Abstract
Background. The role of tissue inhibitor of metalloproteinases-1 (TIMP-1) in estrogen receptor (ER) positive breast cancer remains to be fully elucidated. We evaluated TIMP-1 as a prognostic marker in patients treated with adjuvant tamoxifen and investigated TIMP-1s association with Ki67 and ER/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2) profiles. Material and methods. TIMP-1 expression was evaluated by immunohistochemistry (IHC) on formalin fixed paraffin embedded primary tumor tissue in two independent cohorts comprised of 236 and 192 patients, respectively. Results. No differences in disease free survival (HR 0.98; 95% CI 0.63–1.53; p = 0.92) and overall survival (HR 0.94; 95% CI 0.63–1.43; p = 0.79) were observed according to TIMP-1 status. A significant negative association between TIMP-1 and Ki67 was identified (p = 0.015). TIMP-1 expression did not differ significantly according to ER/PR/HER2 profiles. When analyzed as separate variables PR and HER2 status tended to have a positive but non-significant association with TIMP-1 (PR: p = 0.08; OR 2.54; 95% CI 0.91–7.10, HER2: p = 0.08; OR 0.48; 95% CI 0.21–1.08) whereas ER status was not associated with TIMP-1 expression (p = 0.48; OR 0.68; 95% CI 0.23–1.99). Conclusion. TIMP-1 does not appear to be prognostic in breast cancer patients receiving adjuvant tamoxifen. We identified a negative association between TIMP-1 and Ki67. We did not confirm our previous in vitro findings of a negative association between TIMP-1 and PR.
Matrix metalloproteinases (MMPs) play an important role in tissue remodeling under physiological conditions and in various disease processes including cancer. The MMPs functions are counterbalanced by tissue inhibitor of metalloproteinases (TIMPs). The TIMP family encompasses four small extracellular proteins, all of which inhibit the enzymatic activity of MMPs. Identification of two functional TIMP-1 receptors has provided new insight into MMP independent functions of this protein. Receptor interaction results in anti-apoptotic activity as well as a growth promoting effect [Citation1]. Notably, a similar growth promoting effect has been shown by synthetic MMP inhibitors [Citation2] whereas others have shown a growth inhibitory effect of TIMP-1 in preclinical cancer models [Citation3].
Estrogen receptor (ER) is a predictive marker for response to endocrine therapy in breast cancer [Citation4]. Since the progesterone receptor (PR) is an ER regulated target gene it has been hypothesized that PR is a marker for a functional ER, hence a tumor highly responsive to endocrine therapy [Citation5]. Consequently, ER+/PR− tumors have been described as more aggressive and resistant to endocrine therapy as compared to ER+/PR+ tumors [Citation5]. PR does, however, not add predictive information on endocrine responsiveness when ER status is known [Citation4]. Loss of PR is associated with increased growth factor signaling, e.g. human epidermal growth factor receptor 2 (HER2) over-expression and activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway [Citation5]. This pathway is also known to be activated by TIMP-1 [Citation6] and we have shown that loss of PR may be induced in vitro by TIMP-1 without concomitant loss of ER (Bjerre et al., unpublished observations). Interestingly, the PI3K/Akt pathway has been implicated in the anti-apoptotic functions of TIMP-1 [Citation6], suggesting that TIMP-1 might protect cancer cells from apoptotic cell death induced by, e.g. tamoxifen [Citation7].
A growth promoting effect of TIMP-1 has been supported by clinical studies demonstrating an association with Ki67 [Citation8] and poor prognosis [Citation8–10]. On the other hand, a growth-inhibitory effect of TIMP-1 has also been supported by a clinical study demonstrating an association between TIMP-1, good prognosis and low Ki67 expression [Citation11]. The purpose of this study was to obtain further understanding of the relationship between TIMP-1 and the effect of endocrine therapy in breast cancer. Here we report on the prognostic value of TIMP-1 and the association between TIMP-1 and Ki67 in breast cancer patients treated with adjuvant tamoxifen and we explore the association between TIMP-1 and ER/ PR/HER2 profiles.
Patients and methods
Patients
This study included two different populations of breast cancer patients registered in the Danish Breast Cancer Cooperative Group (DBCG) database. REMARK recommendations were followed wherever applicable [Citation12].
Cohort I included high risk peri- or postmenopausal women diagnosed with breast cancer between 1989 and 2001. A total of 257 patients from a larger cohort of 589 patients were identified retrospectively according to positive ER status (≥ 1% positive cells) on central review and availability of fresh frozen tumor tissue as previously described in [Citation13]. Nineteen of the 257 patients were HER2 + on central review and excluded from further analysis, leaving a total of 238 patients in cohort I. Patients received adjuvant tamoxifen for up to five years without adjuvant chemotherapy according to DBCG guidelines [Citation14]. Thirty-one patients switched from tamoxifen to an aromatase inhibitor. Radiation therapy was administered according to national guidelines. This cohort was used for the studies on prognosis and the association between TIMP-1 and Ki67.
Cohort II was also retrospectively identified and consisted of 202 breast cancer patients who all underwent surgery between February 2004 and January 2007. Patients were identified among high risk patients (age < 35 years or tumor size > 2 cm, ductal grade II–III, ER negative or axillary lymph node metastases) enrolled in the DBCG 2004 program [Citation14]. Patients had documented distant relapse, death (any cause), or were diagnosed with secondary malignant disease before December 1, 2008 and available ER, PR and HER2 status. In the selection process we intentionally enriched for rare combinations of the biomarkers (ER+/PR−/HER2− and ER+/PR+/HER2+). Patients received adjuvant endocrine therapy and/or chemotherapy and radiation therapy according to national guidelines. We used this cohort to study the associations between TIMP-1 and ER/PR/HER2 profiles.
The studies were approved by the Regional Scientific Ethics Committee for Southern Denmark (S-2008-0125) (Cohort I) and the Committee on Health Research Ethics of the Capital Region of Denmark (H-3-2011-162) (Cohort II) before initiation.
Tissue microarray construction
For both cohorts, archival formalin fixed, paraffin embedded primary tumor tissue were used to construct tissue microarrays (TMAs) comprising two 2 mm cores from each patient as described in [Citation15].
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH)
For cohort I, sections from TMA blocks were stained for ER, PR, Ki67 and HER2 utilizing protocols from daily routine clinical practice. Supplementary Table I includes details on antibodies and assays (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.734922).
Table I. Base-line characteristics of included and excluded patients in cohort I.
HER2 was evaluated according to Herceptest Guidelines; HER2 IHC results scored as 2 + were analyzed by FISH and a HER2-to centromere −17 ratio ≥ 2 was regarded as HER2 gene amplification. In cohort II, information on ER, PR and HER2 status as determined by local pathology laboratories were retrieved in the DBCG database. TIMP-1 was detected using the murine monoclonal antibody VT-7 raised against human TIMP-1 [Citation16].
Assessment of IHC
ER, PR, Ki67, HER2, and TIMP-1 were assessed by pathologists (MSL, BBR, AVL) or a trained observer (KLH). The evaluation was performed blinded without knowledge of patient characteristics and outcomes. ER and PR were assessed as a percentage of stained tumor cells. In cohort I, ER expression was dichotomized as 1–89% and 90–100% and PR dichotomized as negative or positive, where < 1% was considered negative. Due to use of local assessment of ER and PR in cohort II, ER and PR expression < 10% were considered negative in this cohort. Ki67 was assessed as the percentage of stained tumor cells in hot spots in the invasive tumor front and dichotomized as Ki67 low (0–13%) or Ki67 high (14+%) [Citation17]. HER2 IHC was scored according to FDA approved guidelines as 0, 1+, 2+, 3+. TIMP-1 was dichotomized as negative (< 1% stained tumor cells) or positive (≥ 1% stained tumor cells). For exploratory analysis in cohort I, TIMP-1 was also divided according to the percentage of stained tumor cells; score 0 = 0%, score 1 = 1–25%, score 2 = 26–50% and score 3 = 51–100%.
Luminal subtyping by immunohistochemistry in cohort I
As an approximation to intrinsic subtyping, Ki67 was used to determine luminal subtype A and B. Luminal A patients had low (0–13%) Ki67 and luminal B patients had high (14+%) Ki67 [Citation17].
Statistical methods
Cohort I.
Associations between TIMP-1 and clinical/pathological variables were investigated by Fishers exact test and Wilcoxon rank sum test. The primary endpoint was disease-free survival (DFS) defined as time from surgery to an event (local, regional, or distant relapse, second malignancy or death of any cause) or censoring (emigration or alive and disease free at the end of 10 years of follow-up or at cut-off date for analysis April 1, 2008) whichever came first. The secondary endpoint was overall survival (OS) defined as time from surgery to death of any cause with censoring at emigration or at the cut-off date June 1, 2010. Time to event endpoints were analyzed by Kaplan Meier estimates and univariate and multivariate Cox proportional hazards models were applied. Cox models were stratified for allocated duration of tamoxifen treatment. In multivariate models, independent variables were TIMP-1, ER, PR, Ki67, nodal status, age at surgery, tumor size and histological type and grade. The assumption of proportional hazards was assessed by Schoenfeld residuals and hazards ratios (HR) with 95% confidence intervals were reported. In exploratory analysis, ER, PR and Ki-67 status were substituted by luminal subtype and heterogeneity of TIMP-1 effects in the luminal A and luminal B subpopulations were investigated. Analyses on TIMP-1 according to score 0–3 and Ki67 were not pre-specified and considered exploratory.
Cohort II.
Associations between ER/PR/HER2 profiles and TIMP-1, nodal status, age at surgery, tumor size, histological type and grade, and recurrence status at December 1, 2008 were investigated by Fishers exact test and Kruskal-Wallis test. Further unadjusted and adjusted analyses of the association between TIMP-1 and clinical/pathological variables were done by Fishers exact test and by logistic regression. All tests were two sided, p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA)
Results
TIMP-1 immunostaining was successful in 236 (99%) of 238 patients in cohort I. Of the 236 patients, 172 (73%) patients were TIMP-1 positive. Included patients had larger tumors and a higher number of positive axillary lymph nodes than excluded patients (). Median follow-up time for censored patients in cohort I was 10 years for DFS and 13.2 years for OS. Median time on tamoxifen was 1.8 years. TIMP-1 status was not associated to PR, ER, Ki-67, age at surgery, tumor size, nodal status and histological type and malignancy grade () when analyzing TIMP-1 as a dichotomized variable. When evaluating TIMP-1 according to the percentage of stained tumor cells TIMP-1 was negatively associated with Ki67 expression (p = 0.015) () whereas TIMP-1 was not associated with any other variables (data not shown). Kaplan-Meier estimates of DFS according to TIMP-1 status are shown in . For DFS, HR for TIMP-1 negative patients compared to TIMP-1 positive patients was 0.98 (95% CI 0.63–1.53; p = 0.92) in univariate analysis stratified by allocated duration of tamoxifen treatment and consequently no difference in DFS was detected. In multivariate analysis further adjusted for PR, ER, Ki67, nodal status, age at surgery, histological type, grade, and tumor size, TIMP-1 was not a prognostic factor for DFS as HR for TIMP-1 negative patients was 0.90 (95% CI 0.57–1.43; p = 0.66) (). As TIMP-1 was associated with Ki67, the latter was excluded in a supplementary analysis which did not change the result for TIMP-1 (HR 0.95; 95% CI 0.61–1.50; p = 0.83). Overall survival did not differ between TIMP-1 negative and TIMP-1 positive patients in the univariate (HR 0.94; 95% CI 0.63–1.43; p = 0.79) and multivariate analysis (HR 0.84; 95% CI 0.54–1.30; p = 0.42). PR negative patients in this cohort had an increased DFS compared to PR positive patients () but negative PR status was not a prognostic factor for overall survival (HR 0.52; 95% CI 0.25–1.09; p = 0.08). High Ki67 and 10 + positive axillary lymph nodes were strong negative prognostic factors for both DFS () and OS (HR 1.74; 95% CI 1.13–2.60; p = 0.01 and HR 3.32; 95% CI 1.99–5.54; p < 0.0001, respectively).
Figure 1. Kaplan-Meier estimates of disease free survival according to TIMP-1 status (A) and luminal subtype (B), n = 236 and n = 235, respectively (one patient with missing Ki67 score).
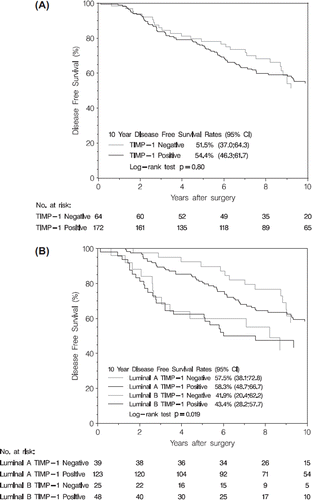
Table II. Clinical and pathological characteristics according to TIMP-1 status, cohort I.
Table III. Association between TIMP-1 and Ki67a.
Table IV. DFS by multivariate analysis in cohort Ia.
We performed an exploratory analysis further adjusted for luminal subtypes. Kaplan-Meier estimates for DFS according to luminal subtype and TIMP-1 status are shown in . In fully adjusted analysis, patients with a luminal A subtype had improved DFS (HR 0.49; 95% CI 0.30–0.7; p = 0.003) compared to patients with luminal B subtype, whereas no prognostic effect of TIMP-1 was found (HR 1.13; 95% CI 0.72–1.79; p = 0.59). There was no heterogeneity of TIMP-1 effect among luminal A and B subtypes (p = 0.52).
In cohort II, TIMP-1 immunostaining was successful in 192 (95%) of the 202 patients and 146 (76%) of those were TIMP-1 positive and equivalent to the findings in cohort I. Due to the selection procedure, ER and PR status were identical (ER+/PR+ or ER−/PR−) in 157 (82%) of the patients and differed (ER+/PR−) in 35 (18%) HER2− patients. TIMP-1 status was not associated to the five ER/PR/HER2 profiles (). In HER2− tumors, absence of TIMP-1 immunostaining was more frequent in ER+/PR− tumors compared to ER+/PR+ tumors (OR 0.36; 95% CI 0.13–0.99; p = 0.066). TIMP-1 status was not associated to triple-negative (ER−/PR−/HER2−) status (OR 1.12; 95% CI 0.52–2.42; p = 0.85) or to hormone receptor status (OR 1.33; 95% CI 0.36–4.89; p = 0.75) in HER2 + tumors. These results were confirmed in logistic regression adjusted for demographic and prognostic variables; In HER2- tumors, TIMP-1 immunostaining was positively associated with ER+/PR+ tumors compared to ER+/PR− tumors (OR 3.11; 95% CI 1.02–9.48; p = 0.047) whereas TIMP-1 status was not associated to triple negative status (OR 1.22; 95% CI 0.60–2.49; p = 0.58) or to hormone receptor status (ER+/PR+ vs. ER−/PR−) in HER2 + tumors (OR 1.52; 95% CI 0.38–6.06; p = 0.17) or to any other variables
Table V. Patient and tumor characteristics according to ER/PR/HER2 profile, cohort II.
When included as separate variables, ER status was not associated to TIMP-1 (p = 0.48, OR: 0.68, 95% CI 0.23–1.99) while PR and HER2 status tended to have an association with TIMP-1 (PR: p = 0.08, OR: 2.54, 95% CI 0.91–7.10, HER2: p = 0.08, OR: 0.48, 95% CI 0.21–1.08).
Discussion
In this study, TIMP-1 did not add independent prognostic information in chemotherapy naïve ER+ patients receiving adjuvant tamoxifen. In the evaluation of TIMP-1 as a prognostic marker in breast cancer, various methodologies have been used. Early studies measured TIMP-1 protein levels in tumor tissue by enzyme linked immunosorbent assay (ELISA) [Citation10], or evaluated mRNA content [Citation9] whereas later studies focused on TIMP-1 protein levels in blood [Citation18,Citation19] or evaluation by IHC [Citation8,Citation11,Citation16]. Most studies find high TIMP-1 protein levels to be associated with poor prognosis whereas only one study reports high TIMP-1 protein levels to be associated with good prognosis [Citation11]. The patient cohorts in these studies were comprised of both ER+ and ER− patients and many received chemotherapy with or without endocrine therapy. In the study by Neri et al. [Citation8], a significant prognostic effect of TIMP-1 is reported. This effect is, however, not present in the subgroup of patients receiving endocrine therapy and as such in line with our findings. To our knowledge, only two publications have specifically investigated the role of TIMP-1 in cohorts of ER+ patients receiving endocrine therapy [Citation18,Citation19]. These studies demonstrated that patients with high TIMP-1 levels in serum or plasma had a reduced response to first or second line endocrine therapy and shorter overall survival. Serum TIMP-1 levels and IHC TIMP-1 levels have been shown to correlate [Citation20], but the studies by Lipton were conducted in patients with metastatic breast cancer of whom many had received prior therapy and thus different from the cohort in our study.
The effects of TIMP-1 studied in an in vitro model system can differ significantly from those observed in vivo and studies suggest that the effects of TIMP-1 are dependent on cellular context and the tumor micro-environment in which they are studied [Citation21,Citation22]. Our previous studies (Bjerre et al., unpublished observations) showing that TIMP-1 induced an ER+/PR− phenotype were carried out in monolayer cell culture with tumor cells only, so in order to see if we could reproduce this finding in a clinically relevant setting, we explored TIMP-1 status according to ER/PR/HER2 profile in cohort II. Our results in cohort II does not support this finding emphasizing that an effect of TIMP-1 identified in a preclinical model not necessarily corresponds with observations in the clinical setting.
As preclinical and clinical studies have found both growth promoting and growth inhibitory functions of TIMP-1, we wanted to investigate the associations between TIMP-1 and Ki67. Our findings suggesting an inverse association between TIMP-1 and Ki67 are in contrast to findings by Neri et al. [Citation8] who report the association for the whole study population and it would have been interesting to see if the association between TIMP-1 and Ki67 also occurred in patients receiving endocrine therapy only, in light of the findings on prognosis discussed above. In our study, a larger number of patients were node-positive and had larger tumors as in the cohort analyzed by Neri et al. The two studies use different antibodies for TIMP-1 and Ki67 and thresholds for positivity. Further, the percentage of TIMP-1 positive patients are quite different and as pointed out by Neri et al. there is a need for standardization of methodologies when evaluating TIMP-1. Our findings on TIMP-1 and Ki67 warrants further studies as we consider our analysis exploratory.
ER+ breast cancer patients may be split according to Ki67 in two prognostically different subtypes, luminal A and B, providing an approximation to intrinsic molecular subtyping [Citation17,Citation23,Citation24]. Having identified the negative association between TIMP-1 and Ki67, we speculated if TIMP-1 could improve prognostic stratification according to luminal subtype. DFS for luminal A patients was significantly better than for luminal B patients, supporting the value of Ki67 as an independent prognostic marker. TIMP-1 status did not provide additional prognostic information, thus supporting our findings in the primary analysis of the prognostic value of TIMP-1 in this cohort.
The strengths of our study on prognosis, TIMP-1, and Ki67 are that we included chemotherapy naïve patients with a long median follow-up time for both DFS and OS (10 and 13.2 years, respectively). On the other hand, the study has limitations. Both cohorts were sampled retrospectively rather than prospectively and our findings on TIMP-1 and Ki67 require validation in an independent cohort. In cohort II we intentionally enriched for rare ER/PR/HER2 profiles which might have introduced bias in our results. We do not find the use of different cut points for ER and PR of clinical relevance in the present study, as only 5% of all breast cancer patients have a borderline ER expression of 1–9% [Citation25]. It was not possible to include an untreated control group in the study on prognosis, and a clearer prognostic effect of TIMP-1 might emerge in comparison with an untreated group and would also allow for evaluation of a potential predictive value of TIMP-1. Patients in cohort I received an inferior endocrine therapy by today's standard as median time on tamoxifen was short (median 1.8 years) and an aromatase inhibitor was not included in the standard regimen.
In conclusion, TIMP-1 was not an independent prognostic marker in this study and we did not observe differences in TIMP-1 status according to ER/PR/HER2 profile. Exploratory analysis revealed a negative association between TIMP-1 and Ki67.
Future studies will be aimed at validating our findings on TIMP-1 and Ki67, investigate the findings on TIMP-1 and luminal subtypes, and evaluate TIMP-1s predictive value.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.734922
Download PDF (160.8 KB)Acknowledgements
The authors wish to thank “Breast Friends” and Danish Cancer Research Foundation for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol 2012;180: 12–6.
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002;295:2387–92.
- Koop S, Khokha R, Schmidt EE, MacDonald IC, Morris VL, Chambers AF, . Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res 1994;54:4791–7.
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011;378: 771–84.
- Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 2005;23:7721–35.
- Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem 2003;278:40364–72.
- Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis 2001;6:469–77.
- Neri A, Megha T, Bettarini F, Tacchini D, Mastrogiulio MG, Marrelli D, . Is tissue inhibitor of metalloproteinase-1 a new prognosticator for breast cancer? An analysis of 266 cases. Hum Pathol 2012;43:1184–91.
- Nakopoulou L, Giannopoulou I, Stefanaki K, Panayotopoulou E, Tsirmpa I, Alexandrou P, . Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol 2002;197:307–13.
- Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, . Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res 2004;10: 2289–98.
- Nakopoulou L, Giannopoulou I, Lazaris AC, Alexandrou P, Tsirmpa I, Markaki S, . The favorable prognostic impact of tissue inhibitor of matrix metalloproteinases-1 protein overexpression in breast cancer cells. APMIS 2003;111: 1027–36.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur J Cancer 2005;41:1690–6.
- Larsen MS, Bjerre K, Giobbie-Hurder A, Laenkholm AV, Henriksen KL, Ejlertsen B, . Prognostic value of Bcl-2 in two independent populations of estrogen receptor positive breast cancer patients treated with adjuvant endocrine therapy. Acta Oncol 2012;51:781–9.
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30–years experience and future promise. Acta Oncol 2008;47:506–24.
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays. J Clin Pathol 2007;60:397–404.
- Willemoe GL, Hertel PB, Bartels A, Jensen MB, Balslev E, Rasmussen BB, . Lack of TIMP-1 tumour cell immunoreactivity predicts effect of adjuvant anthracycline-based chemotherapy in patients (n = 647) with primary breast cancer. A Danish Breast Cancer Cooperative Group Study. Eur J Cancer 2009;45:2528–36.
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes – dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22: 1736–47.
- Lipton A, Leitzel K, Chaudri-Ross HA, Evans DB, Ali SM, Demers L, . Serum TIMP-1 and response to the aromatase inhibitor letrozole versus tamoxifen in metastatic breast cancer. J Clin Oncol 2008;26:2653–8.
- Lipton A, Ali SM, Leitzel K, Demers L, Evans DB, Hamer P, . Elevated plasma tissue inhibitor of metalloproteinase-1 level predicts decreased response and survival in metastatic breast cancer. Cancer 2007;109:1933–9.
- Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, . Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer 2008;122: 2050–6.
- Bigelow RL, Williams BJ, Carroll JL, Daves LK, Cardelli JA. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat 2009; 117:31–44.
- Kuvaja P, Hulkkonen S, Pasanen I, Soini Y, Lehtonen S, Talvensaari-Mattila A, . Tumor tissue inhibitor of metalloproteinases-1 (TIMP-1) in hormone-independent breast cancer might originate in stromal cells, and improves stratification of prognosis together with nodal status. Exp Cell Res 2012;318:1094–103.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, . Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101: 736–50.
- Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, . A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
- Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, . Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol 2012; 30:729–34.