Abstract
Introduction. The risk of developing side effects after radiotherapy is not only dependent on radiation dose, but may also be affected by patient-related risk factors. Here we perform a literature-based meta-analysis to estimate the effect of various clinical risk factors on the incidence of symptomatic radiation pneumonitis (RP). Material and methods. A systematic review of English language articles in the Pubmed, Embase and Cochrane controlled trials registers. Studies with the mesh term “radiation pneumonitis” or the search term “radiation pneumonitis” were included. Additional studies were identified by manual searching of the references. Studies reporting crude incidence or odds ratios (OR) for radiation pneumonitis vs. age, disease location, smoking status, chemotherapy schedule or comorbidity were included. A systematic overview (meta-analysis) was conducted to synthesize data across multiple studies. Results. Significant risk factors for RP were: older age (OR = 1.7, p < 0.0001); disease located in mid-lower lung (OR = 1.9, p = 0.002); presence of comorbidity (OR = 2.3, p = 0.007). Ongoing smoking was found to protect against RP (OR = 0.6, p = 0.008). History of smoking tended to protect against RP (OR = 0.7, p = 0.06). Sequential (rather than concomitant) chemotherapy scheduling (OR = 1.6, p = 0.01) increased RP risk, but treatment intensity and patients selection are likely confounders. Conclusion. This systematic overview revealed several clinical risk factors for RP that have not been unambiguously identified in the literature. These risk factors should be considered when defining dose-volume constraints for radiation treatment plan optimization.
Comorbidity, lifestyle factors and other patient- related factors may affect the risk of developing side effects after radiotherapy [Citation1–3]. However, the literature is plagued by inconsistent findings across studies and even for relatively frequently reported risk factors there may not be general consensus on their significance. One problem is that studies may not have sufficient statistical power to detect a clinically relevant effect size. Here we propose a meta-analysis approach to synthesize effect estimates of the influence of patient-related risk factors on symptomatic radiation pneumonitis. This approach increases statistical power and gives appropriate weight to studies irrespective of their findings.
RP is a relatively common side effect of radiation therapy for thoracic malignancies. In severe cases, it may become life threatening. Multiple studies have established an association between the risk of RP and dose-volume distribution in the lung [Citation4]. However, the clinical utility of these models is limited by a relatively low predictive power and the generalizability of models from a training data set to a validation data set appears to be poor [Citation5]. Clinical data sets generally have low power to discriminate between alternative dose-volume response models for RP [Citation6].
Most predictive models for RP published to date do not systematically consider clinical and treatment-related risk factors beyond dose and volume. Some risk factors may confound analyses linking risk of RP to radiation dose distribution. We therefore conducted a systematic literature-based overview of clinical factors possibly influencing RP incidence in adults after radiotherapy.
Material and methods
We included studies reporting more than five cases of RP after incidental lung irradiation irrespective of primary cancer histology. This presumes the absence of direct interaction between tumor type and the relative effect of RP risk factors. For each candidate risk factor, the odds ratio (OR) for RP in patients with and without the risk factor is estimated within each study and these estimates are subjected to a meta-analysis across studies. This method is insensitive to differences in absolute RP incidence between studies. No limits were imposed on study design. Meta-analysis of radiation dose as predictor of RP was not performed as this is covered in the recent Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) review [Citation4].
Search strategy and selection criteria
Studies published between 1990 and January 2010 were identified from Pubmed, Embase and the Cochrane controlled trials register using the mesh term “radiation pneumonitis” or the search term “radiation pneumonitis”. The search was restricted to English language articles. Additional studies were sought by manual searching of the references in included studies. Studies allowing extraction of RP ORs with confidence interval (any grade and scale for the definition of RP is accepted) for one or more of the following factors were included: age (younger vs. older), disease location (upper vs. middle or lower location of lung cancer), smoking status (current or former smokers vs. non-smokers), chemotherapy schedule (sequential vs. concomitant), comorbidity (present or not present), involved lung (left vs. right), surgery performed and gender.
Study eligibility was checked by title and abstract, excluding studies of interventions against RP, studies on animals and studies without original data. The full text of included studies was searched for data allowing the extraction of ORs as described above. Finally, possible overlap between patient cohorts in the included studies was checked, using only the most recent data in case of multiple reports from the same study. No attempt was made to contact authors or obtain unpublished data. The study selection procedure was performed by IRV.
The following data were extracted: RP definition, RP incidences vs. risk groups, disease site and number of patients included. For each risk factor, all eligible studies were included.
The analysis was performed using the Review Manager software version 5.0.23 [Citation7]. ORs with confidence intervals were either extracted directly from the original reports or calculated from reported events divided by subjects’ data for most risk factors. Logarithms of ORs were then analyzed with inverse variance weighting. For surgery, gender and chemotherapy schedule, all articles presented events divided by subjects’ data and the pooled estimate of the OR was estimated by the Mantel-Haenszel method.
The odds are defined as the probability of a complication divided by the probability of no complication. The logarithm of the OR is often used in analysis since it offers symmetrical confidence intervals.
A heterogeneity test examines whether the variation in OR among studies can be explained by chance alone. Here, Cochran's Q was applied along with the I2 [Citation7,Citation8]. A significant p-value for Q or a high value of I2 indicates heterogeneity among studies, e.g. due to differences in the patient populations or treatments. Random effects modeling can be used to account for such variation. We use fixed effects modeling below unless otherwise noted.
For each risk factor, ORs were plotted as a function of inverse variance and publication year as a graphical test for publication bias. In addition, funnel plots were inspected qualitatively.
The risk of bias was assessed individually for each potential risk factor and is presented in the discussion. No systematic table or scoring was applied to assess risk of bias.
Results
We identified 1021 studies, with 766 studies remaining after removing duplicates. Of these, 566 were excluded after browsing the abstract using the criteria described above. Seven studies were not retrievable, leaving 193 studies for full text screening. ORs could be estimated from 31 studies for at least one candidate risk factor ().
Table I. Summary of data for the 31 included studies including number of patients, tumor site and definition of RP endpoint.
Five studies (1042 patients) compared RP in patients with left vs. right lung tumors [Citation9–13]; five studies (419 patients) reported on comorbidity [Citation14–18], two looked at general preexisting lung disease [Citation14,Citation15] and three looked at chronic obstructive pulmonary disorder [Citation16–18]. None of these studies attempted to quantify the severity of comorbidity. Six studies (994 patients) reported on RP of tumors located in upper vs. middle and lower lung [Citation5,Citation12,Citation13,Citation16,Citation19,Citation20] while studies using other tumor site comparisons (e.g. upper and middle vs. lower lung) were excluded. Surgery was considered in six studies (800 patients), all reporting on RP according to treatment received, not intention to treat [Citation14,Citation15,Citation17,Citation21–23]. Gender was investigated in 13 studies (1724 patients) [Citation9,Citation12,Citation14,Citation17,Citation21,Citation22,Citation24–31].
Smoking status was analyzed separately for current and former smokers. Seven studies specified incidences of RP in current smokers [Citation10,Citation11,Citation18, Citation21,Citation25,Citation30,Citation32] (1996 patients) and four specified the incidence in patients with a history of smoking [Citation15,Citation18,Citation27,Citation30] in 946 patients. Age group data were found in 13 studies (2186 patients) [Citation11,Citation12,Citation14, Citation15,Citation17,Citation18,Citation21,Citation27,Citation29,Citation33–36]. Different cut-off points were used to define young/old patients, ranging from 57 to 70 years at the time of diagnosis. Since the range is limited and no influence of the actual cut-off point is suspected, we pooled all studies in comparing younger vs. older.
Eight studies (1607 patients) compared concoitant and sequential chemotherapy schedules [Citation12,Citation19,Citation26, Citation27,Citation29,Citation31,Citation37,Citation38]. Unfortunately, drugs varied both between and within studies [Citation26], precluding comparison of chemotherapy agents. Only two studies reported RP according to intention to treat [Citation37,Citation38].
displays forest plots for each analysis with the studies ordered according to increasing statistical power (top to bottom), i.e. by the width of the confidence intervals. Publication bias will be suspected when OR estimates approach unity with decreasing width of the confidence interval. Alternatively, risk of publication bias may be assessed from the funnel plots (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.718093). The risk estimate resulting from the pooled analysis is shown by a diamond-shaped mark where the left and right corners represent the lower and upper 95% confidence limits.
Figure 1. Pooled analysis of potential risk factors for development of radiation induced pneumonitis. The forest plot represents the ORs from a study by a rectangle with size proportional to the study weight and fiducial bars marking the 95% confidence interval. Fixed, fixed effects model; IV, inverse variance weighted; M-H, Mantel-Haenszel method; OR, odds ratio.
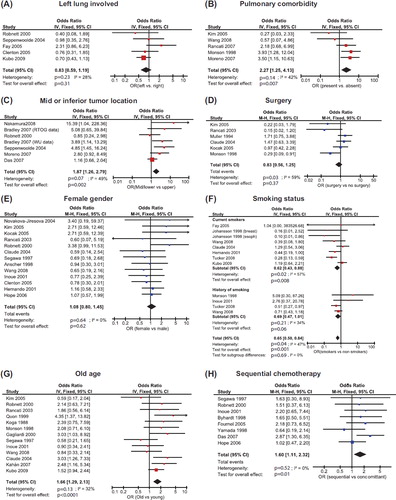
Older age, the presence of comorbidity and tumor location in the middle or inferior part of the lung increase RP risk significantly. In contrast, ongoing smoking is a protective factor and a history of smoking shows a tendency towards protecting against RP. Furthermore, sequential chemotherapy shows a trend towards being associated with higher RP risk than concomitant chemotherapy.
Another potential risk factor, which was not a part of our original analysis or search strategy, is performance status (PS). In the clinical practice, PS is widely used to assess patient’s physical health and potential for severe complications of the treatment. Eleven of the included studies [Citation9,Citation12,Citation14,Citation15,Citation17, Citation18,Citation21,Citation26–29] allowed extraction of events/subjects data stratified by good or bad performance status. We found an OR of 0.74 (95% CI 0.51–1.07, p = 0.11) favoring patients with good performance status (data not shown) when synthesizing these data. The included studies used different scales and cut-points between good and poor PS and there is some heterogeneity in the data (I2 = 48%). Another caveat is that in some series patients with poor PS were more likely to be treated with palliative dose schedules. More studies are warranted on the possible link between PS and radiation pneumonitis.
Discussion
Meta-analysis provides a systematic framework for quantitative synthesis of information from multiple independent studies. Pooling the results of several studies increases statistical power. This is evident in the age comparison, e.g. where only four of 11 studies report a significant or borderline significant risk associated with old age, whereas the pooled analysis demonstrates an effect at the 99.99% confidence level. Older patients may be more likely than younger patients to present with comorbidities, and this may in part explain the excess RP risk. The OR estimate is modest in comparison with many of the OR reported in individual studies that cannot, on their own, demonstrate a significant effect of a risk factor. However, the p-value is highly significant. This again reflects the high power of the analysis. One caveat deserves mention: the lack of a generally accepted a priori cut point for defining young vs. old patients could possibly bias the effect estimate as a result of the individual study authors selecting “optimal” cut-points [Citation39]. In fact, the practice of dichotomizing continuous covariates has been the subject of considerable criticism in recent years [Citation39,Citation40] and is unnecessary for statistical analysis. Biostatisticians’ advice not to dichotomize continuous data, such as age, should therefore be followed in future studies.
One limitation of meta-analysis is that a systematic risk of bias in the included studies may exaggerate or underestimate the effect size and significance of a potential risk factor. Clearly, randomization is impossible for most of the endpoints studied here, and the non-randomized nature and retrospective nature of many of the studies cause a risk of bias due to selective reporting and detection bias as discussed below for the individual risk factors.
Another limitation is the search strategy and selection criteria. As risk factors of RP are often not the primary focus of the original reports it is very difficult to devise an effective search strategy. Here we chose a broad strategy, but limiting studies to more than five events as studies with fewer events in total are unlikely to influence the result. However, assessing the risk of publication bias in this setting is important.
The analysis shows no evidence of involved lung influencing RP incidence. Furthermore, the upper limit of the 95% CI of the OR is very modest at 1.19. Hence, a higher risk of RP in left-sided tumors due to the physiologic link between the lung and the cardiovascular system is not supported by the current literature. Note, however, that Fay et al. is the only multivariate study including a dose descriptor (the volume of lung receiving more than 30 Gy). As this study finds a relatively high OR consistent with a higher risk of RP for left-sided tumors, dose could be a confounder in these analyses. In other words, the non-significant effect of lesion side could be caused by a lower dose to the residual lung in these patients – perhaps due to the physicians actively trying to spare the heart.
Comorbidity increased the risk of RP significantly with no indication of publication bias. Variation in the definition of comorbidity among studies does not cause statistical heterogeneity, probably because COPD was frequent in the two studies considering any preexisting lung disease. Most physicians already use caution when treating patients with preexisting lung conditions. The large point estimate of the OR for RP, however, suggests that more caution is required. Dose constraints derived from unselected patient cohorts may not be safe for patients with preexisting lung conditions.
While lung comorbidity is associated with increased RP risk, no data correlate RP with cardiac comorbidity. Future studies are encouraged to analyze and report RP in cases stratified for cardiac comorbidities, even if the effect is not significant in the individual study.
Several studies have tested the risk of RP vs. location of the lesion, and our analysis shows clear evidence that mid or inferior lung tumors are associated with increased risk of RP compared with upper lung locations (p = 0.002). The definition of position is either based on the lobe or a purely geographical limit. The exact location of the boundary is not expected to influence the OR. The significant heterogeneity is mainly due to the lack of an effect in the large study by Das et al. We did not find an obvious explanation for this discrepancy. Including the study by Das et al. makes the heterogeneity p-value more significant (0.14–0.03) while making the p-value of the overall effect less significant. The overall effect remains significant at the same level in a random effects analysis. OR estimates show a tendency to decrease with increasing study power, which may suggest a publication bias.
Increased RP risk with mid or inferior tumor locations possibly reflect physiological differences, but could also be explained by increased tumor motion during radiotherapy causing more normal lung to be irradiated. Finally, as this analysis is univariate, we cannot rule out that mid or inferior lung lesions can be associated with radiation plans giving larger doses to the normal tissue. This hypothesis could be tested by multivariate analysis including lesion location and more comprehensive dose- volume metrics.
Ongoing smoking protects against RP (p = 0.008) and a history of smoking shows borderline significant protection (p = 0.06). Significant heterogeneity is present in the current-smoker data. Most of the included studies use very low grade RP as endpoint, with only Wang and Tucker using grades higher than one. This could cause added heterogeneity among studies. Note that both studies reporting higher grade RP tend toward smoking being a protective factor and that the dataset by Tucker et al. on its own demonstrates a significant association.
Conceivably, RP symptoms could be masked due to frequent cough or poor general lung function in smokers. However, direct measurement of inflammatory markers in bronchoalveolar lavage fluids demonstrated lower inflammatory radiation response in smokers than in non-smokers [Citation41]. Furthermore, smoking status remained a significant predictive factor in multivariate analysis by Jin et al. [Citation42].
No association of surgery or gender with RP risk was seen. However, the test for study heterogeneity in the surgery vs. RP analysis is significant. This is to be expected since both selection of patients suitable for surgery and the type and outcome of surgery may depend on the hospital as well as the individual surgeon. Random effects analysis does not change the result significantly but widens the OR confidence interval.
Type of surgery was shown to influence the results in a recent study by Albain et al. [Citation43] where the overall survival (OS) was improved in the cohort receiving tri-modality therapy with a radiotherapy dose of 45 Gy followed by lobectomy as compared to chemoradiotherapy alone, whereas the OS was not improved for patients undergoing pneumonectomy. Albain et al. reported general pulmonary toxicity and this study was excluded from the meta-analysis. We note, however, that pneumonitis or other grade 3 or 4 respiratory complications were seen in 9% of the patients in the surgery arm compared to 14% in the chemoradiation alone arm when analyzed by intention to treat. This suggests that if radical operation with lobectomy is possible, it is less likely to cause RP than a course of radical radiotherapy with comparable tumor control. Inclusion of Albain's study in the meta-analysis does not make surgery a protective factor (OR = 0.75, 95% CI 0.53–1.05, p = 0.1. Data not shown).
It may be considered surprising that sequential chemotherapy appears more toxic than concomitant chemotherapy. Protraction of overall treatment time, even of different modalities, would normally be expected to decrease both normal tissue and tumor response. The biological effects of radiation continue for several weeks after the last exposure, so it is indeed likely that chemotherapy/radiotherapy interactions do not disappear by separating the modalities in time. However, the published data may be biased, as concomitant schedules are likely to involve lower intensity chemotherapy than the sequential. Furthermore, the patient cohort receiving sequential chemotherapy likely includes a relatively large proportion of elderly patients, patients with comorbidities etc. in the non-randomized studies. The data are thus to be considered inconclusive, but there is an urgent need to quantify the influence of timing and interactions between chemotherapy and radiotherapy with respect to RP. Our results do, however, indirectly support the current use of concomitant chemotherapy for lung cancer as the documented increase in survival does not appear to be associated with increased pulmonary toxicity. It was not possible to extract the required data from several randomized studies focusing on treatment efficacy, but in an individual patient data meta-analysis of randomized studies concomitant chemotherapy did not increase RP risk (RR = 0.69, p = 0.13 favoring concomitant chemo) [Citation44]. Unfortunately, dose was not included as covariate in this analysis.
Admittedly, publication bias is a concern with any literature-based meta-analysis, but especially in the context of a risk factor analysis. Not all studies include effect estimates for a given risk factor. Therefore, statistically significant factors are more likely to be included in a report than non-significant factors that may be considered unimportant by the study authors. On the other hand, the results extracted for the current analysis are most often not the primary topic of the article and may therefore, potentially, be less susceptible to publication bias. For example, 13 studies report the data on gender vs. RP incidence, but not a single study showed a significant effect of gender on RP risk.
Most publications present univariate analyses only. Our analysis is therefore not capable of taking a potential correlation between clinical risk factors and dose into account. This is unfortunate, as some predictors of RP are likely correlated. For example, tumor location may correlate with radiation dose-volume parameters or the prevalence of comorbidity may correlate with age and smoking status and a surgeon is almost certainly less likely to operate on a patient with COPD thereby causing a selection bias in the analysis. Similarly, compliance to therapy may differ among risk groups. The study of Fay et al., on the other hand, demonstrated multivariate analysis of risk factors and reported their data in a way making it amenable for further analysis in reviews [Citation10]. This level of reporting and the data presentation increases the utility of a published study and is strongly encouraged. Also, risk factors, that do not reach statistical significance in the individual study, should be reported with an associated effect estimate with confidence limits.
The possible consequence of lung DVH as a confounder has been discussed above in relation to the involved lung, location of the lesion and timing of chemotherapy. It is difficult to imagine that old patients, or patients with comorbidity would systematically receive a higher dose to the lung than their healthy/young counterparts. As a result, we expect that, if anything, the significance of these factors should increase when taking the dose distribution into account. Smoking status has been shown to be significant in a multivariate model including dose to the remaining lung [Citation42] suggesting that the result here is not a result of confounding. Finally, we find it unlikely that gender should correlate with dose to the healthy lung.
An individual patient level meta-analysis with detailed three-dimensional (3D) dosimetry would be ideal. However, extraction and processing of the full dose matrix in individual patients remains a major undertaking. The current data can, however, be combined with published dose response data, thus splitting a single dose response curve into two separate curves for patients with/without a given risk factor if the prevalence of that risk factor is known [Citation45]. Improved data banking and more efficient tools for data analysis should now facilitate much more detailed studies of RP and it is reasonable to expect that at least dose is included as a covariate in future studies of potential risk factors. The current method will then be applicable to synthesize data from such multivariate models.
One further barrier for systematic overviews of RP studies is the lack of a consensus on the definition of RP. To compound the misery, the various scales are not directly translatable from one to the other. Finally, some studies restrict the definition of RP to events occurring early, and some studies used a definition of RP including late events [Citation18,Citation28,Citation33]. At least the problem with dichotomizing the five-point toxicity scales can be avoided by the use of polychotomous logistic regression when dose is included as a covariate. With such a method, the response curve for each toxicity grade can be generated from the fit, which includes dose as covariate and is therefore recommended in future studies.
The clinical relevance of the relatively low grade pneumonitis used as endpoint in most studies may be questioned as this is reversible and of limited importance in clinical decision making. In the clinical setting, grade 4–5 pneumonitis is the real issue. It is conceivable that a given risk factor will affect both low and high-grade toxicity [Citation46], but although this assumption is often made in the analysis of treatment effects, it is difficult to validate the assumption from the published data analyzed here.
In almost all clinical cohorts reported, some patients treated with curative intend, will die within the first half year without evidence of disease. That is clinically relevant, because those patients should not have been offered curatively intended treatment and it is of high relevance to study the etiology of these early deaths. However, we believe that at the current stage, a detailed analysis of time to event in a large clinical series is better suited for this kind of study than a literature based meta-analysis.
In conclusion, we have demonstrated a method of pooled analysis of reported toxicity data that makes it possible to synthesize published data across studies to an overall current best estimate of ORs. The analysis shows that several clinical factors influence the risk of RP: Comorbidity (OR = 2.27, p = 0.007); older age (OR = 1.66, p = 0.0001), middle or inferior location of the lesion (OR = 1.87, p = 0.002). Ongoing smoking, on the other hand, is a protective factor (OR = 0.62, p = 0.008) and a history of smoking is borderline significant protective factor (OR = 0.69, p = 0.06). Future studies of RP should include these clinical risk factors together with radiation dose in a multivariate model in order to best improve our understanding of the development of RP.
Supplementary Figure 1
Download PDF (872.9 KB)Acknowledgements
IRV is supported by The Lundbeck Foundation Center for Interventional Research in Radiation Oncology (CIRRO) and The Danish Council for Strategic Research. SMB acknowledges support from the National Cancer Institute grant no. 2P30 CA 014520-34.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Baumann M. Impact of endogenous and exogenous factors on radiation sequelae. In: Dunst JSR, editor. Late sequelae in oncology. Berlin-Heidelberg: Springer Verlag; 1995. pp. 3–12.
- Bentzen SM, Overgaard J. Patient-to-patient variability in the expression of radiation-induced normal tissue injury. Semin Radiat Oncol 1994;4:68–80.
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702–13.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, . Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S70–6.
- Bradley JD, Hope A, El Naqa I, Apte A, Lindsay PE, Bosch W, . A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys 2007;69: 985–92.
- Seppenwoolde Y, Lebesque JV. Partial irradiation of the lung. Semin Radiat Oncol 2001;11:247–58.
- Review Manager (RevMan) [computer program].Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
- Clenton SJ, Fisher PM, Conway J, Kirkbride P, Hatton MQ. The use of lung dose-volume histograms in predicting post-radiation pneumonitis after non-conventionally fractionated radiotherapy for thoracic carcinoma. Clin Oncol (R Coll Radiol) 2005;17:599–603.
- Fay M, Tan A, Fisher R, Mac MM, Wirth A, Ball D. Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:1355–63.
- Kubo A, Osaki K, Kawanaka T, Furutani S, Ikushima H, Nishitani H. Risk factors for radiation pneumonitis caused by whole breast irradiation following breast-conserving surgery. J Med Invest 2009;56:99–110.
- Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000; 48:89–94.
- Seppenwoolde Y, De JK, Boersma LJ, Belderbos JS, Lebesque JV. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:748–58.
- Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee DH, . Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 2005;235:208–15.
- Monson JM, Stark P, Reilly JJ, Sugarbaker DJ, Strauss GM, Swanson SJ, . Clinical radiation pneumonitis and radiographic changes after thoracic radiation therapy for lung carcinoma. Cancer 1998;82:842–50.
- Moreno M, Aristu J, Ramos LI, Arbea L, López-Picazo JM, Cambeiro M, . Predictive factors for radiation-induced pulmonary toxicity after three-dimensional conformal chemoradiation in locally advanced non-small-cell lung cancer. Clin Transl Oncol 2007;9:596–602.
- Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: A retrospective study. Radiother Oncol 2003;67: 275–83.
- Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu C, . Association between systemic chemotherapy before chemoradiation and increased risk of treatment-related pneumonitis in esophageal cancer patients treated with definitive chemoradiotherapy. J Thorac Oncol 2008;3:277–82.
- Das SK, Zhou S, Zhang J, Yin FF, Dewhirst MW, Marks LB. Predicting lung radiotherapy-induced pneumonitis using a model combining parametric Lyman probit with nonparametric decision trees. Int J Radiat Oncol Biol Phys 2007;68:1212–21.
- Nakamura T, Fuwa N, Kodaira T, Tachibana H, Tomoda T, Nakahara R, . Clinical outcome of stage III non- small-cell lung cancer patients after definitive radiotherapy. Lung 2008;186:91–6.
- Claude L, Perol D, Ginestet C, Falchero L, Arpin D, Vincent M, . A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: Clinical and dosimetric factors analysis. Radiother Oncol 2004;71:175–81.
- Kocak Z, Yu X, Zhou SM, D’Amico TA, Hollis D, Kahn D, . The impact of pre-radiotherapy surgery on radiation-induced lung injury. Clin Oncol (R Coll Radiol) 2005; 17:210–6.
- Muller G, Kiricuta IC, Stiess J, Bohndorf W. [Radiation pneumonitis and pulmonary fibrosis after the CT-planned radiotherapy of bronchial carcinoma]. Strahlenther Onkol 1994;170:400–7.
- Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, . Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998;41:1029–35.
- Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, . Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 2001;51:650–9.
- Hope AJ, Lindsay PE, El Naqa I, Alaly JR, Vicic M, Bradley JD, . Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys 2006;65:112–24.
- Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: A retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys 2001;49:649–55.
- Novakova-Jiresova A, Van Gameren MM, Coppes RP, Kampinga HH, Groen HJ. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol 2004; 71:183–9.
- Segawa Y, Takigawa N, Kataoka M, Takata I, Fujimoto N, Ueoka H. Risk factors for development of radiation pneumonitis following radiation therapy with or without chemotherapy for lung cancer. Int J Radiat Oncol Biol Phys 1997;39:91–8.
- Tucker SL, Liu HH, Liao Z, Wei X, Wang S, Jin H, . Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys 2008;72: 568–74.
- Yamada M, Kudoh S, Hirata K, Nakajima T, Yoshikawa J. Risk factors of pneumonitis following chemoradiotherapy for lung cancer. Eur J Cancer 1998;34:71–5.
- Johansson S, Bjermer L, Franzen L, Henriksson R. Effects of ongoing smoking on the development of radiation-induced pneumonitis in breast cancer and oesophagus cancer patients. Radiother Oncol 1998;49:41–7.
- Gagliardi G, Bjohle J, Lax I, Ottolenghi A, Eriksson F, Liedberg A, . Radiation pneumonitis after breast cancer irradiation: Analysis of the complication probability using the relative seriality model. Int J Radiat Oncol Biol Phys 2000;46:373–81.
- Kahan Z, Csenki M, Varga Z, Szil E, Cserháti A, Balogh A, . The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys 2007;68:673–81.
- Koga K, Kusumoto S, Watanabe K, Nishikawa K, Harada K, Ebihara H. Age factor relevant to the development of radiation pneumonitis in radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 1988;14:367–71.
- Quon H, Shepherd FA, Payne DG, Coy P, Murray N, Feld R, . The influence of age on the delivery, tolerance, and efficacy of thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys 1999;43:39–45.
- Byhardt RW, Scott C, Sause WT, Emami B, Komaki R, Fisher B, . Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1998; 42:469–78.
- Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, . Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005;23:5910–7.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829–35.
- Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: An example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol 2011;32:437–40.
- Bjermer L, Franzen L, Littbrand B, Nilsson K, Angstrom T, Henriksson R. Effects of smoking and irradiated volume on inflammatory response in the lung of irradiated breast cancer patients evaluated with bronchoalveolar lavage. Cancer Res 1990;50:2027–30.
- Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, . Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol 2009;91:427–32.
- Albain KS, Swann RS, Rusch VW, Turrisi AT III, Shepherd FA, Smith C, . Radiotherapy plus chemotherapy with or without surgical resection for stage III non- small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009;374:379–86.
- Auperin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, . Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90.
- Appelt AL, Vogelius IR. A method to adjust radiation dose-response relationships for clinical risk factors. Radiother Oncol 2012;102:352–4.
- Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 2007;25: 4096–103.