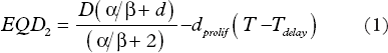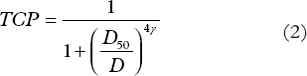Abstract
Purpose. To determine the dose response parameters and the fractionation sensitivity of prostate tumours from clinical results of patients treated with external beam radiotherapy. Material and methods. The study was based on five-year biochemical results from 14 168 patients treated with external beam radiotherapy. Treatment data from 11 330 patients treated with conventional fractionation have been corrected for overall treatment time and fitted with a logit equation. The results have been used to determine the optimum α/β values that minimise differences in predictions from 2838 patients treated with hypofractionated schedules. Results. Conventional fractionation data yielded logit dose response parameters for all risk groups and for all definitions of biochemical failures. The analysis of hypofractionation data led to very low α/β values (1–1.7 Gy) in all mentioned cases. Neglecting the correction for overall treatment time has little impact on the derivation of α/β values for prostate cancers. Conclusions. These results indicate that the high fractionation sensitivity is an intrinsic property of prostate carcinomas and they support the use of hypofractionation to increase the therapeutic gain for these tumours.
The interest in the radiobiology of prostate cancers has increased considerably since the publication of the initial report of Brenner and Hall [Citation1] of a rather low α/β = 1.5 Gy for these tumours. Indeed, according to standard clinical knowledge, a higher fractionation sensitivity than that of normal tissues at risk would suggest that a departure from the conventionally fractionated schedules and the use of higher doses per fraction for these tumours could widen the therapeutic window, leading either to the same tumour control with less complications or to better tumour response for the same level of complications [Citation2]. The original report of a low α/β value for prostate had been based on a comparison of results from low dose rate brachytherapy with those from high dose rate external beam radiotherapy. Not unexpectedly, its results have been disputed on various grounds, such as the relative biological effectiveness of brachytherapy radiation, tumour proliferation, heterogeneity of dose distributions or of tumour cell radiosensitivity, as reviewed by Dasu [Citation3]. Nevertheless, the initial report of a low α/β value for prostates had been followed by an increasing number of publications suggesting that prostate carcinomas might indeed have a high fractionation sensitivity that would favour therapeutic hypofractionation [Citation3–10]. Consequently, several clinical studies exploring the feasibility and effectiveness of hypofractionated schemes have been initiated in recent years [Citation2]. Enough results have now matured to warrant a new evaluation of the clinically relevant α/β value for prostates and this is the aim of the present study.
Material and methods
Clinical studies reporting the outcome of prostate radiotherapy have been identified in the literature using standardised queries or tracking references from relevant papers, as well as in the personal reference database of the authors. From these, only results from studies employing external beam radiotherapy have been selected in order to avoid uncertainties introduced by comparisons with brachytherapy results that employ dose distributions with different heterogeneities. This has in fact been one of the reasons for questioning the original reports of a low α/β for prostate tumours [Citation11]. Furthermore, only studies reporting biochemical tumour control probabilities (TCP) at five years have been retained to minimise heterogeneities. This strategy had allowed the identification of 25 studies encompassing 14 168 patients that have been used for analysis [Citation12–37]. One of these publications reported the results of a hyperfractionated study employing two daily fractions of 1.2 Gy [Citation17]. These results have not been included in the analysis, as it has been argued that incomplete repair between the two daily fractions might interfere with isoeffect calculations [Citation7].
The results of the studies selected for analysis are summarised in for two definitions of biochemical failure, ASTRO and Phoenix. The ASTRO definition of biochemical failure is three consecutive prostate specific antigen (PSA) rises after a nadir with backdating the date of failure. The Phoenix definition of biochemical failure is a rise by 2 ng/ml or more above the nadir PSA without backdating [Citation38]. The ASTRO definition has been criticised for the uncertainties it introduces in patients receiving hormonal treatment, as well as for its dependence on backdating. Nevertheless, it has been extensively used in earlier publications and therefore it was included in a parallel analysis with the Phoenix definition. The results have been analysed not only according to the definition of biochemical failure, but also according to the fractionation schedule and the risk group where this information was available.
Table I. Clinical results from conventionally fractionated studies reporting five-year biochemical control according to the ASTRO definition.
Table II. Clinical results from hypofractionated studies reporting five-year biochemical control according to the ASTRO definition.
Table III. Clinical results from conventionally fractionated studies reporting five-year biochemical control according to the Phoenix definition.
Table IV. Clinical results from hypofractionated studies reporting five-year biochemical control according to the Phoenix definition.
The linear quadratic (LQ) formalism has been used for isoeffective calculations between treatment schedules with various fractionations and durations [Citation39,Citation40]. Thus, the equivalent dose with standard 2 Gy per fraction (EQD2) of a treatment schedule delivering a total dose D in fractional doses d is given by Equation 1.
where α/β is the fractionation sensitivity of the tumour, dprolif is the dose equivalent of proliferation, T is the treatment duration and Tdelay is the time delay before the onset of proliferation. Thames et al. [Citation41] have recently reported clinically relevant dose equivalents of proliferation (mixed risks 0.24 Gy/day, low risk 0.38 Gy/day, intermediate risk 0.14 Gy/day and high risk 0.28 Gy/day) and time delays (Tdelay = 7 weeks) for prostate tumours and these have been used in the present analysis.
Results from clinical studies employing conventional fractionation have been fitted with a logit expression (Equation 2) in order to determine the dose response parameters D50 and γ.
The next step of the analysis was to use the logit parameters to calculate the predicted TCP for each of the hypofractionation schedules under the assumption of various α/β values in Equation 1 and then to calculate the sum of the squares of the differences between the TCP reported in each study and the predicted TCP. This allows the determination of the α/β values leading to the least square deviation between results from conventional fractionation and results from hypofractionation.
Results
shows the dose response curves obtained from schedules with conventional fractionation. The logit parameters for these curves are presented in . It can be seen that the two definitions of biochemical failures lead to different parameters, indicating that they are not equivalent and separate comparisons should be carried out in each group. Furthermore, dose response curves for mixed risks patients are shallower than curves for individual risk groups as the increased patient heterogeneity in this group influences the slope of the dose response curve [Citation42]. Another interesting aspect from the logit analysis of the conventional fractionation schedules is that neglecting the correction for overall treatment time in Equation 1 leads to somewhat shallower dose response curves, but without a significant change of the position of the dose response curve ().
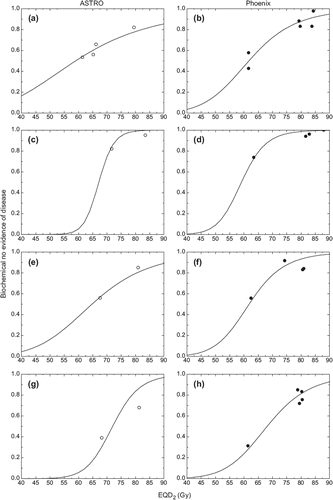
Figure 1. Logit dose response curves obtained from schedules with conventional fractionation. Left panels – ASTRO definition of failure. Right panels – Phoenix definition of failure. a-b mixed risks, c-d low risk, e-f intermediate risk, g-h high risk.
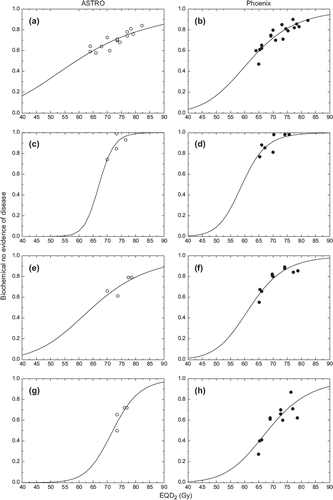
Table V. Logit parameters for the dose response curves in and derived α/β values minimising the sum of squares in .
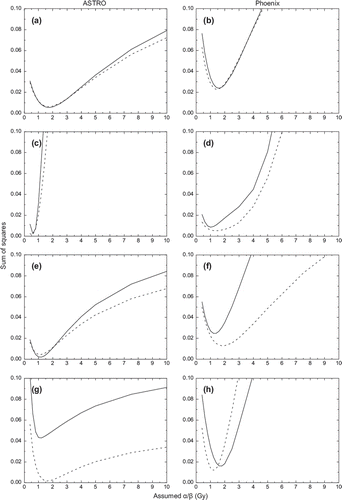
presents the variation of the sum of squares of differences between reported results for hypofractionated schedules and the predicted TCP for various assumptions regarding the α/β value for the individual risk groups considered. It can be seen that for all risk groups the sum of squares is minimised for low α/β values. The optimal α/β values for which the minimum of the sum of squares is achieved for each risk group considered are presented in . These results show that the correction for overall treatment time has a small impact on the derivation of α/β values for prostates.
Figure 2. Variation of the sum of squares with assumptions regarding the α/β value. Left panels – ASTRO definition of failure. Right panels – Phoenix definition of failure. a-b mixed risks, c-d low risk, e-f intermediate risk, g-h high risk. Solid curves – with correction for overall treatment time, dashed curves – without correction for overall treatment time.
shows the relation between dose response curves for conventional fractionation and results from clinical studies employing non-standard fractionation for the optimal α/β values.
Figure 3. Relation between dose response curves for conventional fractionation and results from clinical studies employing non-standard fractionation converted with α/β values leading to minimum values in . Left panels – ASTRO definition of failure. Right panels – Phoenix definition of failure. a-b mixed risks, c-d low risk, e-f intermediate risk, g-h high risk.
Discussion
The fractionation sensitivity of prostates has been a hot topic of debate for the past decade as it has the potential to change the fractionation paradigm in radiation therapy. Current radiotherapy practice assumes that tumours have lower fractionation sensitivity in comparison to late reacting normal tissues, which led to the use of many small fractions to ensure the therapeutic differential between tumours and normal tissues. This certainly is the case for many tumours with an active proliferation pattern. Prostate tumours however have slow proliferation and this raised the question whether their fractionation sensitivity is comparable to or even higher than those of late reacting normal tissues at risk which would in turn favour fractionated patterns employing a few large fractions. Such patterns would be beneficial both to the patients that would face shorter treatments and the radiotherapy departments that could increase their patient throughput. However, the reversal of the fractionation pattern is unsettling given the clinical examples of hypofractionation from other sites that led to an unacceptable rate of late reactions [Citation43]. This is why the initial report of Brenner and Hall [Citation1] of a low α/β value for prostate and all the subsequent proposals have been closely scrutinised for factors that might influence the derivation of the clinically relevant fractionation sensitivity for these tumours. Thus, dose and inter-patient heterogeneity, treatment duration and proliferation, relative biological effectiveness and other factors have been proposed as possible causes of interferences with isoeffect calculations [Citation3]. While attempts were made to carefully account for these factors, it was generally agreed that best results would probably be obtained from randomised studies that minimise the number of differences between the arms of comparison. However, retrospective analyses of data from many patients may offer some advantages, including reducing stochastic fluctuations and the availability of more dose levels that could be used to derive dose response curves.
This study used this latter approach to analyse the results of more than 14 000 patients, making it the largest such analysis this far. Nevertheless, the use of a retrospective analysis approach may be subject to heterogeneity issues and other limitations that need to be taken into account when generalising the applicability of the results. Thus, comparing outcome data across trials increases heterogeneity in the datasets since patients and treatments in different subgroups are not the same. Differences may include risk stratification of the patients from various studies, the availability of adjuvant hormonal therapy that is given to some prostate cancer patients, duration of the follow-up, etc. Indeed, it has previously been shown that androgen suppression therapy may lead to better local control in patients undergoing radiotherapy [Citation44] and therefore better results could be expected from studies employing androgen deprivation in some or all of their patients in comparison to studies refraining from hormonal treatment. However, it has recently been shown that this is not expected to affect the α/β derivation [Citation10]. The duration of the follow-up and its impact on the reported results is another aspect that has to be carefully considered. Thus, Vicini et al. [Citation45] showed that too short follow-ups might lead to the overestimation of the apparent efficacy of the treatments for the ASTRO definition of biochemical failure that relies strongly on backdating. This observation has later been used by Diez et al. [Citation46] to explain the apparently steeper dose response curves that are obtained from non-randomised studies. Thus, they argued that higher dose series tend to have shorter follow-up as they are later introduced and reported and this translates into an overestimation of their efficacy. For these reasons, this study used as many studies as were available and analysed separately the results reported for the two definitions of biochemical failure.
The size and composition of the patient subgroups are other sources of heterogeneity that could influence the results. Thus, controlling a tumour is a process governed by probabilities and therefore subject to stochastic fluctuations, hence results of larger studies are probably less affected by noise than those of smaller studies. The majority of the patients included in the present study (11 330/14 168) were treated with conventional fractionation and this reflects the slow and careful introduction of hypofractionated schedules in clinical trials. The bias towards conventionally fractionated schedules might also translate into somewhat larger confidence intervals ( ± one standard deviation) for the fractionation sensitivities. Risk stratification of the large groups is another aspect that increases the heterogeneity of the analysed data. Indeed, we have earlier shown that the slope of the TCP curves is influenced by heterogeneity in radiosensitivity or population size [Citation42]. Thus, assuming that the various risk groups correspond to tumours with various sizes or intrinsic radiosensitivities that makes them progressively more difficult to control with a given radiation dose, joining all risk groups in an analysis would inevitably lead to shallower dose response curves, as confirmed by the results in where dose response curves for patients with mixed risks are generally shallower than similar curves for each risk group. Furthermore, analysing results originating from patient groups with different compositions (e.g. one with more high-risk patients and one with more low-risk patients) increases the heterogeneity of mixed risks results. It should be noted that stratified data according to risk represents a considerable fraction of the analysed data, but some heterogeneity remains even in the individual risk groups since the criteria for stratification is based on intervals of values for the risk factors. The effect of this type of heterogeneities could only be reduced in randomised studies that control the composition of arms used for comparisons. Nevertheless, the fractionation sensitivity was similar across the various risk groups considered with only a slight correlation between α/β and risk group. This is in agreement with previous findings in smaller patient numbers [Citation10], as well as with analyses of proliferation patterns in individual risk groups [Citation41].
The impact of these sources of heterogeneities could be qualitatively assessed by comparing α/β values obtained in this study from dose response curves with different slopes. Thus, the results in show that similar α/β values were obtained for various risk groups as well as from dose response curves with different slopes, suggesting that heterogeneities have a small impact on the derivation of fractionation sensitivity. Furthermore, the derived α/β values are similar, irrespective of the definition of biochemical failure used for the clinical results. This indicates that the fractionation sensitivity is not an artefact of the definition of failure and that uncertainties associated with the ASTRO definition [Citation38] have little impact on the derivation of the ratio α/β. Nevertheless, failure definitions must be taken into account when analysing clinical data as mixing definitions increases the heterogeneity and decreases the statistical significance of the results.
The logit parameters determined in this study for conventional fractionation schedules are somewhat different from those reported in previous studies [Citation47,Citation48]. The difference could be caused by the inclusion of more results from dose escalation studies in the present analysis that could either decrease the uncertainty at high doses, leading to more relevant dose response parameters, or reflect the heterogeneities mentioned above. Nevertheless, correcting the clinical data sets for overall treatment time during lengthy schedules leads to steeper dose response curves thus indicating further decreases of the uncertainties. Furthermore, dose response curves for the Phoenix definition of biochemical failure are steeper than those for the ASTRO definition, as was also found by Diez and colleagues [Citation46].
The results of this study (α/β = 1–2 Gy) are very close to the initial derivation of Brenner and Hall [Citation1] and other subsequent reports [Citation4–5,Citation8–10] indicating that the practical impact of the potentially adverse factors identified earlier could be smaller than was initially thought. This includes the effect of proliferation of prostate tumour cells, as similar values were obtained when the correction for overall treatment time was taken into account or was neglected. This may also depend on the small values for the dose equivalents of proliferation reported by Thames et al. [Citation41], but these are in agreement with other studies showing variable impact of the overall treatment time for various risk groups [Citation49,Citation50]. It should also be mentioned that calculations have been performed based on the estimated treatment durations, as real treatment times were not available for every patient. Given the low dose equivalents of proliferation, this aspect is however not expected to have a strong impact on the reported results.
The α/β values found in this analysis are generally lower than the fractionation sensitivities of normal tissues previously reported [Citation3]. This suggests that hypofractionation for prostate tumours might bring a therapeutic advantage, especially if combined with better conformal techniques that limit the irradiation of normal tissues. However, there are other reasons for which extreme hypofractionation might fail to bring the expected gain and these should be taken into account when designing the therapeutic schedules. Thus, too few fractions might create problems if hypoxic cells exist in the tumour, as few reoxygenation opportunities would be offered to the cells [Citation51]. Also, too short treatment schedules might prevent compensatory proliferation in acutely reacting tissues, thus increasing the severity of the normal tissue reactions [Citation52]. Nevertheless, studies employing extreme hypofractionations have been initiated and when results will mature the impact of these aspects could be evaluated.
Conclusions
This study presents the largest analysis this far of the fractionation sensitivity for prostate tumours based on clinical results from patients treated with external beam radiotherapy. The analysis yielded very low α/β values for all risk groups and for all definitions of biochemical failures. These results therefore appear to justify the earliest publications that pointed out that α/β values might be as low as approximately 1.5 Gy [Citation1,Citation4]. They also indicate that the high fractionation sensitivity is an intrinsic property of prostate carcinomas and they support the use of hypofractionation to increase the therapeutic gain for these tumours.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–101.
- Dasu A. Radiobiology of prostate cancer. In: Ponsky LE, Fuller DB, Meier RM, Ma C-MC, editors. Robotic radiosurgery. Treating prostate cancer and related genitourinary applications. Berlin: Springer; 2012. pp. 79–101.
- Dasu A. Is the α/β value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol 2007;19:289–301.
- Fowler J, Chappell R, Ritter M. Is α/β for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021–31.
- Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13.
- Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–104.
- Bentzen SM, Ritter MA. The α/β ratio for prostate cancer: What is it, really? Radiother Oncol 2005;76:1–3.
- Williams SG, Taylor JM, Liu N, Tra Y, Duchesne GM, Kestin LL, . Use of individual fraction size data from 3756 patients to directly determine the α/β ratio of prostate cancer. Int J Radiat Oncol Biol Phys 2007;68:24–33.
- Proust-Lima C, Taylor JM, Secher S, Sandler H, Kestin L, Pickles T, . Confirmation of a low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics . Int J Radiat Oncol Biol Phys 2011;79:195–201.
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012;82:e17–24.
- Lindsay PE, Moiseenko VV, Van Dyk J, Battista JJ. The influence of brachytherapy dose heterogeneity on estimates of α/β for prostate cancer. Phys Med Biol 2003;48:507–22.
- Horwitz EM, Hanlon AL, Pinover WH, Anderson PR, Hanks GE. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer 2001;92:1281–7.
- Livsey JE, Cowan RA, Wylie JP, Swindell R, Read G, Khoo VS, . Hypofractionated conformal radiotherapy in carcinoma of the prostate: Five-year outcome analysis. Int J Radiat Oncol Biol Phys 2003;57:1254–9.
- Dearnaley DP, Hall E, Lawrence D, Huddart RA, Eeles R, Nutting CM, . Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005;92:488–98.
- Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Long-term outcomes . Int J Radiat Oncol Biol Phys 2005;63:1463–8.
- Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, . Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 2005;23:6132–8.
- Valdagni R, Italia C, Montanaro P, Lanceni A, Lattuada P, Magnani T, . Is the alpha-beta ratio of prostate cancer really low? A prospective, non-randomized trial comparing standard and hyperfractionated conformal radiation therapy . Radiother Oncol 2005;75:74–82.
- Higgins GS, McLaren DB, Kerr GR, Elliott T, Howard GC. Outcome analysis of 300 prostate cancer patients treated with neoadjuvant androgen deprivation and hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:982–9.
- Peeters ST, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, . Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990–6.
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Konski AA, Movsas B, . Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys 2006;64:518–26.
- Williams SG, Duchesne GM, Gogna NK, Millar JL, Pickles T, Pratt GR, . An international multicenter study evaluating the impact of an alternative biochemical failure definition on the judgment of prostate cancer risk. Int J Radiat Oncol Biol Phys 2006;65:351–7.
- Yeoh EE, Holloway RH, Fraser RJ, Botten RJ, Di Matteo AC, Butters J, . Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: Updated results of a phase III randomized trial. Int J Radiat Oncol Biol Phys 2006;66:1072–83.
- Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, . Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87.
- Eade TN, Hanlon AL, Horwitz EM, Buyyounouski MK, Hanks GE, Pollack A. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys 2007;68:682–9.
- Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys 2007;68:1424–30.
- Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, . Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 2008;71:330–7.
- Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, . Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008;71:1028–33.
- Aizer AA, Yu JB, Colberg JW, McKeon AM, Decker RH, Peschel RE. Radical prostatectomy vs. intensity-modulated radiation therapy in the management of localized prostate adenocarcinoma. Radiother Oncol 2009;93:185–91.
- Goldner G, Bombosch V, Geinitz H, Becker G, Wachter S, Glocker S, . Moderate risk-adapted dose escalation with three-dimensional conformal radiotherapy of localized prostate cancer from 70 to 74 Gy. First report on 5-year morbidity and biochemical control from a prospective Austrian-German multicenter phase II trial. Strahlenther Onkol 2009;185: 94–100.
- Leborgne F, Fowler J. Late outcomes following hypofractionated conformal radiotherapy vs. standard fractionation for localized prostate cancer: A nonrandomized contemporary comparison. Int J Radiat Oncol Biol Phys 2009;74:1441–6.
- Arcangeli G, Saracino B, Gomellini S, Petrongari MG, Arcangeli S, Sentinelli S, . A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2010;78:11–8.
- Buyyounouski MK, Price Jr. RA, Harris EE, Miller R, Tome W, Schefter T, . Stereotactic body radiotherapy for primary management of early-stage, low- to intermediate-risk prostate cancer: Report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Committee. Int J Radiat Oncol Biol Phys 2010;76: 1297–304.
- Rene N, Faria S, Cury F, David M, Duclos M, Shenouda G, . Hypofractionated radiotherapy for favorable risk prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:805–10.
- Alicikus ZA, Yamada Y, Zhang Z, Pei X, Hunt M, Kollmeier M, . Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 2011;117:1429–37.
- Beckendorf V, Guerif S, Le Prise E, Cosset JM, Bougnoux A, Chauvet B, . 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 2011;80:1056–63.
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol 2011;6:3.
- Quon H, Cheung PC, Loblaw DA, Morton G, Pang G, Szumacher E, . Hypofractionated concomitant intensity-modulated radiotherapy boost for high-risk prostate cancer: Late toxicity. Int J Radiat Oncol Biol Phys 2012;82: 898–905.
- Roach III M, Hanks G, Thames Jr. H, Schellhammer P, Shipley WU, Sokol GH, . Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74.
- Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 1982;8:1981–97.
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679–94.
- Thames HD, Kuban D, Levy LB, Horwitz EM, Kupelian P, Martinez A, . The role of overall treatment time in the outcome of radiotherapy of prostate cancer: An analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol2010;96:6–12.
- Dasu A, Toma-Dasu I, Fowler JF. Should single or distributed parameters be used to explain the steepness of tumour control probability curves? Phys Med Biol 2003;48:387–97.
- Cosset JM. Hypofractionnement en radiothérapie: Le retour? Cancer Radiother 2005;9:366–73.
- D’Amico AV, Schultz D, Loffredo M, Dugal R, Hurwitz M, Kaplan I, . Biochemical outcome following external beam radiation therapy with or without androgen suppression therapy for clinically localized prostate cancer. JAMA 2000;284:1280–3.
- Vicini FA, Kestin LL, Martinez AA. The importance of adequate follow-up in defining treatment success after external beam irradiation for prostate cancer. Int J Radiat Oncol Biol Phys 1999;45:553–61.
- Diez P, Vogelius IS, Bentzen SM. A new method for synthesizing radiation dose-response data from multiple trials applied to prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:1066–71.
- Pollack A, Smith LG, von Eschenbach AC. External beam radiotherapy dose response characteristics of 1127 men with prostate cancer treated in the PSA era. Int J Radiat Oncol Biol Phys 2000;48:507–12.
- Cheung R, Tucker SL, Lee AK, de Crevoisier R, Dong L, Kamat A, . Dose-response characteristics of low- and intermediate-risk prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:993–1002.
- Horwitz EM, Vicini FA, Ziaja EL, Dmuchowski CF, Stromberg JS, Gustafson GS, . An analysis of clinical and treatment related prognostic factors on outcome using biochemical control as an end-point in patients with prostate cancer treated with external beam irradiation. Radiother Oncol 1997;44:223–8.
- D’Ambrosio DJ, Li T, Horwitz EM, Chen DY, Pollack A, Buyyounouski MK. Does treatment duration affect outcome after radiotherapy for prostate cancer? Int J Radiat Oncol Biol Phys 2008;72:1402–7.
- Toma-Dasu I, Dasu A, Brahme A. Dose prescription and optimisation based on tumour hypoxia. Acta Oncol 2009;48:1181–92.
- Fowler JF, Harari PM, Leborgne F, Leborgne JH. Acute radiation reactions in oral and pharyngeal mucosa: Tolerable levels in altered fractionation schedules. Radiother Oncol 2003;69:161–8.