Abstract
Gemcitabine has been the standard chemotherapeutic agent in pancreatic cancer. However, two-thirds of pancreatic tumors display low expression of human equilibrative nucleoside transporter 1 (hENT1), which mediates cellular entry of the drug, and do not respond to gemcitabine therapy. The objective was to determine the costs of gemcitabine overtreatment and the cost-effectiveness of hENT1 testing using a Swedish pancreatic cancer cohort. Material and methods. The study population included 87 patients that were diagnosed with pancreatic cancer during 2008–2010 at Skåne University Hospital, Lund. A detailed review of treatments, side effects and resource utilization was performed. The proportion of hENT1-low was estimated at two-thirds based on previous evaluations of tumor samples from the Radiation Therapy Oncology Group (RTOG) trial 9704, the German AIO Pancreatic Cancer Group (AIO-PK) trial 0104, the Low hENT1 and Adenocarcinoma of the Pancreas (LEAP) trial and the authors’ own institution. The cost of the hENT1 test was estimated at €50–200. Results. Sixty patients received gemcitabine and the other 27 best supportive care. Drug administration and hospitalization were the main expenditures. Grade 3 and 4 toxicities occurred in 42%, the most common being neutropenia (18%). The hospital costs related to gemcitabine overtreatment amounted to €5358 per pancreatic cancer patient, corresponding to as much as one-third of the total treatment cost. The health economical costs amounted to €9449 per patient when including indirect costs. Using hENT1 testing to select patients for gemcitabine therapy would save €8.6 million in Sweden each year. Conclusion. Total costs related to gemcitabine overtreatment were high. Individualizing gemcitabine treatment is cost-saving and would reduce unnecessary treatment-related toxicity.
Pancreatic cancer is a highly lethal malignancy. Due to its infiltrative growth pattern, tendency for distant spread, and the long period of clinical silence prior to symptoms and diagnosis, the prognosis is usually very poor [Citation1,Citation2]. The disease is responsible for an estimated 266 000 deaths per year worldwide and the mortality-incidence ratio is estimated at 0.95 [Citation3]. The only potentially curative treatment is surgical resection, but most tumors are either unresectable or have already metastasized to a distant site at the time of diagnosis, thereby excluding surgical resection with radical attempt [Citation2].
The nucleoside analog gemcitabine (2’,2’-difluoro 2’-deoxycytidine) has been the current first-line chemotherapeutic option in pancreatic cancer. The addition of other cytotoxic agents to gemcitabine has shown only modest survival benefits [Citation2,Citation4]. Gemcitabine is transported into the tumor cell mainly via the human equilibrative nucleoside transporter 1 (hENT1). Albeit other transporters, such as human concentrative nucleoside transporters (hCNT1 and 3), are implicated in gemcitabine uptake [Citation5,Citation6], experimental and clinical data indicate that hENT1 appears to be the main cellular gateway for gemcitabine [Citation7]. In the randomized Radiation Therapy Oncology Group (RTOG) trial 9704 [Citation8], 538 patients were randomized to either gemcitabine or 5-fluorouracil following pancreatic resection with postoperative concurrent chemoradiation. A total of 198 specimens were eligible for hENT1 analysis using immunohistochemistry. It was found that hENT1 expression was associated with increased overall and disease-free survival in patients receiving gemcitabine, but not in the group receiving 5-fluorouracil, implying that hENT1 is a predictive marker of gemcitabine response [Citation9]. Similar findings have been reproduced by others, including patients with unresectable disease [Citation10–13].
Inside the cell, gemcitabine is phosphorylated by deoxycytidine kinase (dCK) to gemcitabine monophosphate and the active metabolites gemcitabine di- and triphosphate (dFdCDP and dFdCTP), which exert cytotoxic effects through several mechanisms. dFdCTP can be incorporated into DNA or RNA, inhibiting tumor growth. Alternatively, the diphosphate intermediate can arrest tumor growth by targeting ribonucleotide reductase, and thereby reduces the availability of deoxyribonucleotide pools essential for DNA synthesis. Gemcitabine is considered to be inactivated by deoxycytidine deaminase to 2,2’-difluorodeoxyuridine (dFdU). The monophosphate of gemcitabine can also be deaminated by deoxycytidylate (dCMP) deaminase, resulting in accumulation of 2´,2´-difluorodeoxyuridine monophosphate (dFdUMP), a thymidylate synthase inhibitor [Citation14].
A number of variables affect the cellular sensitivity to gemcitabine. Differences in expression levels of uptake receptors such as hENT1, but also variations in metabolic enzymes and target molecules are important for drug sensitivity [Citation15]. For example, deficient dCK activity and overexpression of ribonucleotide reductase have been associated with increased resistance to gemcitabine [Citation16,Citation17]. Intracellular signaling pathways, most notably the Akt-pathway, may also be involved in gemcitabine chemoresistance [Citation18]. Drug resistance may be acquired during gemcitabine treatment or may represent an intrinsic property of the tumor cells.
Approximately 65% of all pancreatic ductal adenocarcinomas demonstrate low hENT1 expression. This estimate is based on an immunohistochemistry in vitro diagnostic assay using 363 retrospectively collected tumor samples from the RTOG 9704 study, the German AIO Pancreatic Cancer Group (AIO-PK 0104) study and the authors’ own institution () [Citation19]. The hENT1 scoring algorithm accurately identified patients least likely to benefit from gemcitabine therapy (hENT1-high vs. -low; HR = 0.58, p = 0.018). Further, the predictive value of the hENT1 assay was confirmed by showing no association with clinical outcome in the 5FU-treated control group (hENT1-high vs. –low HR = 0.92, p = 0.7). The distribution of hENT1 expression was similar between these series and 241 metastatic biopsy specimens from the Low hENT1 and Adenocarcinoma of the Pancreas (LEAP; NCT 01124786) trial (64% hENT1 low) [Citation20]. Thus, two-thirds of pancreatic cancer patients receiving gemcitabine may get unnecessary and ineffective treatment with side effects and potential economic consequences. The objective of this study was to determine the total costs associated with gemcitabine overtreatment in a Swedish setting, as well as assess the cost-effectiveness of testing for hENT1 before initiating gemcitabine therapy.
Table I. Two-thirds of primary and metastatic tumor tissues express low levels of hENT1 [Citation19].
Patients and methods
A retrospective cohort study design was used that included all patients who were diagnosed with pancreatic ductal adenocarcinoma between 1 January 2008, and 31 December 2010 at Skåne University Hospital, Lund, Sweden. The human ethics committee at Lund University approved the study (EPN dnr 2010/298). Records from the emergency, surgical and oncological departments were retrieved. Cases were identified by International Classification of Diseases, Tenth Revision (ICD-10), diagnostic code C25. Only patients within the primary catchment population of the hospital (approximately 300 000) were included and all referrals were excluded. A total of 87 patients with pancreatic cancer were identified during the study period, corresponding to an annual incidence of 9.7 per 100 000 citizens. Surgery was performed in 17 patients, including pancreatic resection in eight patients and palliative gastrointestinal procedures in nine patients. Gemcitabine was administered in 60 patients and 27 patients were treated with best supportive care.
Gemcitabine treatment
Baseline evaluations for each patient included medical history, physical examination, Eastern Cooperative Oncology Group (ECOG) performance status, blood chemistries and computed tomography (CT) evaluations of the chest and abdomen. Gemcitabine was delivered intravenously as a 30 minute infusion at a dose of 1000 mg/m2, given as adjuvant for six months on days 1, 8, and 15 every 28 days or for three months in palliative patients. Doses of gemcitabine were reduced to 75% when the neutrophil count was in the range of 0.5–0.9 × 109/L, or if the platelet count was in the range of 50–99 × 109/L. The treatment was postponed if the neutrophil count was < 0.5 × 109/L or if the platelet count was < 50 × 109/L. During therapy, all patients visited an oncologist regularly for toxicity evaluations and assessment of disease status. Treatment was discontinued at signs of disease progression on radiological imaging, unacceptable toxicity or on patient's or doctor's request. The National Cancer Institute (NCI) Common Toxicity Criteria scale (updated to version 4.0) was used to grade gemcitabine-related side effects.
Costs
Hospital costs per patient were calculated, including expenses for the chemotherapeutic agent, vascular access, outpatient visits, ward days and radiological investigations. These costs include the primary treatment, but also chemotherapy-related side effects. Sick leave days were retrieved from the medical charts. According to the National Social Insurance Office [Citation21] the cost of sick leave was €82 per day and the subsequent loss of production was estimated at €266 per day according to health economists [Citation22]. We assumed that a hENT1 test would cost €50–200 (not including the cost for the automatic slide staining platform), given the costs of other commercially available immunohistochemistry kits, e.g. HercepTest for determining HER2 status in breast and gastric cancer [Citation23,Citation24]. We also made the assumption that patients receiving best supportive care would not need to be tested for hENT1. The internal price list of Skåne University Hospital was employed to translate resource use into cost [Citation23]. All costs were calculated in Swedish krona (SEK) and converted into € using the yearly average exchange rate for 2011 (€1 = 9.0287 SEK).
Statistical analysis
Data were analyzed using SPSS version 19 (IBM Corp., Somers, NY, USA) and are presented as median with range for continuous variables and frequencies and proportions for categorical variables. Mann-Whitney U-test was used to make between-group comparisons of continuous data and χ2-tests for categorical data. Survival was analyzed with Kaplan-Meier analysis and the log-rank test. Significance was assumed where p < 0.05.
Results
The baseline characteristics of patients are summarized in . The median age was 69 (46–82) years in the gemcitabine group and 79 (55–92) years for best supportive care (p < 0.001). Significantly more patients in the gemcitabine group had ECOG performance status 0 to 1 (p = 0.004). Fewer invasive procedures (diagnostic and therapeutic) were performed in patients receiving best supportive care. Median survival from initial diagnosis was 10.2 months in the gemcitabine group and 3.3 months in patients receiving best supportive care (p < 0.001), though the selection bias was considerable.
Table II. Characteristics of the study population.
A total of 632 gemcitabine infusions (median 9, range 1–23) were administered. Second-line chemotherapy was administered in 14 patients (23%) consisting of 5-fluorouracil and folinic acid and oxaliplatin (FLOX) in 11 patients, 5-fluourouracil and folinic acid in two patients and capecitabine in one patient. Adverse events during the treatment with gemcitabine were analyzed. details the grade 3 and 4 toxicities. Overall, gemcitabine therapy was well-tolerated, although severe toxicities were observed in 42%. Neutropenia (18%) was the most common individual toxicity event. Non-hematological toxicities were experienced in 27%. Nineteen patients (32%) required hospitalization at the oncological ward either for in-hospital administration of gemcitabine therapy or for management of side effects. Twenty patients were under the age of 65 years (median age of 59 years). Eleven of these were eligible for sick leave, while nine were ineligible due to early retirement. The number of utilized sick leave days could be retrieved in 10 patients. A total of 1534 sick leave days were used with a median of 101 (63–396) days per patient.
Table III. Grade 3 and 4 toxicities of gemcitabine.
Cost of overtreatment and the impact of hENT1 testing
The resource utilization in the gemcitabine group is shown in . The major hospital expenses were cost of chemotherapy administration and hospitalization. Assuming that two-thirds of the patients would receive gemcitabine despite a low hENT1 status resulted in a mean cost of €5358 per pancreatic cancer patient due to overtreatment. Adding the cost of sick leave and loss of production, the total cost per patient was estimated at €9449. The cost saving that may be achieved by testing for hENT1 before initiating gemcitabine treatment with a €50–200 test is shown in . Using hENT1 testing to select patients for gemcitabine therapy would save €8.6 million in Sweden each year [population (2011): 9 482 855 individuals]. Thus, the cost savings would be about €905 000/106 inhabitants and year, a level probably representative for health economical costs in Western Europe.
Figure 1. Estimated cost savings for individualizing gemcitabine treatment in Sweden with a €50–200 hENT1 test. Modeled on hospital costs (a) and overall costs (b).
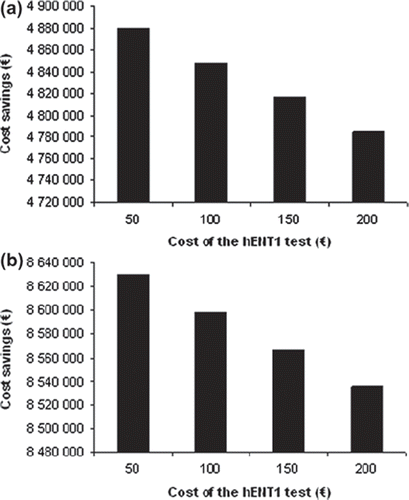
Table IV. Costs for gemcitabine treatment.
Discussion
This is the first health economical evaluation of gemcitabine overtreatment in pancreatic cancer. The strength is the detailed analysis of expenses, including primary treatment and complications, as well as indirect costs related to loss of production. Furthermore, chemotherapeutic regimens for pancreatic cancer patients are similar across different health care institutions in Sweden, and are thus less likely to have affected the overall results. Using the present data, the cost of gemcitabine overtreatment was estimated at €5358 per pancreatic cancer patient. Taking the recent publication on national costs associated with pancreatic cancer in Sweden into consideration [Citation25], the cost for overtreatment would represent as much as one-third of the direct treatment cost. However, apart from cost savings, individualizing gemcitabine therapy reduces unnecessary and potentially toxic side effects of treatment. Gemcitabine is generally considered to be associated with relatively moderate side effects, though we found that grade 3 to 4 toxicity occurred in 42%. Interestingly, side effects appear to be independent of the hENT1 status of the tumor, as has been shown, e.g. in post hoc analysis of the RTOG 9704 trial [Citation9]. Since hENT1 status has been related with outcome, but not with toxicity, other possible tests, such as polymorphisms and activity of the catabolic enzyme cytidine deamine (starting from blood samples, by genotyping PCR and by HPLC/spectrophotometric tests, respectively), which have been related with gemcitabine toxicity, may need to be developed and prospectively tested [Citation26,Citation27].
Ways to render gemcitabine more effective can be separated into two main groups: firstly by modification of the gemcitabine structure in order to make it independent of the otherwise necessary nucleoside transporters for cellular uptake [e.g. conjugation with elaidic acid (CO-101) (NCT01124786)], and secondarily by increasing the expression of hENT1 on the tumor cells. An illustrative example of the latter scenario, as demonstrated by in vitro studies, is treatment with the thymidylate synthase inhibitor, 5-fluourouracil, before initiating gemcitabine treatment [Citation14]. Currently, however, single-agent gemcitabine still represents the most established chemotherapeutic agent used in pancreatic cancer. By including hENT1 status estimates from four different cohorts (n = 605), it was concluded that only one-third of tumors would have a high expression of hENT1 [Citation19]. We have also found a complete concordance of the hENT1 receptor status when comparing the primary pancreatic cancer and lymph node metastasis [Citation19]. In this scenario, we thus demonstrated that hENT1 testing prior to treatment is cost-effective for the society (cost-savings €8.6 million per year) and reduces unnecessary toxicity.
The limitations of the present study should be duly noted. Although we used a detailed register-based approach there may be some uncertainty, especially regarding the costs of the hENT1 test. Sensitivity and specificity estimates for hENT1 testing were not included. Furthermore, data on quality-adjusted life years were not incorporated into the present study and may need to be considered in future health economical estimations. Based on future discrimination of patients potentially being non-responders of traditional gemcitabine treatment, alternative interventions and chemotherapeutic alternatives should be explored.
In conclusion, the mean hospital costs related to gemcitabine overtreatment amounted to €5358 per pancreatic cancer patient and total societal costs to €9449 when including indirect costs. The cost saving associated with hENT1 testing reached 8.6 million per year in Sweden. This study exemplifies how modern medicine could be customized to the individual patient with the hope of maximizing treatment efficacy, avoiding unnecessary toxicity and decreasing costs and by the use of companion diagnostics together with treatment.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Carpelan-Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 2005;54: 385–7.
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378:607–20.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Sun C, Ansari D, Andersson R, Wu D. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? A meta-analysis of 26 randomized controlled clinical trials. World J Gastroenterol 2012;18: 4944–58.
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: Identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). Mol Membr Biol 2001;18:65–72.
- Garcia-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: Role of hCNT1 in 2’,2’-difluorodeoxycytidine-induced cytotoxicity. Clin Cancer Res 2003;9: 5000–8.
- Spratlin JMJ. Human Equilibrative Nucleoside Transporter 1 (hENT1) in pancreatic adenocarcinoma: Towards individualized treatment decisions. Cancers 2010;2:11.
- Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 2008;299:1019–26.
- Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 2009;136:187–95.
- Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res 2006;66: 3928–35.
- Marechal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res 2009;15:2913–9.
- Morinaga S, Nakamura Y, Watanabe T, Mikayama H, Tamagawa H, Yamamoto N, et al. Immunohistochemical analysis of Human Equilibrative Nucleoside Transporter-1 (hENT1) predicts survival in resected pancreatic cancer patients treated with adjuvant gemcitabine monotherapy. Ann Surg Oncol 2012;19(Suppl 3):S558–64.
- Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res 2004;10:6956–61.
- Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, et al. Gemcitabine chemoresistance in pancreatic cancer: Molecular mechanisms and potential solutions. Scand J Gastroenterol 2009;44:782–6.
- Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2’,2’-difluorodeoxycytidine (gemcitabine). Drug Resist Updat 2002;5:19–33.
- Bergman AM, Eijk PP, Ruiz van Haperen VW, Smid K, Veerman G, Hubeek I, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res 2005;65:9510–6.
- Kroep JR, Loves WJ, van der Wilt CL, Alvarez E, Talianidis I, Boven E, et al. Pretreatment deoxycytidine kinase levels predict in vivo gemcitabine sensitivity. Mol Cancer Ther 2002;1:371–6.
- Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del Tacca M, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 2005;68:110–8.
- Boeck S, Raponi M, Richardson B, Towne P, Isaacson J, Ranger Moore J, et al. Development of the SP120 rabbit monoclonal antibody for determining hENT1 status and predicting response to gemcitabine in pancreatic ductal adenocarcinoma. J Clin Oncol 2012;30(Suppl):abstr 4041.
- Raponi M, Boeck S, Richardson B, Towne P, Isaacson J, Ranger-Moore J, et al. Two-thirds of pancreatic ductal adenocarcinomas have low expression of hENT1 as determined by the SP120 rabbit monoclonal antibody immunohistochemistry test. World GI 2012 (abstract).
- Bra att veta om sjuklön. National Social Insurance Office, Sweden. Faktablad. [cited 2012 Feb 28]. Available from: http://www.forsakringskassan.se
- Tingstedt B, Isaksson J, Andersson R. Long-term follow-up and cost analysis following surgery for small bowel obstruction caused by intra-abdominal adhesions. Br J Surg. 2007;94:743–8.
- Södra regionsvårdsnämnden. Regionala priser och ersättningar för södra sjukvårdsregionen 2011. [cited 2012 Mar 6]. Available from http://www.skane.se/sv/Webbplatser/Sodra-regionvardsnamnden/
- Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol 2004;22:854–63.
- Tingstedt B, Andersson E, Flink A, Bolin K, Lindgren B, Andersson R. Pancreatic cancer, healthcare cost, and loss of productivity: A register-based approach. World J Surg 2011; 35:2298–305.
- Farrell JJ, Bae K, Wong J, Guha C, Dicker AP, Elsaleh H. Cytidine deaminase single-nucleotide polymorphism is predictive of toxicity from gemcitabine in patients with pancreatic cancer: RTOG9704. Pharmacogenomics J2012;12: 395–403.
- Okazaki T, Javle M, Tanaka M, Abbruzzese JL, Li D. Single nucleotide polymorphisms of gemcitabine metabolic genes and pancreatic cancer survival and drug toxicity. Clin Cancer Res 2010;16:320–9.