Abstract
Background. Since May 2001, imatinib mesylate has become the first-line therapy for chronic myeloid leukemia (CML) but the survival pattern by age, sex, and ethnicity is not clear. Material and methods. We analyzed the Surveillance, Epidemiology, and End Results (SEER*Stat) database to compare survival rates in CML among Caucasians, African-Americans (AA), and other races, and also within each race to see survival differences from the pre-imatinib (1973–2000) to post-imatinib eras (2002–2008). We used Z-tests in SEER*Stat to compare relative survival rates categorized by race, gender, and age groups (all ages, < 50, 50+ years). Results. The three-year relative survival rates among Caucasians, AA, and other races in the pre-imatinib era were 44.9 ± 0.6%, 46.8 ± 1.8%, and 48.0 ± 2.2%, respectively, and in the post-imatinib era 64.4 ± 0.8%, 67.3 ± 2.4%, and 69.6 ± 1.6%, respectively. The relative survival increased from the pre-to post-imatinib era for all ethnic groups. In the post-imatinib era, three-year relative survival rates among young AA women were significantly lower (Z-value = −2.54, p = 0.011) than young Caucasian women, 80.5 ± 4.5% (n = 105) vs. 90.3 ± 1.4% (n = 589). Conclusions. The relative survival rates of CML patients have improved in the post-imatinib era. However, the improvement in survival rates has been modest in this population-based data compared to those reported from randomized trials. Improvement in survival among older patients is lower than in younger patients. Young (<50 years) AA women with CML had lower relative survival rates compared to young Caucasian women in the post-imatinib era.
Chronic myelogeneous leukemia is a clonal myeloproliferative disorder comprising approximately 15–20% of all leukemias. CML is characterized by a consistent cytogenetic abnormality, the Philadelphia chromosome (Ph). Ph chromosome is the product of the translocation t(9;22) (q34;q11). This results in a unique gene product, BCR-ABL1 fusion, which leads to overactive tyrosine kinase. The deregulated tyrosine kinase activity is the hallmark of CML as it is responsible for myeloid cell proliferation.
Discovery of tyrosine kinase inhibitors has revolutionalized the treatment of CML. Imatinib mesylate (Gleevec®) is the first tyrosine kinase inhibitor approved as the front line treatment of CML since May 2001. This was based on the results of International Randomized study of Interferon and STI571 (IRIS) trial [Citation1]. A recent update of the IRIS trial showed six-year overall survival of 88% in patients with CML treated with imatinib 400 mg daily in the first line setting [Citation2]. Treatment with imatinib is superior to stem cell transplantation as the first line treatment of CML [Citation3]. Little is known about the effectiveness of imatinib in different age groups and ethnic groups. A single institution study showed a possible racial disparity in CML survival in a sample of 26 patients; non-Caucasians reported to have poorer response to treatment with imatinib [Citation4]. However, this has not been verified in a large population based study. This study was conducted to evaluate survival differences in CML by race, gender, and age categories in pre- and post-imatinib eras using the Surveillance, Epidemiology and End Results (SEER) database.
Methods
The Surveillance, Epidemiology and End Results (SEER) Program from the National Cancer Institute is a population-based cancer registry covering more than 25% of the US population across several disparate geographical regions [Citation5]. The SEER Program obtains fairly accurate and complete mortality data by collecting information on all deaths occurring in the US from the National Center for Health Statistics on an annual basis.
Using the April 2011 release of the SEER 17 registries database + hurricane Katrina impacted Louisiana cases, we analyzed the relative survival rates in CML categorized by race, gender, and age [Citation6]. As imatinib was approved in May 2001, patients diagnosed from 1973–2000 and 2002–2008 were categorized into the pre- and post-imatinib eras, respectively. We used April 2001 as the study cut-off, as well as for defining cut-offs for pre-era patients that were lost to follow-up. Patients followed through December 2008 were included for the post-era.
Statistical analysis
Relative survival rates were used as measures of CML survival which measures a net cancer survival in the absence of other causes of death. It has been defined as the ratio of the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in a comparable general population free from the cancer in question. We used Z-tests [Citation7] in SEER*Stat to compare the relative survival rates among Caucasians (CC), African Americans (AA), and other races categorized by gender and age groups. The young and old populations are defined by age groups <50 and 50 + years, respectively. The Ederer II method was used for the expected cumulative survival. All p-values correspond to two-tailed tests, with p < 0.05 considered statistically significant.
Results
Case distribution by age
There were 10744 and 6425 patients diagnosed with chronic myeloid leukemia in the pre- (1973–2000) and post-imatinib (2002–2008) eras, respectively. A comparison of race, gender, and age specific survival rates in the pre- and post-imatinib eras is given in . Patients younger than 50 years represented 28.3% and 30.5% of the sample in the pre- and post-eras, respectively. Proportions of young AA and other race patients presenting with CML were higher compared to young Caucasians in both eras. Similarly, there was a male predominance in each category.
Table I. Demographics and 3-year relative survival for comparison groups in the pre- and post-imatinib eras.
Age category and survival
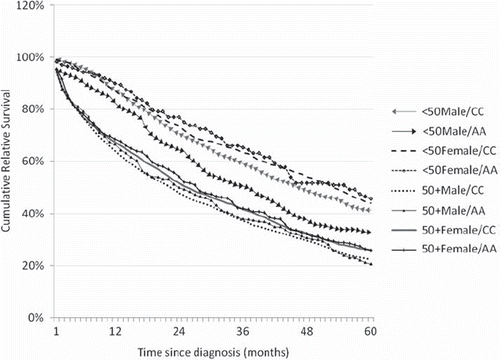
The overall three-year relative survival pooled across age, gender, and race categories increased from 45.2 ± 0.5% in the pre-era to 65 ± 0.7% in the post-era. Similarly, survival rates are estimated at 60.1 ± 1% and 86.7 ± 0.9% for the young patient population and 39.2 ± 0.6% and 55.2 ± 0.9% for the older patient population in the pre- and post-eras, respectively. For each stratified group, the relative survival in the older population was lower compared to the young population in the pre-era, as well as in post-era (); this difference was statistically significant (p < 0.01) for each cohort (). From the pre- to post-eras, relative survival improved and was statistically significant for each cohort (). In the post-era, the highest survival was found among young Caucasian women (90.3 ± 1.4%) and the lowest survival among older men of other race (52.2 ± 5.2%).
Table II. Columns 2 and 3 include Z-value comparing 50+ and <50 cohorts in the pre- and post imatinib era for 3-year relative survival. P-values are suppressed as all of these are significant at <0.01 level. Columns 4 and 5 include Z-value/p-value comparing post- and pre-imatinib eras’ cohorts for 3-year relative survival.
Ethnicity and survival
In post-imatinib era, the three-year relative survival rates among young AA women and young Caucasian women were 80.5 ± 4.5% (n = 105) and 90.3 ± 1.4% (n = 589), respectively (). Thus, the survival rates of young AA women were significantly lower (Z-value = −2.54, p = 0.01) compared to young Caucasian women (, ). The survival rates of the older population were lower than the younger population for each group ().
Figure 1. Cumulative relative survival rates by calendar period of diagnosis in the post-imatinib era (2002–2008).
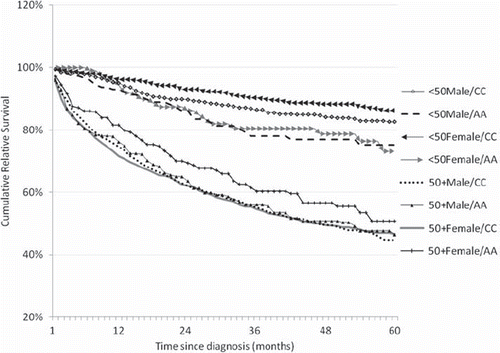
Table III. Comparison between different groups and Z-test values for 3-year relative survival in pre- and post-imatinib eras.
Although the survival rate of young AA men was significantly lower (Z-value = −2.69, p = 0.01) compared to young Caucasian men (, ) in the pre-imatinib era, this difference disappeared in the post-imatinib era.
Discussion
Chronic myeloid leukemia is a clonal hematopoetic stem cell disorder. Based on the results of the IRIS study, imatinib gained FDA approval for treatment of advanced stages of CML in May 2001. In our population based study, survival for CML patients has improved since approval of imatinib. However, the overall three-year relative survival rate of 65% in post-imatinib era is significantly lower compared to the findings of published studies [Citation1,Citation2,Citation8,Citation9]. A recent study from England suggested similar findings of lower efficacy of imatinib in CML in a small population based study [Citation10]. Improvement in survival for patients older than 50 years has been modest (39.2% in the pre-imatinib to 55.2% in post- imatinib era). This is consistent with the findings of other studies [Citation11–13]. The study by Wiggins et al. showed that the poorer survival rates in older patients may be secondary to the lower rate of imatinib use in this population [Citation11]. The barriers of imatinib use in older CML patients may include patient's preference, co-morbid conditions, higher rates of toxicities, among other factors. Further studies may be needed to evaluate these issues. Optimal benefits of tyrosine kinase inhibitors in CML can be obtained by improving their use in older patients who form the bulk of the CML patient population. Younger patients, on the other hand, had excellent three-year relative survival rates (86.7% in post-imatinib compared to 60.1% in pre-imatinib era).
In the post-imatinib era, the relative survival rate among young AA women was significantly lower than young Caucasian women ( and , ). One may argue that socioeconomic status and poor compliance might be factors responsible for this. Since we used aggregate survival rates for comparisons between groups in this study, we could not adjust for socio-economic status (SES). However, this is to some extent internally controlled. For example, we did not observe differences in survival rates for the same cohort of AA patients over a relatively prolonged pre-imatinib era (28 years). Similarly, if SES were important, this would have probably affected survival in other subgroups of AA patients also. Furthermore, survival differences among young Caucasian men and young AA men ( and , ) that existed in the pre-imatinib era appear to have normalized in the post-imatinib era. In addition, the study by Wiggins et al. has shown that imatinib use does not vary significantly by gender, race/ethnicity, socio-economic status, urban/rural residence, number of comorbidities, or medical insurance status in recent years [Citation11]. Thus, one possible explanation for the lower survival among young AA women may be higher rates of imatinib resistance in this group. Socioeconomic status and access to healthcare have been known to impact survival in the past [Citation14,Citation15], which may explain the lower survival in AA men that we observed in the pre-imatinib era.
Conclusions
Even though relative survival rates of CML patients have improved since the use of imatinib, the survival rates seem considerably lower in this population based data compared to those reported from randomized trials. Young (<50 year) African American women with CML had lower three-year relative survival rates compared to young Caucasian women in the post-imatinib era.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003;349:1423–32.
- Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009;23:1054–61.
- Baccarani M, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl 5):v165–7.
- George S, Horvath L, Molokie R, Berry J, Rondelli D, Hoffman R, editors. Response to Therapy with imatinib mesylate in patients with CML is poor in non-caucasian patients. ASH Annual Meeting Abstr; 2004;104:2937.
- Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2003;8:541–52.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub (1973–2008 varying) – Linked To County Attributes – Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission.
- Brown CC. The statistical comparison of relative survival rates. Biometrics 1983;39:941–8.
- Kantarjian HM, Talpaz M, O’Brien S, Jones D, Giles F, Garcia-Manero G, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood 2006;108:1835–40.
- de Lavallade H, Milojkovic D, Khorashad J, Reid A, Olavarria E, Kaeda J, et al. Outcome, prognostic factors and long-term follow-up in 207 chronic phase CML patients receiving front-line imatinib 400 mg at a single institution. ASH Annual Meeting Abstr 2007;110:1045.
- Lucas CM, Wang L, Austin GM, Knight K, Watmough SJ, Shwe KH, et al. A population study of imatinib in chronic myeloid leukaemia demonstrates lower efficacy than in clinical trials. Leukemia 2008;22:1963–6.
- Wiggins CL, Harlan LC, Nelson HE, Stevens JL, Willman CL, Libby EN, et al. Age disparity in the dissemination of imatinib for treating chronic myeloid leukemia. Am J Med 2010;123:764.e1–9.
- Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: Disclosing the impact of advances in therapy on the population level. Haematologica 2008;93:1544–9.
- Thygesen LC, Nielsen OJ, Johansen C. Trends in adult leukemia incidence and survival in Denmark, 1943–2003. Cancer Causes Control 2009;20:1671–80.
- Cella DF, Orav EJ, Kornblith AB, Holland JC, Silberfarb PM, Lee KW, et al. Socioeconomic status and cancer survival. J Clin Oncol 1991;9:1500–9.
- Singh GK, Miller BA, Hankey BF, Edwards BK. Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. NCI Cancer Surveillance Monograph Series. Number 4. Bethesda: National Cancer Institute; 2003.