0 or 1 to dichotomized and weights 0–2 to trichotomized factors. The single scores of the three significant factors were added up to the score index which was then visually validated by determining the survival after re-irradiation for each of the resulting four prognostic classes: excellent (0 points), good (1 point), moderate (2 points) and poor (3–4 points).
Results
Radiation treatment
Radiotherapy was well tolerated by all patients and could be completed without interruptions > 4 days due to side effects or other medical problems. Acute toxicity observed included alopecia, headaches, nausea and skin erythema which were mild in most patients. We observed radiographically diagnosed and histologically confirmed radiation-induced necrosis after re-irradiation in one patient only. No other severe early or late side effects > CTCAE Grade 2 could be documented.
Survival after re-irradiation
Median survival after re-irradiation was eight months (range 1–168 months) for glioblastoma, 20 months (range 1–99 months) for WHO Grade III gliomas, and 24 months (range 2–132 months) for low-grade gliomas.
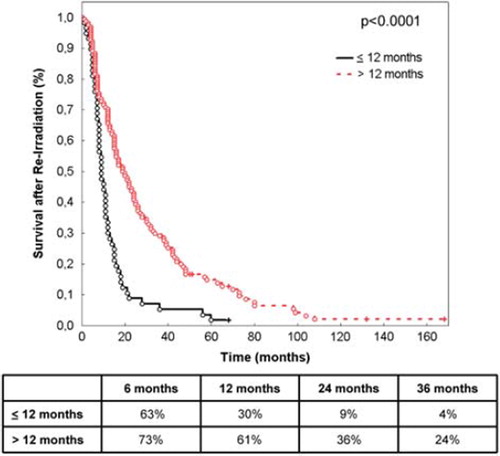
The strongest prognostic factors significantly impacting survival after re-irradiation were histology (p < 0.0001; ), age (< 50 vs. ≥ 50 years of age, p < 0.0001; ) and time between initial radiotherapy and re-irradiation (≤ 12 vs. > 12 months, p = 0.0001; ).
Figure 1. Survival after re-irradiation according to primary histology. The table shows survival at 6, 12, 24 and 36 months.
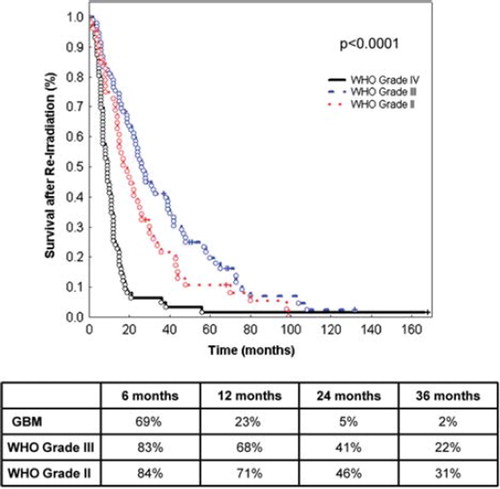
Figure 2. Survival after re-irradiation according to age < 50 and ≥ 50 years of age. The table shows survival at 6, 12, 24 and 36 months.
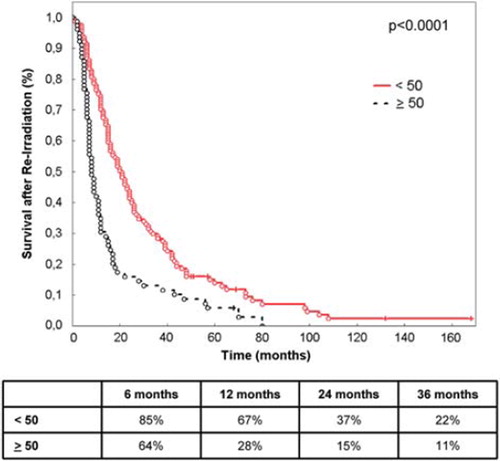
Figure 3. Survival after re-irradiation according to time between primary radiotherapy and re-irradiation (≤ 12 months vs. > 12 months). The table shows survival at 6, 12, 24 and 36 months.
Tumor volume at re-irradiation (< 47 ml vs. > 47 ml; p = 0.84), Karnofsky Performance Score (p = 0.25), presence of neurological symptoms (p = 0.99) as well as gender (p = 0.57) did not impact survival after re-irradiation.
In multivariate analysis, only histology and age remained significant at p = 0.013.
Prognostic score for survival after re-irradiation
The prognostic variables identified as significantly influencing survival after re-irradiation in univariate analysis were used as a basis to generate the prognostic score. To calculate a patient's score, a sum of the following values was calculated: for histology, glioblastoma was rated as 2, WHO Grade III tumors as 1, and low-grade WHO Grade II tumors as 0. With respect to age, patient's younger than 50 were given 0 points, and age 50 or older was scored with 1. Time between initial radiation and re-irradiation was counted as 0 if 12 or more months, and as 1 if the time interval was less than 12 months. These categories are shown in .
Table II. Factors identified as signficiantly influencing survival after re-irradiation used for the generation of the prognostic score.
Taking into account these significant prognostic factors, we generated a prognostic score: an additive scale (range 0–4 points) comprised of these three factors could distinguish patients with excellent (0 points), good (1 point), moderate (2 points) and poor (3–4 points) survival after re-irradiation. For example, patients in group 0 represent, e.g. low-grade histology, time between initial RT and re-irradiation > 12 months, age under 50 years), and show the most survival benefit with a median survival of 25 months.
Of the 233 patients, 62 were scored 0, 51 were scored 1, score 2 was defined for 41 patients, and score 3 and 4 for 45 and 34 patients, respectively. The difference in outcome was highly significant at p < 0.0001 ().
Discussion
In the present work we generated a prognostic index to determine classes of patients showing a significant benefit from re-irradiation for recurrent gliomas. The work is based on 233 patients treated with a homogeneous schedule of FSRT in a single institution representing the largest group of patients treated with re-irradiation for this diagnosis to our knowledge. The subclassification into the four scoring groups demonstrated a highly significant correlation with survival after re-irradiation.
At recurrence, the choice of treatment for patients with recurrent gliomas is limited. Available treatments include surgical resection, systemic treatments with chemotherapy or novel molecular targeted agents, or a second course of radiation therapy [Citation1,Citation3,Citation6,Citation11–15]. The value of surgery has been discussed controversially in the past, and only few studies have reported outcome after surgery for patients with recurrent gliomas [Citation14,Citation16]. In general, outcome was poor, and surgical resection often associated with a major risk of side effects due to the infiltrative nature of the disease. Only recently, Park and colleagues generated a valuable tool to determine a prognostic score for outcome after surgery for recurrent gliomas [Citation9]. This work took into account relevant factors influencing neurosurgical resection, such as tumor volume, location of the tumor and association with eloquent brain areas, as well as patient related factors such as Karnofsky Performance Score. The authors developed a score index, similar as in this study, ranging between 0 = good and 3 = poor, and they could an show that patients with a score of 3 (e.g. location in eloquent area, poor performance status, large lesion volume) showed only modest benefit from a surgical approach, stressing that treatment decisions must be made on an individual basis taking into account the above mentioned relevant factors.
For radiation therapy, several study groups have reported safety and efficacy with different treatment schemes including hypofractionated radiotherapy, radiosurgery or fractionated precision techniques [Citation1,Citation4,Citation6,Citation15,Citation17]. Our group has shown previously that FSRT is a highly effective method for treatment of recurrent gliomas, without substantial toxicity [Citation1]. Since the majority of lesions develops within the previous high-dose treatment area, single doses of 2 Gy were chosen to minimized the risk of severe treatment-related side effects, and the total dose of 36 Gy was chosen to stay below a total cumulative dose of 100 Gy (to normal tissue) [Citation18]. Therefore, this regimen is considered standard in our institution when patients present with the defined criteria such as lesions up to 4 cm in diameter, time between initial radiotherapy and re-irradiation of six months or more. However, until now, it had not been systematically investigated as to which patients really benefit from a second course of irradiation, since a multitude of factors determine outcome in these patients.
Therefore, we updated our patient population treated with FSRT for recurrent gliomas and evaluated significant prognostic factors for survival after re-irradiation. Since progression-free survival is often difficult to determine due to the intricate patterns of imaging after radiation including edema or post- radiotherapy contrast-enhancement, as well as potential other treatment-related differences in imaging, survival after re-irradiation was chosen as a “hard endpoint” correlating with treatment efficacy.
A scoring system based on the significant factors age, time between initial radiotherapy and histologic classification was generated distributing the 233 patients into the scoring groups 0–4. While patients scored 3–4 represented the worst outcome, patients scored 0 clearly showed best outcome after re-irradiation.
For treatment decisions, patients scored 0–2 show a clear benefit from re-irradiation. Patients scored 3 and 4 demonstrated significantly lower survival after re-irradiation. However, taking into account the WHO Grade III and IV histology generally associated with the scores 3 and 4, the median survival times of, e.g. eight months for glioblastoma, still account for a substantial part of overall survival, considering overall survival times of about 16 months [Citation19,Citation20]. We included significant variables determined in univariate analysis, which are known to be prognostic factors. However, in multivariate analysis, only two factors remained significant. This is most likely due to the evident correlation between “time between radiation” and primary histology, meaning, e.g. patients with low-grade histology mostly demonstrate a longer time interval until recurrence, whereas glioblastoma patients develop their recurrence much faster, and thus demonstrate a shorter time between a first and second radiotherapy. However, based on the clinical experience and data published in the literature, we based the classification on the three most significant variables prognostic for survival: histology, age, and time between first and second radiation.
Since the risk for treatment-associated side effects with high-precision radiotherapy are practically minimal, the choice for re-irradiation can be made generously even in patients with score 3 and 4.
Therefore, the calculated scores must be kept in mind for treatment decisions, and can be used as a helpful accessory in the clinical setting. Since the scoring system was developed and assessed in the largest group of patients presently available and treated homogeneously for recurrent gliomas, the predication developed within this analysis can be considered as an effective tool for evaluating the indication for re-irradiation in patients with recurrent gliomas.
Two limitations of this study were recognized for future research. The main limitation of the score might be that patients with various primary histologies were included and factors are likely to correlate with each other. However, at recurrence, the histologic diagnosis especially in tumors with malignization, play only a diminutive role in the context of tumor volume, performance-score of the patients, age and especially the velocity of tumor progression over time. This justifies taking together the three histological subgroups, with the objective of drawing the novel prognostic score on a sound data basis. Additionally, full validation of the proposed score, e.g. using an independent data might be performed in the future.
It must be kept in mind that decision-making remains to be performed on an individual basis. This score can be helpful in patient counseling, and for the design of further clinical trials. However, individual treatment decisions may include other patient-related factors not directly influencing outcome. However, besides the need of further validation, we think that a prognostic score derived form this large population should be used as a helpful practical tool, but not as a sole argument for treatment recommendations.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol 2005;23:8863–9.
- Bauman GS, Sneed PK, Wara WM, Stalpers LJ, Chang SM, McDermott MW, . Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys 1996;36:433–41.
- Arcicasa M, Roncadin M, Bidoli E, Dedkov A, Gigante M, Trovo MG. Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys 1999;43:789–93.
- Combs SE, Gutwein S, Thilmann C, Debus J, Schulz-Ertner D. Reirradiation of recurrent WHO grade III astrocytomas using fractionated stereotactic radiotherapy (FSRT). Strahlenther Onkol 2005;181:768–73.
- Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R. Hypofractionated stereotactic reirradiation of recurrent glioblastomas: A beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol 2009;185:235–40.
- Henke G, Paulsen F, Steinbach JP, Ganswindt U, Isijanov H, Kortmann RD, . Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol 2009;185: 113–9.
- Pollack IF. Neuro-oncology: Therapeutic benefits of reirradiation for recurrent brain tumors. Nat Rev Neurol 2010;6:533–5.
- Rasmussen KH, Hardcastle N, Howard SP, Tome WA. Reirradiation of glioblastoma through the use of a reduced dose rate on a tomotherapy unit. Technol Cancer Res Treat 2010;9:399–406.
- Park JK, Hodges T, Arko L, Shen M, Dello ID, McNabb A, . Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol 2010; 28:3838–43.
- Karger CP, Hipp P, Henze M, Echner G, Hoss A, Schad L, . Stereotactic imaging for radiotherapy: Accuracy of CT, MRI, PET and SPECT. Phys Med Biol 2003;48:211–21.
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, . Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999;17:2572–8.
- Wick A, Pascher C, Wick W, Jauch T, Weller M, Bogdahn U, . Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 2009;256:734–41.
- Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, . Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 2007;25:3357–61.
- Barker FG, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, . Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998;42:709–20.
- Niyazi M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, Geisler J, . Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 2012;82:67–76.
- Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg 1993;36:271–5.
- Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D. Stereotactic radiosurgery (SRS): Treatment option for recurrent glioblastoma multiforme (GBM). Cancer 2005;104:2168–73.
- Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 2008;70:1350–60.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66.
- Combs SE, Wagner J, Bischof M, Welzel T, Edler L, Rausch R, . Radiochemotherapy in patients with primary glioblastoma comparing two temozolomide dose regimens. Int J Radiat Oncol Biol Phys 2008;71:999–1005.