Abstract
Background. Long-term data on breast cancer detection in mammography screening programs are warranted to better understand the mechanisms by which screening changes the breast cancer pattern in the population. We aimed to analyze 17 years of breast cancer detection rates inside and outside screening in two Danish regions, emphasizing the influence of organizational differences of screening programs on the outcomes. Material and methods. We used data from two long-standing population-based mammography screening programs, Copenhagen and Fyn, in Denmark. Both programs offered biennial screening to women aged 50–69 years. We identified targeted, eligible, invited and participating women. We calculated screening detection and interval cancer rates for participants, and breast cancer incidence in non-screened women (= targeted women excluding participants) by biennial invitation rounds. Tumor characteristics were tabulated for each of the three groups of cancers. Results. Start of screening resulted in a prevalence peak in participants, followed by a decrease to a fairly stable detection rate in subsequent invitation rounds. A similar pattern was found for breast cancer incidence in non-screened women. In Fyn, non-screened women even had a higher rate than screening participants during the first three invitation rounds. The interval cancer rate was lower in Copenhagen than in Fyn, with an increase over time in Copenhagen, but not in Fyn. Screen-detected cancers showed tumor features related with a better prognosis than tumors detected otherwise, as more than 80% were smaller than 20 mm and estrogen receptor positive. Conclusion. Data from two long-standing population-based screening programs in Denmark illustrated that even if background breast cancer incidence and organization were rather similar, performance indicators of screening could be strongly influenced by inclusion criteria and participation rates. Detection rates should be interpreted with caution as they may be biased by selection into the screening population.
Breast cancer is the most common cancer among women worldwide, representing a major public health issue [Citation1]. Over the last three decades its epidemiology has varied considerable: while the mortality has been declining in high-income countries [Citation2], the breast cancer incidence has been increasing until the early 2000s, where after a downturn was reported in many developed countries [Citation3,Citation4]. Several factors have likely contributed to the observed trends, including a significant drop in the hormone therapy use, widespread use of effective systemic treatment modalities, and mammographic screening programs having past the phase of prevalence peaks [Citation2–4].
The goal of screening mammography is to reduce breast cancer mortality by means of early detection of tumors. Achieving the expected reduction in mortality will depend on the quality of screening procedures and participation. Women who regularly participate in screening may profit from it by detection of cancer at an earlier stage, which are associated with a better prognosis than those detected outside screening [Citation5]. Screening might though also have negative side effects as false positive tests and overdiagnosis. The benefit of attending a screening program is reduced for women diagnosed with interval cancer (i.e. cancers diagnosed after a negative screening result and before the next invitation to screening). These tumors are detected clinically, thus with a delay in time of diagnosis and worse prognosis features. The interval cancer rate has been recognized as a valid indicator of screening quality and the sensitivity of the screening procedure [Citation6].
Denmark is one of the countries with the highest breast cancer incidence in the world [Citation1]. Mammography screening was introduced in 1991 in the municipally of Copenhagen, and in the county of Fyn in 1993. Nationwide roll out of screening took place in 2007–2010. Although the background cancer incidence does not differ greatly across Danish regions [Citation7], some organizational differences exist between the above mentioned screening programs in criteria for definition of eligible women and in invitation procedures. The existence of the Danish unique identification number allows the link of screening data with population-based registers and the identification of all diagnosis of breast cancer, both detected at screening, as interval cancers, and outside screening. The chance to have accurate information from screened and non-screened women from two regions with long-term screening performance allowed to look for effects of organizational differences in areas with fairly similar background incidence rates. In addition, with nearly 20 years of follow-up, to our knowledge, this is the longest studied scenario showing trends overtime of screen-detected breast cancers, interval cancers and breast cancers diagnosed outside screening. Differences reported both for background cancer incidences and screening protocols as well as for screening performance indicators in European countries [Citation8–11], call for nationwide evaluations of screening tradeoffs.
The aim of the current work was to determine the rates of screen-detected, interval cancers and cancers diagnosed outside screening over 17 years in Denmark (Copenhagen and Fyn), emphasizing the influence of organizational differences in the screening programs to changes of breast cancer detection patterns in the population.
Material and methods
Setting and study population
In this study we used data from the first eight invitations rounds of the Copenhagen organized screening program (April 4, 1991 to April 14, 2008) and from the first six invitation rounds of the organized screening program in Fyn (November 1, 1993 to December 31, 2005). Both programs offered, by a personal letter, biennial screening to women aged 50 to 69. Screening took place in central clinics, in Fyn supplemented by a mobile van. At first screen two-view mammography was used, at subsequent screens one-or two-view depending on breast density, and from 2004 onwards all women were offered two-view mammography. All mammograms were independently double read by specially trained radiologists. During the study period some organizational and technological changes occurred. The most important being the introductions of high frequency ultrasound devices and stereotactic guided biopsies, of two-view mammography for all screens, and of digital mammography [Citation12].
We distinguished between the target population, all women within the catchment area, target age and time period; the eligible population, target population excluding women ineligible due to breast cancer related reasons; the invited population, eligible population excluding those not invited for other reasons; and the participating population, invited women who participated.
Copenhagen, Target: The Copenhagen program targeted women aged 50–69 at the start of each invitation round and residing in the catchment area at any time during the invitation round. This means that the Copenhagen program targeted fixed birth cohorts in each invitation round. Copenhagen, Eligible: From its implementation, the program aimed at including information from the Danish Breast Cancer Cooperative Group (DBCG) to avoid inviting women operated for breast cancer within the past 18 months. As this register was not fully updated at the time, women operated within the past 18 months were not systematically excluded from invitation. As the program progressed, information was collected on women with breast cancer surgery within the last 18 months, bilateral mastectomy or breast protheses where mammography was technically not possible. Once identified, these women were exempted from invitation. Copenhagen, Invited: Furthermore, women could notify the program if they did not want to be invited. From Copenhagen, invitation data were available.
For the Fyn program some assumptions had to be made as the program did not systematically keep files on targeted and/or invited women. Fyn, Target: The Fyn program targeted women aged 50–69 and residing in the catchment area at the date of invitation. This means that the Fyn program targeted a dynamic population, and this had to be taken into account in identifying the target population. Fyn, Eligible: The Fyn county had its own IT-system, which enabled exclusion from invitation of women with breast cancer during the last five years, and from 1997 also women with excisions from in situ or non-specified neoplasms. Fyn, Invited: As in Copenhagen, women could notify the program if they did not want to be invited. Furthermore, non-participants in the previous round were not invited unless they contacted the program. Invited women with a mammography within the last six months could cancel their screening participation, as they were controlled elsewhere in the health care system.
Data collection
The Danish Civil Registration System (CRS) was used to identify the target population. The CRS is based on the Danish unique identification number. This register is updated every day and includes demographic information, including migration and death, for all persons ever residing in Denmark since 1968. Each of the two programs kept Mammography Registers on the outcome of screening. We furthermore used data from the Danish Cancer Register (DCR), and the DBCG.
Individual screening data were retrieved from the Mammography Registers and linked by use of the unique identification number with the target populations. This linkage allowed us to distinguish between participants and other targeted women. The Mammography Registers included information on date of screening mammogram, outcome of screening and (where relevant) date of screen-detected cancer diagnosis, including carcinoma in situ (CIS) and invasive cancers. Interval cancers were defined as breast cancers diagnosed within two years of a negative screen or before next screen, whichever came first. Interval cancers and cancers among other targeted women were retrieved by merging the Mammography Registers with the DCR, for the identification of invasive cancers and with the DBCG, for the identification of CIS.
Tumor-related information was retrieved from the DCR and DBCG. From DCR, we obtained data on morphology (International Classification of Diseases -10 Classification), and from DBCG we obtained data on tumor size, lymph node involvement, and estrogen receptor (ER) status.
Statistical analysis
For targeted women in each screening round, six outcomes were possible: 1) participation in screening without breast cancer diagnosis; 2) participation with screen-detected breast cancer; 3) participation with interval breast cancers; 4) false positive rate (screen-positive women – screen-detected cases); 5) non-screened women without breast cancer diagnosis and; 6) non-screened women with breast cancer. To take the varying length of invitation rounds into account, we calculated incidence rates for each invitation round. Detection rate in participants was calculated as screen-detected breast cancers divided by person years among participants. Interval cancer rate in participants was calculated as interval cancers divided by person years at risk in up to two years among participants declared negative at screening. Incidence rate in non-screened women was calculated as diagnosed breast cancers in non-screened women divided by the person years accumulated by these women. The women’s screening round was defined as the time (first, second, etc) that she participated in the screening program, regardless of the number of the program’s invitation round. Data were processed using SAS (version 9.2), SPSS (version 18.0) and R statistical software programs (version 2.12.2).
Ethics
Use of screening data and tumor-related information was approved by the Danish Data Inspection Agency (2008-41-2191).
Results
The number of targeted, eligible, invited and participating women presented clear differences in both studied screening programs (). In Copenhagen, the proportion of targeted women identified as non-eligible was small, the proportion of eligible women who had declined invitation was larger, and so was the proportion of invited women who did not participate. By contrast, in Fyn more targeted women were identified as non-eligible, a large proportion of women were not invited because they had declined invitation or because they had not participated previously, and consequently more of the invited women participated in Fyn than was the case in Copenhagen. For these reasons, we present breast cancer rates for participants and non-screened women (including non-eligible, non-invited, and non-participants), respectively.
Table I. Number of target population, eligible population, invited women and participants by invitation round in Copenhagen and Fyn screening programs, Denmark.
From 1991, with the implementation of a breast cancer population-based screening program in Copenhagen, it had accounted 214,088 screening participations and 139,461 targeting of non-screened women. It should be noted that a given woman can have been targeted in more than one invitation round. From 1993 onwards, the Fyn program has accounted 272,634 screening participations and 90,692 targeting of non-screened women ().
Table II Number of breast cancers (invasive and carcinoma in situ) detected at screening in participants, diagnosed as interval cancers in participants or diagnosed in non-screened women. Proportion per 1000 women, and rate per 1000 person years. Copenhagen and Fyn screening programs, Denmark.
The target populations of Copenhagen and Fyn had fairly similar breast cancer incidence rates (). Copenhagen had a clear prevalence peak in screen-detected cancers 5.79 per 1000 person years or 11.88 per 1000 participants. Hereafter the rate decreased, and it remained fairly stable across the subsequent invitation rounds (). The breast cancer incidence in non-screened women showed a pattern similar to that of screened women though at a lower level. From the second invitation round onwards, the incidence rate in non-screened women increased steadily. From invitation round four and onwards, the rates for participating and non-screened women overlapped. The interval cancer rate started at 0.97 per 1000 person years, and this rate increase slightly over time being 1.47 per 1000 person years in the eighth invitation round.
Figure 1. Incidence rates of breast cancer in target population in Copenhagen (1991–2008) and Fyn (1993–2005). Incidence rates of screen-detected breast cancer and interval cancer in participants, and of breast cancers in non-screened women in Copenhagen and Fyn, Denmark.
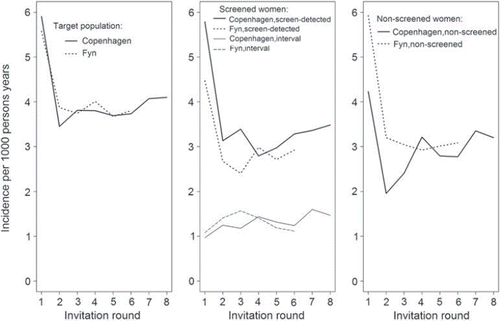
The start of screening in Fyn resulted also in a prevalence peak among participating women at 4.47 per 1000 person years or 9.66 per 1000 participants, where after the incidence decreased and remained fairly stable over time (). A similar pattern was seen for non-screened women. However, in Fyn at the start of screening the incidence in non-screened women was higher than the incidence in participants. From invitation round four and onwards, the two sets of rates overlapped. The interval cancer rate in Fyn started at 1.08 per 1000 person years, increasing to a higher level in the next three invitations round where after it decreased again.
In Copenhagen, interval cancers constitute 25% [555/(1622 + 555)] of breast cancers in participants. Of these, 168 (30%) were detected within 0–11 months of the previous negative screen and the remaining 70% within 12–23 months. In Fyn, interval cancers accounted for 30% [702/(1668 + 702)] of breast cancers in participants, with 31% detected within the first 11 months and 69% within 12–23 months of the negative screen. The division of the breast cancer rate among screened women into screen-detected and interval cancer can be used as an indicator of sensitivity. In the first invitation round of the Copenhagen program this proportion was 86% = 5.79/(5.79 + 0.97). In the subsequent invitation rounds this proportion varied between 66% and 74% with no particular trend. In the first invitation round of the Fyn program the proportion was 81% = 4.47/(4.47 + 1.08), and in subsequent invitation rounds the proportion varied between 61% and 72%, also with no particular trend.
Copenhagen had a high false positive rate of 5.6% during the first invitation round (). This rate decreased over subsequent invitation rounds to stabilize at 1.4–1.5% from the sixth round onwards. Fyn had a much follow false positive rate starting out at 1.7% during the first invitation round, and stabilizing at 0.8% during the last two invitation rounds.
Detection data for participants by women’s screening round showed a peak at first screening followed by a dramatic decrease and a steady increase as the population aged by increasing screen number. The interval cancer rate was stable across women’s screening round ().
Table III. Screen-detected breast cancers and interval cancers. Proportion per 1000 participants by women’s screening round in Copenhagen and in Fyn screening programs, Denmark.
In situ cases constituted 15% of the screen- detected cases in Copenhagen, and 12% in Fyn. Screen-detected cancers showed tumor features related with a better prognosis, as more than 80% were smaller than 20 mm and expressed ER (). The Copenhagen profile of screen-detected cancers was better than the one from Fyn, as 41% in Copenhagen vs. 35% in Fyn were less than/equal to 10 mm. Among interval cancers the proportion of large invasive cancer (> 30 mm) was more than twice as high as in screen-detected cancers but still lower than in non-screened women. In Copenhagen nearly 4% of cases in non-screened women were CIS, while the percentage in Fyn was 6%. In Copenhagen, 78% of interval cancers and 76% of cancers in non-participants were ER positive, these percentages being 76% and 77%, respectively, in Fyn.
Table IV. Comparison of tumor characteristics between Copenhagen and Fyn regions (Denmark), of screen-detected cancers, interval cancers and cancers detected in non-screened women.
Discussion
As expected due to the lead time, the start of organized screening in Denmark resulted in a prevalence peak of screen-detected breast cancers. It was highly surprising, however, that the start of screening resulted also in a “prevalence peak” among non-screened women. In Fyn it was furthermore so that the non-screened women had a higher breast cancer incidence than the screening participants during the first three invitation rounds. Screen-detected tumors in Copenhagen presented a better profile than those detected in Fyn, as 41% in Copenhagen vs. 35% in Fyn were less than/equal to 10 mm, and 15% vs. 12%, were respectively in situ carcinomas.
The strengths of this study were the long study period allowing an overview over nearly 20 years, and the completeness and accuracy of the data. The current work was based on data from organized population-based screening and on a data from a population-based cancer register. This allowed accurate identification of breast cancer cases detected both inside and outside of screening, and the use of unique personal identification numbers ensured complete linkage. All screening data were obtained from computerized records and came from two screening programs in Denmark whose performance indicators reached the European standards [Citation12,Citation14]. Within the Danish health care system women with symptoms of breast cancer can be referred for mammography by their general practitioner. This system was in place already before the organized screening programs started. The use of opportunistic screening mammography has been very limited in Denmark [Citation13].
The study had some limitations as well. First, we could not divide interval cancers into those deriving from false negative screens and those developed after screening, this information would provide better insight into the screening process. Nonetheless, it has been reported from other screening settings that false negative cases account for about one third of interval cancers or less [Citation15–17]. Secondly, misclassification of the detection mode cannot be excluded. Some interval cancers could be classified as screen-detected if symptomatic women waited for the screening visit instead of making an immediate appointment with a physician. This misclassification would attenuate differences on tumor characteristics between screen-detected and interval cancers.
At the start of the Copenhagen program, only women treated for breast cancer within the past 18 months were considered non-eligible, and 95% of the target population was invited. Only 71% of the invited women participated. However, up to now none of the studies from the Scandinavian countries have indicated that non-participants in screening should be particularly burdened by known breast cancer risk factors [Citation7,Citation18]. The “prevalence peak” in non-screened women was therefore unlikely to be explained by a selection bias. A possible explanation for the “prevalence peak” might be increased awareness of breast cancer along with the start of the screening program. Women with symptoms might then have sought help before they were invited to the program. The clear deficit in incidence among non-screened women in the second invitation round speaks in favor of the hypothesis.
The situation at the start of the screening program in Fyn was different. Here, 12% of the targeted women were identified as non-eligible. In addition to women already diagnosed with breast cancer within the last five years, the non-eligible women in Fyn from 1997 onwards included also those with a prior excision of in situ or non-specified neoplasms, and women with a recent mammogram could cancel participation. As women with a previous benign breast lesions are at an increased risk of breast cancer, the non-eligible women thus represented a high risk group. Furthermore, the participation rate among invited women in Fyn was 85%, considerably higher than in Copenhagen. As Fyn is a mixed urban-rural area, and Copenhagen is the capital this difference reflects the well-known urban-rural gradient in screening participation [Citation19]. The high ineligibility rate in Fyn combined with the high participation rate in invited women meant that non-screened women in Fyn formed a selected group at higher risk of breast cancer than the screening participants. Our data thus illustrated that in addition to differences in screening performance, the organizational structure as invitation procedures and the screening setting as participation rate may contribute to variations in breast cancer occurrence both inside and outside of screening.
Throughout the study period, the proportion of ineligible women was higher in Fyn than in Copenhagen. This means that the participants in Fyn formed a breast cancer wise more healthy population than the participants in Copenhagen. Such a selection bias may explain why the cancer detection rate was lower in Fyn than in Copenhagen, particularly in the first three invitation rounds. There might though also be true performance differences between the programs. The Fyn program started later than the Copenhagen program and deliberately aimed at a lower recall rate. As showed previously, this policy resulted in a lower cumulative risk of false positive tests in Fyn than in Copenhagen, being 9% and 16%, respectively [Citation20]. The interval cancer rates were though higher in Fyn than in Copenhagen during the first three invitation rounds. While the specificity seem to be higher in Fyn than in Copenhagen indicated by the lower false positive rates, the sensitivity seem to have been lower indicated by the interval cancer rate. These differences were in particular seen during the first three invitation rounds.
These very different observations from otherwise similar mammography screening programs in a small homogeneous country suggest caution in comparison of screening outcomes from different programs. In the European Guidelines [Citation6] an acceptable detection rate at initial screen is set to two times the population background incidence rate, and to 1.5 for subsequent screens. Our analysis has shown that if certain groups of high risk women are exempted from the programs, then detection rates will not be comparable across programs even when the population background incidence rate is controlled for as suggested in the European Guidelines.
Interval cancer rates were slightly higher than those reported in Sweden [Citation9], The Netherlands [Citation21], Finland [Citation22], Italy [Citation11] and Norway [Citation23], but similar than those found in UK for the 0–24 months after screen [Citation8,Citation24]. Consistent with percentages found in previous works [Citation23,Citation25], nearly 70% of interval cancers appeared during the 12–23 months after the last screening participation. In addition, interval cancers represented 25% of all cancers in screened women in Copenhagen and 30% in Fyn, very similar to percentages reported from Norway [Citation23], Sweden [Citation9] and in a pooled data from six European countries [Citation10], and quite lower than reported from The Netherlands and the UK [Citation21,Citation24]. The higher interval cancer rates reported in the current work reflect the high background incidence of breast cancer in Denmark, but not a poor quality of screening performance.
The trend in screen-detected cancers by women’s screening round resembled data previously reported by Bord s et al. [Citation9]. The interval cancer rate did, however, not increase in subsequent screenings as previously reported by these authors [Citation9]. It could be expected that the sensitivity increased by women’s screening round, as previous mammograms were here available for comparisons. This would result in a decreasing interval cancer rate, but we did not see such a pattern. A possible explanation could be that most interval cancers are fast growing tumors, not visible at previous screen and truly arousing in the screening interval. Some works that carried out a radiological revision of both screening and diagnostic mammograms indicates that around 40% of interval cancers are true interval cancers [Citation15,Citation16].
As expected, pathological characteristics of screen-detected tumors were related to better prognosis. At diagnosis, screen-detected cancers were smaller, more frequently lymph node-negative and showed a higher percentage of tumors expressing ER than clinically-detected tumors. These findings are in line with several publications [Citation10,Citation15,Citation21,Citation23]. Although traditional prognostic factors as tumor size or lymph node involvement indicated worse prognosis among cancers in non-participants than for interval cancers, the closer percentages of tumors lacking ER may be a reflection of the more aggressive molecular pattern associated previously with these cancers, especially when true interval cancers were taken into account [Citation15]. However, since we were not able to differentiate between false negative and true interval cancers, these differences could be attenuated given that all of them were considered together as interval cancers.
In conclusion, in spite of a high breast cancer incidence in Danish women, screening has allowed detection of breast cancers at early stages. Nonetheless, the proportion of breast cancers detected outside screening remained high, and these cases had less favorable prognostic characteristics than the screen-detected cases. Our analysis demonstrated that high data quality is needed for evaluation of mammography screening. All data should be stored with personal identifiers, and all files, including the administrative files on targeted women and invitations, should be kept. While the organized programs in Copenhagen and Fyn operated in fairly similar ways there were nevertheless important differences. Our data illustrated that organizational aspects and screening setting of the programs, as invitation criteria and participation rates, strongly influenced the detection rate which is used as the standard indicator for the outcome of screening. It is therefore pertinent that evaluation of screening starts from the target population, and that the eventual selection in participating women is taken into account in the interpretation of the screening outcome.
Acknowledgements
This study was partially funded by CIBER de Epidemiología y Salud P blica (CIBERESP).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Autier P, Boniol M, La Vecchia C, Vatten L, Gavin A, Hery C, . Disparities in breast cancer mortality trends between 30 European countries: Retrospective trend analysis of WHO mortality database. BMJ 2010;341:c3620.
- Euler-Chelpin M. Breast cancer incidence and use of hormone therapy in Denmark 1978–2007. Cancer Causes Control 2011;22:181–7.
- Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, . The decrease in breast- cancer incidence in 2003 in the United States. N Engl J Med 2007;356:1670–4.
- Bordas P, Jonsson H, Nystrom L, Lenner P. Survival from invasive breast cancer among interval cases in the mammography screening programmes of northern Sweden. Breast 2007;16:47–54.
- Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L, editors. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities; 2006.
- Svendsen AL, Olsen AH, Euler-Chelpin M, Lynge E. Breast cancer incidence after the introduction of mammography screening: What should be expected? Cancer 2006;106: 1883–90.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer 2011;104:571–7.
- Bordas P, Jonsson H, Nystrom L, Lenner P. Interval cancer incidence and episode sensitivity in the Norrbotten Mammography Screening Programme, Sweden. J Med Screen 2009;16:39–45.
- Tornberg S, Kemetli L, Ascunce N, Hofvind S, Anttila A, Seradour B, . A pooled analysis of interval cancer rates in six European countries. Eur J Cancer Prev 2010;19:87–93.
- Bucchi L, Ravaioli A, Foca F, Colamartini A, Falcini F, Naldoni C. Incidence of interval breast cancers after 650,000 negative mammographies in 13 Italian health districts. J Med Screen 2008;15:30–5.
- Utzon-Frank N, Vejborg I, Euler-Chelpin M, Lynge E. Balancing sensitivity and specificity: Sixteen year’s of experience from the mammography screening programme in Copenhagen, Denmark. Cancer Epidemiol 2011;35:393–8.
- Jensen A, Olsen AH, Euler-Chelpin M, Helle NS, Vejborg I, Lynge E. Do nonattenders in mammography screening programmes seek mammography elsewhere? Int J Cancer 2005; 113:464–70.
- Njor SH, Olsen AH, Bellstrom T, Dyreborg U, Bak M, Axelsson C, . Mammography screening in the county of Fyn. November 1993–December 1999. APMIS Suppl 2003; (110):1–33.
- Domingo L, Sala M, Servitja S, Corominas JM, Ferrer F, Martinez J, . Phenotypic characterization and risk factors for interval breast cancers in a population-based breast cancer screening program in Barcelona, Spain. Cancer Causes Control 2010;21:1155–64.
- Hofvind S, Geller B, Skaane P. Mammographic features and histopathological findings of interval breast cancers. Acta Radiol 2008;49:975–81.
- Vitak B, Olsen KE, Manson JC, Arnesson LG, Stal O. Tumour characteristics and survival in patients with invasive interval breast cancer classified according to mammographic findings at the latest screening: A comparison of true interval and missed interval cancers. Eur Radiol 1999;9:460–9.
- Zackrisson S, Andersson I, Manjer J, Janzon L. Non-attendance in breast cancer screening is associated with unfavourable socio-economic circumstances and advanced carcinoma. Int J Cancer 2004;108:754–60.
- Euler-Chelpin M, Olsen AH, Njor S, Vejborg I, Schwartz W, Lynge E. Women’s patterns of participation in mammography screening in Denmark. Eur J Epidemiol 2006;21: 203–9.
- Njor SH, Olsen AH, Schwartz W, Vejborg I, Lynge E. Predicting the risk of a false-positive test for women following a mammography screening programme. J Med Screen 2007;14: 94–7.
- Fracheboud J, de Koning HJ, Beemsterboer PM, Boer R, Verbeek AL, Hendriks JH, . Interval cancers in the Dutch breast cancer screening programme. Br J Cancer 1999;81: 912–7.
- Sarkeala T, Hakama M, Saarenmaa I, Hakulinen T, Forsman H, Anttila A. Episode sensitivity in association with process indicators in the Finnish breast cancer screening program. Int J Cancer 2006;118:174–9.
- Hofvind S, Bjurstam N, Sorum R, Bjorndal H, Thoresen S, Skaane P. Number and characteristics of breast cancer cases diagnosed in four periods in the screening interval of a biennial population-based screening programme. J Med Screen 2006;13:192–6.
- Lawrence G, O’Sullivan E, Kearins O, Tappenden N, Martin K, Wallis M. Screening histories of invasive breast cancers diagnosed 1989–2006 in the West Midlands, UK: variation with time and impact on 10-year survival. J Med Screen 2009;16:186–92.
- Nederend J, Duijm LE, Voogd AC, Groenewoud JH, Jansen FH, Louwman MW. Trends in incidence and detection of advanced breast cancer at biennial screening mammography in The Netherlands: A population based study. Breast Cancer Res 2012;14:R10.