Abstract
Background. Carcinosarcomas are a highly malignant type of endometrial carcinomas where extra uterine spread and recurrences are frequent. There is no consensus regarding the best treatment of this group of malignancies. Material and methods. In a complete geographic series of 322 cases of primary uterine carcinosarcomas prophylactic pelvic irradiation and/or chemotherapy was used as postoperative treatment in the majority of the cases. Vaginal brachytherapy was also added as a boost. The primary surgery was extended hysterectomy in 23 cases (10%), and simple hysterectomy in 220 cases (90%). In 46 cases (14%) no major surgery was possible. Results. In the complete series 123 recurrences (38%) were recorded. Locoregional recurrences (11%) and distant recurrences (28%) were most frequent. Type and extent of surgery was not associated with the risk of tumor recurrence. Extended surgery did not reduce the risk of local and regional recurrences. In the complete series, the five-year overall survival rate was 30% and the recurrence-free survival (RFS) rate was 27%. The five-year pelvic disease control was 82% in stage I, 68% in stage II, and 76% for more advanced stages. The five-year locoregional RFS rate was 63% for patients treated with surgery alone, 68% after addition of adjuvant chemotherapy, 86% after adjuvant radiotherapy, and 95% after combined chemotherapy and radiotherapy. Conclusion. Radiotherapy seems to be the most important constituent of the adjuvant therapy. Serious late tissue reactions, requiring surgery, from the bladder and intestine occurred in 2.5% of the irradiated cases. The death of three patients could be related to radiotherapy and of four patients due to the cytotoxic treatment. This population-based series may serve as a baseline for improvements by, e.g. standard care programs and referral to a few specialist centers for this rare and serious disease.
Sarcomas of the uterine body are rare tumors and represent 1% of all gynecological malignancies and 2–5% of all uterine tumors [Citation1,Citation2]. The incidence of uterine sarcomas is reported in the literature to 1.7 per 100 000 women and year [Citation2]. The age of the patients varies with type of histology. Patients with carcinosarcomas are on average older than patients with other histological types of sarcomas. Mixed malignant mesodermal tumors (MMMT; carcinosarcomas) are most frequent followed by leiomyosarcomas (LMS) and endometrial stromal sarcomas (ESS). Other rare types account for less than 5% of the uterine sarcomas. The overall prognosis of primary uterine sarcomas is unfavorable and the five-year survival rate is reported to be 30–40%. Fifty percent of the patients with uterine sarcomas present with FIGO stage I disease at diagnosis [Citation3]. Uterine sarcomas spread hematogenously, and the prognosis in advanced stages is extremely poor. However, the rate of recurrence is also high in early stage disease (FIGO stage I–II) [Citation4,Citation5]. In 35% of the cases the recurrences are localized in the pelvis and in 65% as distant sites. During recent years carcinosarcomas have been reclassified and moved from the uterine sarcoma group to endometrial carcinomas of high-risk type [Citation6].
Surgery is the cornerstone in treatment of uterine sarcomas including carcinosarcomas [Citation7]. Extended, radical surgery is not routine, and the value of pelvic and paraaortic lymphadenectomy is questioned [Citation4]. Residual tumor after surgery is an important prognostic factor [Citation8,Citation9]. The benefits of postoperative radiotherapy and chemotherapy are still under debate [Citation3,Citation7,Citation10–12]. Tumor stage, age of the patient, parity, type of histology and the mitotic count are known prognostic factors [Citation4,Citation13,Citation14]. Depth of myometrial invasion, tumor grade, and lymphovascular space invasion are also significant prognostic factors.
In the present retrospective study of a large series of carcinosarcomas postoperative adjuvant pelvic radiotherapy has been standard and an integrated part of the primary therapy. Chemotherapy has also been used in the adjuvant setting and for advanced tumors, operable and non-operable, in stage III–IV. The clinical outcome is presented with focus on type of adjuvant therapy. Recurrences, progression-free survival, overall survival are presented as well as side effects related to the combined treatment with surgery, pelvic irradiation and/or chemotherapy.
Patients and methods
Two complete geographic series of uterine carcinosarcomas in FIGO stages I–IV, treated during the years 1973–2007 in Gothenburg and Örebro Medical Regions were included in this study, evaluating the type of surgery and postoperative adjuvant radiotherapy and/or chemotherapy. In all, 322 patients were included in the study (). The mean age at diagnosis of the patients was 71.2 years (range, 30–97 years). All histological slides were reviewed at the Departments of Pathology, Gothenburg and Örebro University Hospitals. One hundred and fifty-eight tumors were in FIGO stage I (49%), 39 tumors in stage II (12%), 67 tumors in stage III (21%), and 58 tumors in stage IV (18%). The primary surgery was total abdominal hysterectomy in 220 cases (68%), and Wertheim-Meigs surgery in 23 cases (7%). In 33 cases (10%) only multiple biopsies were taken due to advanced disease (). The surgery was performed at 19 local departments of Gynecology and Obstetrics, but all patients were then referred to the Departments of Gynecological Oncology, Gothenburg or Örebro University Hospitals, for postoperative evaluation and treatment.
Table I. Tumor characteristics of the complete series (n= 322).
Table II. Surgical technique used in the complete series.
The pathology specimens were reviewed at the departments of pathology at the university hospitals of Örebro and Gothenburg. The reference pathologists for gynecological oncology were responsible for this review as for all types of gynecological pathology during this time period. This work was part of the routine pathology and the examinations were mainly based on H/E sections, but immunostaining was also used when regarded as necessary for the correct diagnosis. At the review of this series 330 cases had a localization of the malignancy in the uterine body, and in 322 cases these tumors were classified as carcinosarcomas. The prognostic factors reported for these tumors were: grade of the epithelial component, morphology (polypoid and non-polypoid type), presence of heterologous components, tumor necrosis, myometrial infiltration, lymphovascular space invasion (LVSI), tumor size, DNA ploidy and S-phase fraction. Number of mitotic figures was not available in this series. Since this was a retrospective study data on all prognostic factors were not present in all cases.
The time interval between surgery and external beam radiotherapy was four to eight weeks (median 48 days). The main type of radiotherapy was adjuvant pelvic irradiation. Two or four-field techniques were used. Photon beams were used and given daily, five days a week. The doses and fractionation of radiotherapy are presented in . Most patients treated with external beam therapy also received intracavitary vaginal brachytherapy as a boost to the upper two-thirds of the vaginal walls. The dose per fraction varied from 2.5 to 7.0 Gy (specified at 5 mm below the surface of the vaginal wall). The number of fractions varied from 2 to 6. In 27 patients preoperative intrauterine brachytherapy was given, and in five of these patients postoperative external beam radiotherapy was also administered. Most treatments were given at an outpatient basis.
Table III. Type of radiotherapy, targets and schedules used.
In 92 patients cisplatin-containing chemotherapy was administered. In four patients a taxane was also part of the chemotherapy. Anthracyclines and DTIC were also frequently included in the chemotherapy regimens. Chemotherapy was given as the only postoperative therapy or treatment in 42 patients and in combination with radiotherapy in 50 patients.
All patients were followed up for at least 10 years and no cases were lost to follow-up. The mean follow-up time was 51.4 months (range, 2–189 months) for all patients alive. During all visits symptoms and signs related to the therapy were recorded.
The first follow-up visit was after one month, then every three months during the first year, every four months during the second and third years, and every six months up to five years and then annually up to 10 years. All data were collected in computerized databases at the Department of Gynecological Oncology, Gothenburg or Örebro.
In the statistical analyses, survival curves were generated using the Kaplan-Meier technique and differences were tested with the χ2 or log-rank tests. The χ2-test was also used for comparison of proportions and the independent t-test and ANOVA statistics for comparing means. Logistic regression analysis was used in multivariate analyses of the risk of tumor recurrences and late tissue reactions and Cox proportional regression analysis with regard to survival data. P-values < 0 .05 were regarded as statistically significant. The Statistica (StatSoft, Inc., Tulsa, USA) software package (version 10, 2010) was used for the statistical analyses.
Results
In the complete series of 322 uterine carcinosarcomas 123 recurrences (38%) were recorded. Central pelvic and regional (pelvic lymph nodes) recurrences were recorded in 11%, and distant metastases in 28% (). The median time from treatment to recurrence was 13 months (range, 2–82 months).
Table IV. Sites of recurrences.
Primary surgery with standard hysterectomy was performed in 220 cases and extended surgery (Wertheim-Meigs) was used in 23 cases (7%). In 50 cases (16%) lymphadenectomy or lymph node sampling was performed. In 256 cases bilateral salpingo-oophorectomy was part of the surgical procedure. In 33 cases explorative laparotomy with tissue sampling, but without hysterectomy, was performed.
Type of surgery was not associated with tumor recurrences. After standard hysterectomy the locoregional recurrence rate was 11% and after extended hysterectomy (Wertheim-technique) it was 17%. Distant recurrences were recorded in 31% and 26%, respectively. Lymph node dissection as part of the primary surgery was not associated with recurrence rate or site of recurrences (). Pelvic lymph node dissection was performed in only 50 patients (16%) as part of Wertheim-Meigs surgery, and this extensive procedure was not standard treatment in this series of carcinosarcomas.
Table V. Type of surgery and tumor recurrences.
Presence of positive peritoneal cytology had no influence on the recurrence-free survival (RFS) rate (Cox analysis; p= 0.704). In 149 of 276 (54%) evaluable samples of peritoneal fluid malignant cells were present. In 46 cases no surgery was performed.
Radiotherapy (external beam therapy ± brachytherapy) was administered in 204 cases (63%) all together. In 181 cases (56%) it was as postoperative adjuvant therapy, in 27 cases (8%) as preoperative radiotherapy. In 118 cases (37%) no radiotherapy was given. Pelvic irradiation (anterior-posterior fields or four-field) was the technique used for the adjuvant external beam therapy. Adjuvant vaginal brachytherapy was also used as a boost.
In stage I–II the locoregional recurrence rate was 8% after surgery plus radiotherapy and 19% after surgery alone (χ2 = 2.657; p = 0.103). Tumor recurrences versus radiotherapy or not are presented in . For surgery plus adjuvant chemotherapy the recurrence rate was 14%. Distant metastases were recorded in 31% after radiotherapy alone, and in 33% after radiotherapy plus chemotherapy (χ2 = 0.053; p= 0.817). Age was significantly associated with the risk of all types of tumor recurrences (OR= 1.007; p= 0.00014).
Table VI. Tumor recurrences versus radiotherapy in stages I–II.
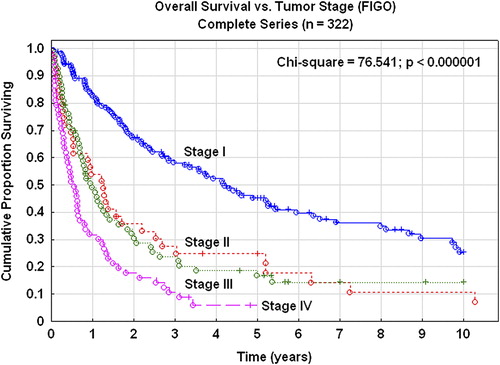
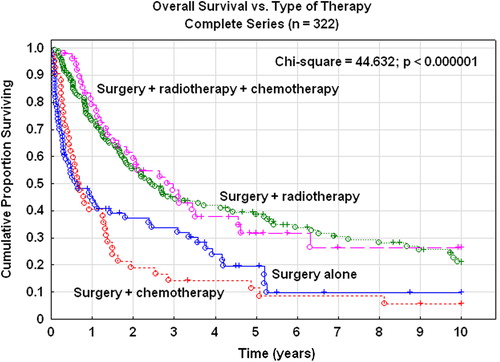
The five-year overall survival rate of the complete series was 30%. In stage I the survival rate was 45% and in stage II 25% (). In the complete series 191 patients (59%) died due to their carcinosarcomas and 52 patients (16%) due to other diseases. The five-year RFS rate was 27%. In stages I–II it was 39%, and in stages III–IV the RFS was only 9%. The five-year pelvic disease control was 82% in stage I, 68% in stage II, and 76% for more advanced stages. Patients treated with adjuvant radiotherapy or radiotherapy plus chemotherapy had a superior overall () and RFS compared with patients treated with chemotherapy alone or no adjuvant therapy (χ2; p< 0 .000001). These significant differences in survival rates were true for the complete series of all stages, but also in stages I–II (χ2; p = 0.032) and stages III–IV (χ2; p< 0 .000001) when analyzed separately.
Combined therapy with radiotherapy and chemotherapy was superior (log-rank test; p= 0.0015) of chemotherapy alone in patients with stage III–IV tumors with regard to RFS. A Cox multivariate proportional regression analysis showed a risk ratio of 0.62 (95% CI 0.46–0.85) when radiotherapy was added to chemotherapy after correction for tumor stage (stage III vs. IV).
In 138 tumors analysis of DNA-ploidy was available and 37% of the carcinosarcomas were diploid and 63% non-diploid (aneuploid or tetraploid). The mean S-phase fraction was 13.2% (95% CI 11.6–14.7). Patients with non-diploid tumors had a significantly (log-rank test; p = 0.040) worse progression-free survival than patients with diploid tumors. However, S-phase fraction and DNA-index did not reach statistical significance (p> 0 .05) in univariate analyses. Number of mitoses was not included in the analysis of this series.
Tumors of the polypoid type had a more favorable prognosis than tumors not showing this characteristic morphology and the risk ratio with regard to RFS was 0.57 (95% CI 0.39–0.83). On the other hand, tumors with a broad base morphology had a risk ratio of 1.60 (95% CI 1.08–2.38) compared with tumors of other types of morphology. Deep myometrial infiltration (> 50 %) (risk ratio 1.93, 95%CI 1.46–2.54) and LVSI (risk ratio 1.49, 95% CI 1.07–2.08) significantly deteriorated the RFS rate. On the other hand, tumors with homologous or heterologous elements (p= 0.605) or presence of necrosis or not (p= 0.274) had similar prognosis. Tumor grade of the epithelial component of the carcinoma had a highly significantly (χ2; p= 0.019) influence on RFS rate.
The medium tumor size of this series was 6.0 cm with a range of 1–18 cm. Tumor size was highly significantly associated with overall recurrence rate (OR= 1.043; p= 0.0043), locoregional recurrence rate (OR= 1.260; p< 0 .000001), and distant recurrence rate (OR = 1.100; p< 0 .000001). Overall survival rate and RFS rate were also significantly (p= 0.023; p= 0.0089) associated with tumor size.
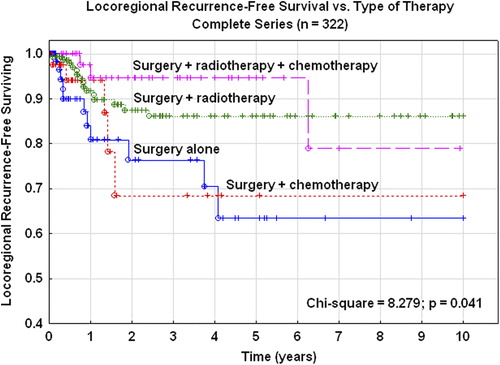
In the complete series the five-year locoregional RFS rate was 63% for patients treated with surgery alone, 68% after adjuvant chemotherapy, 86% after adjuvant irradiation, and 95% after combined chemotherapy and radiotherapy (). These differences were statistically significant (χ2 = 8.279; p= 0.041).
Acute tissue reactions during irradiation were common, e.g. diarrhea, and were recorded in 55% of the cases in the Örebro-series. Late tissue reactions after irradiation (any type and grade) were recorded in 19%. In five cases (2.5%) serious late reactions from the intestine or bladder, e.g. strictures or fistulas requiring surgery, were recorded. In three cases (1.5%) the deaths of the patients were associated with the radiotherapy ().
Table VII. Radiation reactions.
Ninety-two patients received chemotherapy as the only treatment or in combination with radiotherapy. In 62 patients (67%) all cycles of chemotherapy were completed according to the treatment plan. Bone marrow toxicity was infrequent and only five patients discontinued the treatment due to this side effect. Deterioration of the physical status and tumor progression were more common reasons for an incomplete chemotherapy course. In four patients the cytotoxic treatment per se was associated with the death of the patient ().
Table VIII. Cytotoxic side effects.
Discussion
Carcinosarcomas of the uterine body earlier classified to belong to the uterine sarcoma group which is a heterogeneous group of female malignancies with unfavorable prognosis despite the fact that 50% of the cases are diagnosed in stage I. Today, carcinosarcomas belong to the group of high-risk endometrial carcinomas [Citation6]. Interestingly however, a recently published study from Japan showed that gene expression profiles of carcinosarcomas resembled uterine sarcomas more than endometrial carcinomas [Citation15]. Carcinosarcomas are also clinically more aggressive than high-grade endometrial carcinomas. Anyhow, there is lack of standardized treatment consensus for this group of tumors. Surgery is regarded to be the cornerstone in primary therapy, but the extent of surgery and the benefit of lymph node dissection are under debate [Citation16]. The value of adjuvant postoperative therapy is controversial and this is true for both radiotherapy and chemotherapy [Citation12,Citation14]. A number of non-randomized, retrospective studies have proposed a benefit of radiotherapy, especially with regard to local and regional tumor control [Citation3,Citation17–20]. A number of other studies have not shown a convincing improvement in tumor control or survival rate [Citation21]. The role of chemotherapy, both as adjuvant treatment and in combination regimens, has attracted interest during the last years [Citation11,Citation22–24]. However, as for radiotherapy, there are diverging results and conclusions from existing studies, both randomized and non-randomized. The randomized phase III GOG 150 study did not find a statistically significant advantage in recurrence rate or survival for adjuvant chemotherapy (cisplatin-ifosfamid and mesna) over whole abdominal irradiation as post-surgical therapy in stage I–IV carcinosarcomas [Citation24]. Postoperative sequential treatment with chemotherapy and radiotherapy has been proposed [Citation25]. Palliative chemotherapy to patients with advanced, unresectable disease who are symptomatic is a reasonable treatment [Citation26,Citation27]. Uterine carcinosarcomas spread hematogenously, but the rate of locoregional recurrences is also high (30–40%) despite early stages. The concept of pelvic and vaginal irradiation is therefore still of interest and may be further evaluated. Since more than 60–70% of the recurrences are distant metastases adjuvant chemotherapy is also of great interest as single treatment or in combination with radiotherapy. The optimal chemotherapy regimen for carcinosarcomas is not known, but a combination of paclitaxel and carboplatin seems reasonable to use as for other types of high-risk endometrial carcinomas [Citation28].
In the present retrospective study a large series of uterine carcinosarcomas were treated with primary surgery and postoperative adjuvant pelvic radiotherapy and/or chemotherapy as the main line. A smaller number of the patients did not receive any radiotherapy or chemotherapy. Since this study was not randomized this smaller group of patients may not be fully comparable with respect to a number of background factors when compared with the main group receiving adjuvant therapy. Due to primary advanced disease a smaller number of patients did not undergo primary surgery, but only palliative measures, chemotherapy or chemotherapy in combination with radiotherapy. Since this was not a randomized study a mixture of the effects of patient selection and true treatment effects of radiotherapy and chemotherapy limit the possibilities to draw firm conclusions with regard to the optimal treatment of carcinosarcomas. However, we believe that the results from this series can give us a baseline for improvements of the care of this group of patients.
Tumor stage, type of histology, grade of the epithelial component, depth of myometrial infiltration, tumor size, LVSI, and DNA-ploidy were all confirmed to be important prognostic factors in this series. The number of mitosis is also a known prognostic factor in uterine sarcomas and carcinosarcomas. In a prior study from our center [Citation14] the mean number of mitosis was 30 per 10 high-power field (HPF) for carcinosarcomas, which was significantly higher than for leiomyosarcomas and stromal sarcomas. In the present study the number of mitosis was not analyzed. Age was significantly associated with tumor recurrences. Extended surgery according to Wertheim-Meigs seemed to be of no benefit compared with conventional total abdominal hysterectomy. Adjuvant postoperative pelvic irradiation with or without vaginal irradiation was part of standard therapy in this series of patients. The outcome data, e.g. recurrence rate, recurrence-free and overall survival rate compare well with data presented from other studies. The five-year locoregional RFS survival was 63% after surgery alone, but increased to 68% with adjuvant chemotherapy, to 86% with adjuvant radiotherapy, and to 95% with a combination of radiotherapy and chemotherapy. Benoit et al. [Citation7] reported 67% in a series from France. Our results support the importance of radiotherapy, but even better results with a combination of radiotherapy and chemotherapy. Adjuvant treatment with chemotherapy alone gives an inferior locoregional tumor control, not significantly different from surgery alone. With regard to distant metastases no significant differences were noted for the various treatment groups. The addition of chemotherapy to surgery or to surgery plus radiotherapy did not improve the control of recurrences with distant metastases.
However, still the prognosis is poor for this group of patients with uterine carcinosarcomas showing a five-year overall survival rate of only 30%. For high-risk endometrial carcinomas it has been shown that addition of chemotherapy to postoperative pelvic radiotherapy ± vaginal brachytherapy significantly increased the survival rate in a randomized multicenter study. Since carcinosarcomas are thought to belong to endometrial carcinomas and constitute a new high-risk group within this diagnosis a combination of radiotherapy and chemotherapy seems to be a reasonable treatment choice for this group until further studies are available [Citation29].
Radiotherapy increased early and late tissue reactions and side effects but was overall rather well tolerated. In most series 5–10% serious late radiation reactions from bladder and intestine are expected to occur. In our series 19% of the patients reported late tissue reactions, but only 2.5% grade 3–4 lesions, requiring surgery. In three cases with intestinal complications (ileus, perforation and fistula) death may be a result of the radiotherapy administered.
Further studies are needed to define the role of radiotherapy, but also chemotherapy and a combination of both. Pelvic radiation “sandwiched” with ifosfamid-containing chemotherapy has been found to be efficacious for surgically staged carcinosarcomas with no residual disease [Citation30]. More attention must also be paid to vaginal brachytherapy and in some cases external beam therapy may be omitted or perhaps replaced by chemotherapy. Targeted drug therapy [Citation31–33] should be further evaluated in forthcoming trials. Different types of uterine sarcomas must be studied separately and carcinosarcomas, so far, together with high-risk endometrial carcinomas.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours. In: Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003.
- Disaia PJ, Creasman WT. Sarcoma of the uterus. In: Disaia PJ, Creasman WT, editors. Clinical gynecologic oncology. St. Louis: Mosby; 2002.
- Livi L, Paiar F, Shah N, Blake P, Villanucci A, Amunni G, et al. Uterine sarcoma: Twenty-seven years of experience. Int J Radiat Oncol Biol Phys 2003;57:1366–73.
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currei JL, et al. Prognostic factors in early stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 1993;71:1702–9.
- Chi DS, Mychalczak B, Saigo PE, Recigno J, Brown CL. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol 1997;65: 493–8.
- WHO. Pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press;2003.
- Benoit L, Arnould L, Cheynel N, Goui S, Collin F, Fraisse J, et al. The role of surgery and treatment trends in uterine sarcoma. Eur J Surg Oncol 2005;31:434–42.
- Arrastia CD, Fruchter RG, Clark M, Maiman M, Remy JC, Macaset M, et al. Uterine carcinosarcomas: Incidence and trends in management and survival. Gynecol Oncol 1997;65: 158–63.
- Silverberg SG, Major FJ, Blessing JA, Fetter B, Askin FB, Liao S-Y, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 1990; 9:1–9.
- Villena-Heinsen C, Diesing C, Fischer D, Griesinger D, Maas G, Diedrich N, et al. Carcinosarcomas – a retrospective analysis of 21 patients. Anticancer Res 2006;26:4817–23.
- Pautier P, Rey A, Haie-Meder C, Kerbrat P, Dutel JL, Gesta P, et al. Adjuvant chemotherapy with cisplatin, ifosfamide, and doxorubicin followed by radiotherapy in localized uterine sarcomas: Results of a case-control study with radiotherapy alone. Int J Gynecol Cancer 2004;14: 1112–7.
- Odunsi K, Moneke V, Tammela J, Ghamande S, Seago P, Driscoll D, et al. Efficacy of adjuvant CYVADIC chemotherapy in early-stage uterine sarcomas: Results of long-term follow-up. Int J Gynecol Cancer 2004;14: 659–64.
- Nordal RR, Kristensen GB, Stenwig AE, Nesland JM, Pettersen EO, Tropé CG. An evaluation of prognostic factors in uterine carcinosarcoma. Gynecol Oncol 1997;67: 316–21.
- Sorbe B, Johansson B. Prophylactic pelvic irradiation as part of primary therapy in uterine sarcomas. Int J Oncol 2008; 32:1111–7.
- Chiyoda T, Tsuda H, Tanaka H, Kataoka F, Nomura H, Nishimura S, et al. Expression profiles of carcinosarcomas of the uterine corpus – are these similar to carcinoma or sarcoma?Genes Chromosomes Cancer 2012;51:229–39.
- Morice P, Rodrigues A, Pautier P, Rey A, Camatte S, Atallah D, et al. Surgery for uterine sarcoma: Review of the literature and recommendations for the standard surgical procedure. Gyn Obstet Fert 2003;31:147–50.
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol 2004;93:204–8.
- Le T. Adjuvant pelvic radiotherapy for uterine carcinosarcomas in a high risk population. Eur J Surg Oncol 2001;27: 282–5.
- Ferrer F, Sabater S, Farrus B, Guedea F, Rovirosa F, Anglada L, et al. Impact of radiotherapy on local control and survival in uterine sarcomas: A retrospective study from the Group Oncologic Catala-Occita. Int J Radiat Oncol Biol Phys 1999;44:47–52.
- Knocke TH, Kucera H, Dorfler D, Pokrajac B, Potter R. Results of postoperative radiotherapy in the treatment of sarcoma of the corpus uteri. Cancer 1998;83:1972–9.
- Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcomas. Gynecol Oncol 1997;65: 493–8.
- Kushner DM, Webster KD, Belinson JL, Rybicki LA, Kennedy AW, Markman M. Safety and efficacy of adjuvant single-agent ifosfamide in uterine sarcoma. Gynecol Oncol 2000;78:221–7.
- Toyoshima M, Akahira J, Matsunaga G, Niikura H, Ito K, Yaegashi N, et al. Clinical experience with combination paclitaxel and carboplatin therapy for advanced or recurrent carcinosarcomas of the uterus. Gynecol Oncol 2004;94: 774–8.
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcomas (CS) of the uterus. Gynecol Oncol 2007;107:177–85.
- Menczer J, Levy T, Piura B, Chetrit A, Altaras M, Meirovitz M, et al. A comparison between different postoperative modalities of uterine carcinosarcomas. Gynecol Oncol 2005;97: 166–70.
- Kanjeekal S, Chambers A, Fung FMK, Verma S. Systematic therapy for advanced uterine sarcoma: A systematic review of the literature. Gynecol Oncol 2005;97:624–37.
- Galaal K, Godfrey K, Naik R, Kucukmetin A, Bryant A. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcomas. Cochrane Database Syst Rev 2011;19:CD006812.
- Makker V, Abu-Rustum NR, Alektiar KM, Aghajanian CA, Zhou Q, Iasonos A, et al. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I–IV uterine carcinosarcomas. Gynecol Oncol 2008;111:249–54.
- McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer 2002;12:687–90.
- Einstein MH, Klobocista M, Hou JY, Lee S, Mutyala S, Mehta K, et al. Phase II trial of adjuvant pelvic radiation “sandwiched” between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma. Gynecol Oncol 2012;124:26–30.
- Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/ amplification in endometrial carcinosarcomas. Gynecol Oncol 2006;100:101–6.
- Raspollini MR, Susini T, Amunni G, Paglierani M, Castiglione F, Garbini F, et al. Expression and amplification of HER-2/neu oncogene in uterine carcinosarcomas: A marker for potential molecularly targeted treatment?Int J Gynecol Cancer 2006;16:416–22.
- Gurumurthy M, Somoye G, Cairns M, Parkin DE. An update on the management of uterine carcinosarcomas. Obstet Gynecol Surv 2011;66:710–6.